Comment on Bougie et al, page 922
Quinine is a universal remedy for the common symptom of nocturnal leg cramps and may be the most common cause of drug-induced thrombocytopenia and multisystem disorders.
Thrombocytopenia is the most common drug-induced hematologic disorder, and therefore platelets have been the most useful tool for understanding the molecular mechanisms of drug-dependent antibodies. Although an early hypothesis was that drug-dependent antibodies are formed when drugs are covalently bound to a protein, inducing drug (hapten)–specific antibodies, this mechanism is not consistent with the reversible, low-affinity binding of quinine to platelets. Another hypothesis was that antibodies reacted directly with quinine to form immune complexes that reacted with platelet Fc receptors; this hypothesis gained attention because of its clever description of platelets as merely “innocent bystanders.” The innocent bystander hypothesis was discarded 20 years ago by the demonstration that drug-dependent antibodies reacted with platelets via their Fab, rather than their Fc, domains.1 FIG1
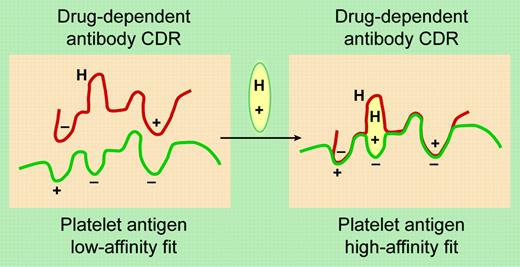
A proposed model for DDAb binding to an epitope on a platelet GP. The drug (oval symbol) has elements that bind to the charged and hydrophobic (H) domains on the glycoprotein epitope and the antibody's complementarity-determining region (CDR). See the original figure in the article beginning on page 922; adapted from the original by Frank Forney.
A proposed model for DDAb binding to an epitope on a platelet GP. The drug (oval symbol) has elements that bind to the charged and hydrophobic (H) domains on the glycoprotein epitope and the antibody's complementarity-determining region (CDR). See the original figure in the article beginning on page 922; adapted from the original by Frank Forney.
In this issue of Blood, Bougie and colleagues describe new and unexpected observations on quinine-dependent antibodies. Two distinct antibodies were identified in the serum of patients with quinine-dependent thrombocytopenia. First, platelet-reactive antibodies were demonstrated by their binding to platelets only in the presence of quinine, as illustrated in the figure. In the absence of the drug, the antibody complementarity-determining region (CDR) fits poorly with the epitope on the platelet antigen. However the antibody and antigen are brought together in the presence of the drug, which binds to both the antibody and antigen. These are the antibodies that cause quinine-induced thrombocytopenia. This mechanism explains why platelet counts are normal in spite of persistent high titers of quinine-dependent antiplatelet antibodies in the absence of quinine and also why the profound thrombocytopenia occurs suddenly when quinine is ingested.
Second, and unexpectedly, Bougie and colleagues demonstrated distinct quinine-specific antibodies that reacted with quinine-conjugated albumin. In contrast to the quinine dependence of the platelet-reactive antibodies, this reaction was inhibited by soluble quinine. Remarkably, both of these antibodies had identical, exquisite specificity for the molecular structure of quinine. These quinine-specific antibodies may have no role in the etiology of thrombocytopenia, but they may allow simpler assays to detect drug-dependent antibodies.
A simpler test to confirm the diagnosis is important because quinine-induced thrombocytopenia is common and also commonly overlooked. Patients may take quinine only occasionally for leg cramps or may be exposed to quinine only in beverages, and therefore they may not report quinine in response to routine questions about current medications.2-4 Overlooking quinine as an etiology for thrombocytopenia or multisystem disorders, which may include acute anuric renal failure, acute hepatitis, and disseminated intravascular coagulation, causes a risk for inappropriate therapy and recurrent episodes.2,3,5
More questions remain. Why do most patients take quinine medications or drink quinine-containing beverages for many years without harm while a few patients develop dangerous immune-mediated reactions? Why do most patients develop quinine-dependent antibodies that cause only thrombocytopenia, while other patients have acute, severe multiorgan failure? Since quinine is produced from cinchona that in turn is extracted from the bark of cinchona trees, can cinchona-containing products also cause “quinine”-dependent thrombocytopenia and systemic disorders? Although cinchona-containing herbal remedies are widely distributed,4 no adverse reactions have yet been described.
The answers to these questions will help us to understand the basis for quinine- and other drug-induced disorders. We can count on the investigators of the Blood Center of Wisconsin to help us better understand drug-induced disorders. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal