Abstract
In leukemogenesis, several genetic changes conferring a proliferative and/or survival advantage to hematopoietic progenitor cells in addition to a block in differentiation are required. Here, we demonstrate that overexpression of the wild-type (wt) Flt3 receptor tyrosine kinase collaborates with NUP98-HOX fusions (NUP98-HOXA10 and NUP98-HOXD13) to induce aggressive acute myeloid leukemia (AML). We used a mouse transplantation model to show their synergism in cotransduced bone marrow cells as well as in a cellular model of leukemic progression. Furthermore, our data support the finding that Meis1 overexpression leads to marked elevation in Flt3 transcription and extend it to the context of NUP98-HOX–induced leukemia. Together, these results support a multistep model where the synergism between NUP98-HOX and wt-Flt3 is the result of the ability of Flt3 to increase proliferation of myeloid progenitors blocked in differentiation by NUP98-HOX fusions and reveal a direct role for wt-Flt3 in the pathobiology of AML. Given the similarities in the leukemogenic role of native HOX and NUP98-fused HOX genes, our results underscore the clinical significance of the recurrent co-overexpression of wt-FLT3 and HOX in human leukemia and suggest that specific FLT3 inhibitors could be useful in treatment of HOX-induced AML or acute lymphoblastic leukemia (ALL).
Introduction
The identification and functional characterization of fusion oncogenes involving transcription factors have resulted in the discovery of pathways involved in leukemic transformation. However, it is becoming increasingly clear that these defects themselves are not sufficient to cause acute leukemia. Thus, an emerging paradigm is that the fusion oncogenes block differentiation pathways of hematopoietic precursors, giving rise to a preleukemic population of cells that are then susceptible to the acquisition of cooperating genetic changes that drive their proliferation, such as those involving receptor tyrosine kinases.1 Due to the difficulties in the use of small molecules to inhibit the products of fusion oncogenes encoding DNA-binding proteins, the identification of collaborating events leading to more suitable therapeutic targets in leukemia is of high clinical relevance.
Homeobox-containing genes, a family of transcription factors first identified for their function in early development, play a major role in cancer. Indeed, they are linked to a wide array of leukemias by virtue of their deregulated overexpression, as for example through translocations involving the HOX regulator MLL.2 Moreover, they are also directly associated with chromosomal translocations. In this context, Abd-B HOX genes are the most common fusion partners of nucleoporin 98 (NUP98).3,4 NUP98-HOX fusions containing different Abd-B HOX genes have been identified in patients with myeloid leukemia (acute myeloid leukemia [AML]), posttherapy AML, and chronic myeloid leukemia.3
The leukemogenic potential of NUP98-HOX fusions has been demonstrated in murine bone marrow (bm) transplantation models.5-8 However, the long latency of the disease provides evidence that HOX fusions, as well as their native counterparts, require additional genetic events for the induction of overt leukemia. Indeed, we and others have shown that overexpression of the HOX cofactor Meis1 cooperates with multiple native and NUP98-fused HOX proteins to accelerate the onset of AML.8-10
Interestingly, leukemias associated with high levels of HOX show significantly higher levels of the FMS-like tyrosine kinase receptor (FLT3) mRNA11,12 and are associated with an increase of FLT3 mutations,11 thus warranting the investigation of FLT3 as a novel putative HOX collaborator. Moreover, the presence of high levels of FLT3 in leukemia associated with increased expression of HOX and MEIS112 suggests that FLT3 may in part serve as an important mediator of the observed collaboration between the 2 genes. This possibility is supported by recent data demonstrating the up-regulation of Flt3 by Meis1 in a mouse model of HOXA9-induced AML.13
FLT3 is expressed in 70% to 100% of patients with AML, essentially in all patients with B-cell acute lymphoblastic leukemia (B-ALL) and in approximately 30% of T-cell ALL (T-ALL).14,15 FLT3-activating mutations also represent the most common somatic genetic alteration in AML and are associated with poor prognosis in both adult and pediatric AML patients.16-18 Furthermore, synergism between FLT3-activating mutations and several fusion oncogenes containing transcription factors has been demonstrated.19-21 Thus, the idea of collaboration of FLT3 with native and fused HOX genes is appealing in the context of a progression model of leukemogenesis in which FLT3 provides a proliferative advantage to preleukemic myeloid progenitors blocked in differentiation by HOX.
Although a large number of studies point to a significant role of FLT3-activating mutations in human AML, the role of increased levels of wild-type (wt) FLT3 in the etiology of the disease is still unclear. Furthermore, overexpression of wt-FLT3 is a common event in leukemia associated with FLT3-activating mutations, and moreover, high levels of wt-FLT3 have been reported in a large proportion of AML patients without FLT3 mutations.15,22,23
In the present study, we investigated the possibility of collaboration between NUP98-HOX fusions and wt-Flt3 in the induction of AML. Herein, we reveal synergy between HOX fusions (NUP98-HOXD13 and NUP98-HOXA10) and wt-Flt3, thus showing for the first time a direct functional role for wt-Flt3 in the pathobiology of AML. We also demonstrate that Flt3 expression is induced by Meis1 overexpression in NUP98-HOX–derived preleukemic cells, thus expanding the role of Flt3 as a mediator of Meis1 leukemic effects in the context of NUP98-HOX–induced AML. These results highlight the likely functional significance of identifying elevated levels of wt-FLT3 in leukemia and provide new directions for targeted molecular therapy directed at FLT3.
Materials and methods
Retroviral vectors
The following vectors have been previously described24 : MSCV-IRES-GFP (green fluorescent protein [GFP] virus), MSCV FLAG-NUP98-HOXD13-IRES-GFP (ND13 virus), MSCV-FLAG-NUP98-HOXA10-IRES-GFP (NA10 virus), and MSCV-HA-Meis1a-IRES-YFP (Meis1 virus). The MSCV-Flt3 IRES-YFP (Flt3 virus) was constructed using a mouse Flt3 cDNA kindly provided by Dr Connie J. Eaves (Terry Fox Laboratory, British Columbia Cancer Agency, University of British Columbia, Canada).
Generation of transduced 5-FU bm cells and NUP98-HOX preleukemic cell lines
Primary mouse bm cells were transduced as previously described.7 Establishment and characterization of the NUP98-HOX preleukemic cell lines has recently been reported.24 In brief, these lines were established from bm cells from (C57Bl/6Ly-Pep3b × C3H/HeJ) F1 (PepC3) mice freshly transduced with the NA10 or ND13 virus, and maintained for a period of 4 weeks or more in liquid culture (Dulbecco modified Eagle medium [DMEM] supplemented with 15% fetal bovine serum [FBS], 10 ng/mL human interleukin-6 (hIL-6), 6 ng/mL murine (m) IL-3, and 100 ng/mL murine stem cell factor [mSCF]). To generate NUP98-HOX/Meis1 and NUP98-HOX/Flt3 cell lines, the NUP98-HOX lines were transduced by cocultivation on irradiated E86-Meis1 or E86-Flt3 viral producers, respectively, for a period of 2 days in the presence of 5 μg/mL of protamine sulfate (Sigma, Oakville, ON, Canada). All culture media and growth factors were obtained from StemCell Technologies (Vancouver, BC, Canada). Cells were maintained at a cell density below 1 × 106/mL. Cells were counted with the Vi-Cell XR Cell Viability Analyzer (Beckman Coulter, Fullerton, CA).
bm transplantation and monitoring of recipients
Parental strain mice were bred and maintained as approved by the University of British Columbia Animal Care Committee. Donors of primary bm cells were PepC3 mice (Ly5.1+/Ly5.2+) and recipients were (C57Bl/6J × C3H/HeJ) F1 (B6C3) mice (Ly5.2+/Ly5.2+). Cells were injected into the tail vein of irradiated (900 cGy of 137Cs γ-radiation) recipient mice. Unless otherwise indicated, transplants consisted of a life-sparing dose of 2.5 × 105 B6C3 bm cells together with 7.5 × 105 NUP98-HOX cells (GFP+) or 5 × 103 to 7.5 × 105 NUP98-HOX cells transduced with the Meis1 or Flt3 virus (GFP+/YFP+) after selection by flow cytometry. Mice given transplants of freshly cotransduced bm cells received the equivalent of 3 × 104 to 5 × 104 cotransduced cells. Donor-derived engraftment and reconstitution were monitored by flow cytometry analysis of GFP and/or yellow fluorescent protein (YFP) expression in the peripheral blood of the transplants. For phenotypic analysis, single-cell suspensions were stained with the following monoclonal antibodies: PE-labeled Gr-1, Mac-1, B220, Sca-1, c-Kit, and Ly5.1 (all obtained from Pharmingen, San Diego, CA). Morphologic and histologic analyses were performed as previously described.7
In vitro assays
Cell proliferation was assessed in 10% FBS in DMEM supplemented with the cytokine cocktail described, or with murine Flt3 ligand (FL; StemCell Technologies) with or without the Flt3 inhibitor AG1295 (catalog no. T3932; Sigma) at a concentration of 10 μM. Cells were washed 3 times with phosphate-buffered saline (PBS) before plating in specified concentrations of FL-supplemented media.
Real-time RT-PCR
Quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was done as previously described.25 The relative expression changes were determined with the 2-ΔΔCT method,26 and the housekeeping glyceraldehyde-3-phosphate dehydrogenase (Gapdh) gene transcript was used to normalize the results. Primers were ordered from and manufactured by Invitrogen (Carlsbad, CA). Primer sequences (5′ to 3′) are as follows: Flt3 (NM_010 229) forward primer ATCCTTCCCCAACCTGACTT, reverse primer TTGCCACCCATGTTCTGATA; Meis1 (NM_010 789) forward primer GCACAGGTGACGATGATGAC, reverse primer AGGGTGTGTTAGATGCTGGAA; Flt3 ligand (NM_013 520) forward primer CAGACCAACATCTCCCACCT, reverse primer TAGGGCTATGGGACTCCTTG; and Gapdh (NM_008 084) forward primer AACTTTGGCATTGTGGAAGG, reverse primer ATGCAGGGATGATGTTCTGG.
Stimulation experiments and Western blot analysis
NA10 or ND13 cells transduced with Meis1 or Flt3 were washed 3 times with PBS and cytokine-starved for 16 hours in DMEM containing 0.5% FBS. The cells were then stimulated with a suboptimal concentration of growth factors (3% FBS, 2 ng/mL hIL-6, 1.2 ng/mL mIL-3, 20 ng/mL mSCF, and 10 ng/mL FL) or FL alone (20 ng/mL) for 5 minutes at 37°C. For the stimulation experiments, 1 × 106 stimulated and nonstimulated cells were lysed with 80 μL 1 × sodium dodecyl sulfate (SDS) sample buffer, passaged 5 times through a 26-gauge needle to sheer DNA. The samples were boiled for 1 minute, separated on a 10% SDS–polyacrylamide gel electrophoresis (PAGE), transferred to polyvinylidene fluoride (PVDF) and probed with antibodies for p-Akt, p-Erk1/2, or GAPDH as a loading control. For all other Western blotting, 1 × 106 cells were lysed with 150 μL lysis buffer (50 mM Tris-HCl [pH 8], 0.1% Tween-20, 0.1% SDS, 150 mM NaCl, 0.5 mM EDTA, 10 mM DTT, and 1 mM PMSF, plus protease inhibitor cocktail; Sigma, catalog no. P8340) and incubated for 10 minutes on ice followed by brief sonication to reduce viscosity. NuPage buffer × 4 (catalog no. NP0007; Invitrogen) (50 μL) was added and samples were heated for 10 minutes at 70°C. Lysates were loaded onto 4% to 12% NuPage Novex BIS-Tris SDS-polyacrylamide gels (catalog no. NP0321BOX; Invitrogen) and electroblotted in MOPS transfer buffer to nitrocellulose membrane (catalog no. LC2009; Invitrogen). Rabbit polyclonal anti-Flt3 (catalog no. sc-340; Santa Cruz Biotechnology, Santa Cruz, CA), goat polyclonal anti-Meis1/2 (C-17, catalog no. sc-10 599; Santa Cruz Biotechnology), rabbit polyclonal anti–phospho-Erk (catalog no. 9101; Cell Signaling Technology, Beverly, MA), rabbit polyclonal anti–phospho-Akt (catalog no. 9271; Cell Signaling Technology), or mouse monoclonal anti-GAPDH (catalog no. TRK5G4-6C5; RDI, Flanders, NJ) and horseradish peroxidase–conjugated donkey antimouse or goat antirabbit antibodies (catalog nos. A9044 and A0545, respectively; Sigma) or donkey antigoat antibodies (catalog no. sc-2020; Santa Cruz Biotechnology) in 0.1% Tween-20, 5% bovine serum albumin (BSA) Tris-buffered saline (TBS) were used for protein detection. Proteins were visualized using enhanced chemiluminescence (ECL) reagent (Renaissance, Boston, MA).
Wt-Flt3 collaborates with NUP98-HOXA10 to induce leukemia in mice. Kaplan-Meier survival curves of mice given transplants of 5-FU–treated bm transduced with Flt3, NUP98-HOXA10, or NUP98-HOXA10 and Flt3. The median survival time for mice given transplants of bm cells expressing NUP98-HOXA10 + Flt3 was 114 days. The log-rank test was used to confirm the statistical significance between the NUP98-HOXA10 and NUP98-HOXA10 + Flt3 curves (P = .02).
Wt-Flt3 collaborates with NUP98-HOXA10 to induce leukemia in mice. Kaplan-Meier survival curves of mice given transplants of 5-FU–treated bm transduced with Flt3, NUP98-HOXA10, or NUP98-HOXA10 and Flt3. The median survival time for mice given transplants of bm cells expressing NUP98-HOXA10 + Flt3 was 114 days. The log-rank test was used to confirm the statistical significance between the NUP98-HOXA10 and NUP98-HOXA10 + Flt3 curves (P = .02).
Southern blot analysis
Genomic DNA was isolated using DNAzol reagent as recommended by the manufacturer (Invitrogen), and Southern blot analyses were performed as previously described.7 In short, DNA was digested with the long terminial repeat (LTR)–specific NheI restriction endonuclease and probed with a GFP-specific probe to confirm the integrity of the NUP98-HOXA10 and NUP98-HOXD13 GFP (4.5 kb) or Flt3 YFP (3.1 kb) proviruses, or DNA was digested with HindIII restriction endonuclease and probed with an Flt3-specific probe. The Flt3 probe used for the Southern was a 689-bp EcoRI-XhoI fragment from the MSCV-Flt3-IRES-YFP Vector construct corresponding to the 3′-end of the cDNA insert.
Statistical analysis
All statistical measures were calculated using GraphPad Prism Version 4.00 statistical software (San Diego, CA). Comparison of survival curves were performed using the log-rank test.
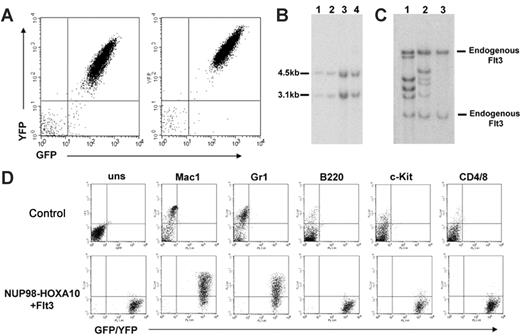
Mice given transplants of bm cotransduced with NUP98-HOXA10 and Flt3 succumb to AML. (A) Analysis of GFP (NUP98-HOXA10) and YFP (Flt3) expression in leukemic bm cells (2 representative profiles shown). (B) Southern blot analysis of genomic DNA harvested from the bm of leukemic mice. DNA digested with the LTR-specific NheI restriction endonuclease was probed with a GFP-specific probe confirming the integrity of the NUP98-HOXA10-GFP (4.5 kb) and Flt3-YFP (3.1 kb) proviruses. Lanes 1 and 2 show NUP98-HOXA10 + Flt3 transplant 4, bm, and spleen, respectively. Lanes 3 and 4 show NUP98-HOXA10 + Flt3 transplant 1, bm, and spleen, respectively. (C) Southern blot analysis of genomic DNA harvested from the spleen of leukemic mice. DNA was digested with a restriction endonuclease (HindIII) and probed with an Flt3-specific probe. Lane 1 shows NUP98-HOXA10 + Flt3 transplant 3; lane 2, NUP98-HOXA10 + Flt3 transplant 4; and lane 3, C57-negative control. (D) Immunophenotyping of bm cells. Top panel shows flow cytometric profiles on bm from a B6C3 control mouse; bottom panel shows bm from a representative NUP98-HOXA10 + Flt3 leukemic mouse. Expression of GFP/YFP is shown on the x-axis and the presence of the cell-surface antigens is shown on the y-axis. uns indicates unstained.
Mice given transplants of bm cotransduced with NUP98-HOXA10 and Flt3 succumb to AML. (A) Analysis of GFP (NUP98-HOXA10) and YFP (Flt3) expression in leukemic bm cells (2 representative profiles shown). (B) Southern blot analysis of genomic DNA harvested from the bm of leukemic mice. DNA digested with the LTR-specific NheI restriction endonuclease was probed with a GFP-specific probe confirming the integrity of the NUP98-HOXA10-GFP (4.5 kb) and Flt3-YFP (3.1 kb) proviruses. Lanes 1 and 2 show NUP98-HOXA10 + Flt3 transplant 4, bm, and spleen, respectively. Lanes 3 and 4 show NUP98-HOXA10 + Flt3 transplant 1, bm, and spleen, respectively. (C) Southern blot analysis of genomic DNA harvested from the spleen of leukemic mice. DNA was digested with a restriction endonuclease (HindIII) and probed with an Flt3-specific probe. Lane 1 shows NUP98-HOXA10 + Flt3 transplant 3; lane 2, NUP98-HOXA10 + Flt3 transplant 4; and lane 3, C57-negative control. (D) Immunophenotyping of bm cells. Top panel shows flow cytometric profiles on bm from a B6C3 control mouse; bottom panel shows bm from a representative NUP98-HOXA10 + Flt3 leukemic mouse. Expression of GFP/YFP is shown on the x-axis and the presence of the cell-surface antigens is shown on the y-axis. uns indicates unstained.
Results
Wt-Flt3 collaborates with NUP98-HOX in the induction of AML
To investigate the possibility of synergism between enhanced levels of wt-Flt3 and NUP98-HOX in AML, we exploited the mouse bm transplantation model. Lethally irradiated mice received transplants of unsorted freshly transduced 5-FU bm cells coinfected with the NUP98-HOXA10-GFP and Flt3-YFP viruses (2 independent experiments were conducted with frequencies of 5.2% [n = 5] and 1% [n = 4] GFP+/YFP+ or infected singly with the NUP98-HOXA10-GFP virus (81% GFP+ [n = 5]), or with the Flt3-YFP virus (8% YFP+ [n = 3]) as controls. All recipients of Flt3 singly transduced bm remained healthy over the observation time extending 270 days (Figure 1). Two of 5 recipients of NUP98-HOXA10–transduced bm died of a myeloproliferative disorder at days 178 and 228 after transplantation (Figure 1). The disease was characterized by elevated white cell counts but a low percentage of blasts in bm (data not shown) and consistent with our previously reported NUP98-HOXA10 experiments.8 Quantitative RT-PCR revealed no induction of endogenous Flt3 gene expression in these mice (data not shown). In sharp contrast, 8 of 9 mice that received bm cells cotransduced with NUP98-HOXA10 and Flt3 succumbed to aggressive AML with a median latency of 114 days (Figure 1). Fluorescence-activated cell sorter (FACS; Figure 2A) and Southern blot analyses (Figure 2B) demonstrated the presence of the NUP98-HOXA10 and Flt3 provirus in the leukemic bm cells. Moreover, Flt3 proviral integration patterns revealed that the leukemias arose through independent clones within the same experiments (Figure 2C). The primary leukemia was transplantable, with secondary transplant recipients rapidly succumbing to AML (median latency of 24.5 days; data not shown) with characteristics similar to those seen in the primary animals.
The diagnosis of AML in the recipients of double-transduced bm was supported by elevated peripheral nucleated cell counts (mean of 1.5 × 108/mL), splenomegaly with a mean weight of 0.84 g (range, 0.33-2.13 g), and more than 20% poorly differentiated myeloblast cells, as revealed by examination of Wright-Giemsa staining of bm preparations (data not shown). Histopathologic analysis of the diseased mice showed massive myeloblast infiltration in the spleen and other organs such as the liver and kidneys (data not shown). A myelomonocytic phenotype of the leukemic cells was further confirmed by flow cytometry, which revealed that the majority of the leukemic bm cells were Mac-1+ and Gr-1+, but negative for B220, c-Kit, CD4, CD8 (Figure 2D), and Sca-1 (data not shown).
Altogether, these experiments clearly demonstrate that constitutive expression of wt-Flt3 and NUP98-HOXA10 can functionally collaborate to induce rapid, aggressive AML.
Overexpression of wt-Flt3 induces the leukemic conversion of NUP98-HOX–derived preleukemic cells
To further assess the collaboration between wt-Flt3 and NUP98-HOX fusions, we exploited a novel model of leukemic progression. These preleukemic NUP98–HOXD13 and NUP98–HOXA10–derived myeloid lines (ND13 and NA10, respectively) contain early granulomonocytic progenitors with extensive in vitro self-renewal capacity and short-term myeloid repopulating activity but essentially no spontaneous conversion to leukemia-initiating cells.24 These cells can, however, be converted to leukemia-initiating cells with high efficiency by engineered overexpression of Meis1, thus providing a powerful platform to test candidate genes for collaborative potential with NUP98-HOX genes.24
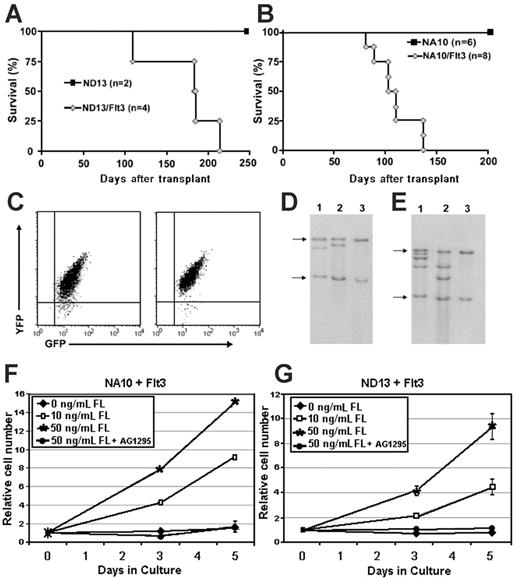
To this end, 2 NA10 and ND13 progenitor cell lines were transduced with the Flt3-YFP retroviral vector (NUP98-HOX/Flt3 cells) to determine whether the synergistic role of wt-Flt3 and NUP98-HOXA10 observed in primary bm experiments is reproducible in a preleukemic cell-line model and extends to other NUP98-HOX fusion genes. Parental NA10 and ND13 cells had low but detectable levels of Flt3 expression by Western blot analysis (Figure 3B) but did not show any significant growth even in the presence of high (50 ng/mL) concentrations of FL (Figure 4A-B). Elevated levels of Flt3 protein were clearly detectable after Flt3 transduction (Figure 3B), and these cells showed a strong proliferative response to FL that was blocked by the Flt3 inhibitor AG1295 (Figure 4A-B). Importantly, growth of the preleukemic or transduced cells was not inhibited by AG1295 when stimulated with IL-3, thus ruling out nonspecific toxicity from the inhibitor (mean 4.5-fold expansion in 3 days in the presence or absence of inhibitor). The leukemogenic potential of the NUP98-HOX/Flt3 cells was tested by injecting double-transduced cells (4.0 × 104 GFP+/YFP+) into lethally irradiated recipients. As shown in Figures 5A and 5B, mice injected with ND13/Flt3 cells (n = 4) or NA10/Flt3 cells (n = 8) succumbed to disease with a median latency of 184.5 and 107 days after transplantation, respectively. As expected, mice receiving NA10 or ND13 parental cells showed transient myeloid repopulation (data not shown) and remained healthy beyond an observation period longer than 250 and 200 days, respectively (Figures 5A-B), which is consistent with our previous finding for these preleukemic cells.24 Moreover, recipients of NA10 or ND13 preleukemic cells transduced with an “empty” YFP-control virus also remained healthy over a similar observation period, making it highly unlikely that insertional mutagenesis events accounts for the leukemic conversion observed with transduction with Flt3 (data not shown). FACS analysis confirmed that the bm cells of the leukemic animals were double transduced (Figure 5C), and Southern blot analysis showed that different animals carried leukemic cells of independent origin (Figures 5D-E). Analysis of the diseased mice supported the diagnosis of AML with characteristics similar to those described for the primary animals that received transplants of cotransduced 5-FU bm (data not shown). This leukemia was also transplantable, with secondary transplant recipients rapidly succumbing to AML (median latency of 77 days; data not shown). Furthermore, leukemic cells harvested from primary diseased mice were still dependent on the presence of FL to grow ex vivo and, importantly, their growth was inhibited by the addition of the Flt3 inhibitor AG1295 (Figure 5F-G).
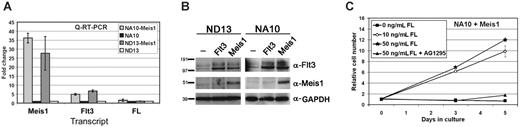
Elevated expression of Flt3 by transduction of Meis1 confers a survival and proliferative advantage to the NUP98-HOX cells. (A) Quantitative RT-PCR showing the levels of Meis1, Flt3, and FL transcripts in the NA10 and ND13 preleukemic cells before and after Meis1 or Flt3 transduction. Levels are shown as fold changes relative to the parental lines. The error bars represent standard deviation from 2 independent experiments. (B) Western blot analysis showing an increase of Flt3 protein levels in the NUP98-HOX cells upon transduction with the Flt3 or Meis1 retroviruses. Both bands represent Flt3; the top band is the glycosylated form. The levels of Meis1 and GAPDH are also shown. The transduced cells were sorted for GFP/YFP expression and at least 85% of the tested cells were double positive. (C) Ex vivo dose-response growth curves where bm cells harvested from diseased NA10/Meis1 mice were grown in 10% FBS and 0, 10, or 50 ng/mL FL in the presence or absence of Flt3 inhibitor AG1295 for a period of 5 days. The error bars represent the standard deviation from 3 independent experiments.
Elevated expression of Flt3 by transduction of Meis1 confers a survival and proliferative advantage to the NUP98-HOX cells. (A) Quantitative RT-PCR showing the levels of Meis1, Flt3, and FL transcripts in the NA10 and ND13 preleukemic cells before and after Meis1 or Flt3 transduction. Levels are shown as fold changes relative to the parental lines. The error bars represent standard deviation from 2 independent experiments. (B) Western blot analysis showing an increase of Flt3 protein levels in the NUP98-HOX cells upon transduction with the Flt3 or Meis1 retroviruses. Both bands represent Flt3; the top band is the glycosylated form. The levels of Meis1 and GAPDH are also shown. The transduced cells were sorted for GFP/YFP expression and at least 85% of the tested cells were double positive. (C) Ex vivo dose-response growth curves where bm cells harvested from diseased NA10/Meis1 mice were grown in 10% FBS and 0, 10, or 50 ng/mL FL in the presence or absence of Flt3 inhibitor AG1295 for a period of 5 days. The error bars represent the standard deviation from 3 independent experiments.
Overexpression of wt-Flt3 confers responsiveness to FL. (A-B) Shown are comparisons of growth curves for parental and Flt3-transduced cells stimulated with 50 ng/mL FL in the presence or absence of Flt3 inhibitor AG1295 for a period of 5 days.
Overexpression of wt-Flt3 confers responsiveness to FL. (A-B) Shown are comparisons of growth curves for parental and Flt3-transduced cells stimulated with 50 ng/mL FL in the presence or absence of Flt3 inhibitor AG1295 for a period of 5 days.
Taken together, these results indicate an ability of wt-Flt3 to collaborate with 2 different NUP98-HOX fusions. Moreover, they support a multistep model in which the synergism between NUP98-HOX and Flt3 is the result of the ability of Flt3 to induce the proliferation/self-renewal of preleukemic myeloid progenitors blocked in differentiation by NUP98-HOX.
Transplantable, preleukemic NUP98-HOX cells can be converted into leukemic cells by wt-Flt3. (A) Survival curve of mice given transplants of ND13 or ND13/Flt3 cells. (B) Survival curve of mice given transplants of NA10 or NA10/Flt3 cells. (C) FACS analysis of GFP (ND13/NA10) and YFP (Flt3) expression in leukemic bm cells (2 representative profiles shown). (D) Southern blot analysis of genomic DNA harvested from the bm of ND13/Flt3 leukemic mice and probed with an Flt3-specific probe. Lanes 1 and 2 show ND13/Flt3 transplants 1 and 2, respectively; Lane 3, C57-negative control mouse. Arrows denote endogenous Flt3. (E) Southern blot analysis of genomic DNA harvested from bm of NA10/Flt3 leukemic mice and probed with an Flt3-specific probe. Lanes 1 and 2 show NA10/Flt3 transplants 1 and 2, respectively; Lane 3, C57-negative control mouse. (F-G) In vitro dose-response growth curves where bm cells harvested from diseased NA10/Flt3 or ND13/Flt3 mice were grown in 10% FBS and 0, 10, or 50 ng/mL FL in the presence or absence of Flt3 inhibitor AG1295 for a period of 5 days. The error bars represent the standard deviation from 3 independent experiments.
Transplantable, preleukemic NUP98-HOX cells can be converted into leukemic cells by wt-Flt3. (A) Survival curve of mice given transplants of ND13 or ND13/Flt3 cells. (B) Survival curve of mice given transplants of NA10 or NA10/Flt3 cells. (C) FACS analysis of GFP (ND13/NA10) and YFP (Flt3) expression in leukemic bm cells (2 representative profiles shown). (D) Southern blot analysis of genomic DNA harvested from the bm of ND13/Flt3 leukemic mice and probed with an Flt3-specific probe. Lanes 1 and 2 show ND13/Flt3 transplants 1 and 2, respectively; Lane 3, C57-negative control mouse. Arrows denote endogenous Flt3. (E) Southern blot analysis of genomic DNA harvested from bm of NA10/Flt3 leukemic mice and probed with an Flt3-specific probe. Lanes 1 and 2 show NA10/Flt3 transplants 1 and 2, respectively; Lane 3, C57-negative control mouse. (F-G) In vitro dose-response growth curves where bm cells harvested from diseased NA10/Flt3 or ND13/Flt3 mice were grown in 10% FBS and 0, 10, or 50 ng/mL FL in the presence or absence of Flt3 inhibitor AG1295 for a period of 5 days. The error bars represent the standard deviation from 3 independent experiments.
Flt3 levels are increased by the overexpression of Meis1 in NUP98-HOX progenitor cells
The leukemogenic potential of wt-Flt3 highlights the importance of identifying the mechanisms responsible for its increased level in leukemia. In this regard, for example, leukemias associated with MLL translocations show increased cooverexpression of FLT3, HOX, and MEIS1.12 Given the similar ability of Meis1 and Flt3 to collaborate with HOX, their coordinated up-regulation is interesting. A link between Meis1 and Flt3 expression has been suggested by Wang et al,13 who have shown that Meis1 programs the transcription of Flt3 in a HOXA9-derived myeloid precursor. To determine if there is a relationship between Flt3 and Meis1 expression in our NUP98-HOX model, we determined the levels of Flt3 in the NUP98-HOX cells before and after increased expression of Meis1 through retroviral transduction. Quantitative RT-PCR showed that, although the NUP98-HOXD13 and NUP98-HOXA10 cell lines already expressed low levels of Flt3, following their transduction with Meis1, a 20- to 30-fold increase in Meis1 mRNA transcripts was associated with a concomitant 5- to 7-fold increase in Flt3 transcripts (Figure 3A). This was also accompanied by a clear increase in Flt3 protein, as shown by Western blotting, that was similar to the level achieved upon Flt3 viral transduction (Figure 3B). The expression of endogenous FL was also assessed by quantitative RT-PCR in the NUP98-HOXD13 and NUP98-HOXA10 cell lines. FL expression could be detected in all cell lines but no difference was found between parental cell lines and cell lines transduced with either Meis1 or Flt3 (Figure 3A).
To test whether the elevated expression of Flt3 conferred any survival and/or proliferative advantage in the setting of Meis1 overexpression, leukemic cells from diseased mice transplanted with double-transduced NUP98-HOXA10/Meis1 cells were cultured in media supplemented with 10% FBS with or without FL at varying concentrations. All cell samples were at least 85% double-positive for NUP98-HOXA10 (GFP+) and Meis1 (YFP+) prior to culturing (data not shown). A dose-dependent growth response was observed with addition of FL to the Meis1-transduced NUP98-HOXA10 cells, and their growth was inhibited by the addition of the Flt3 inhibitor AG1295 (Figure 3C). The NUP98-HOXA10/Meis1 cells could be maintained in the presence of 10% FBS and 50 ng/mL FL for several weeks (not assessed further; data not shown).
Taken together, these results demonstrate a strong positive correlation between the levels of Meis1 and Flt3 and suggest that Meis1 regulates the expression of Flt3 in the NUP98-HOX cells. Furthermore, these results demonstrate that increased Meis1 expression is associated with robust up-regulation of Flt3 and subsequent response to FL and that NUP98-HOXA10/Meis1–transduced leukemic cells from diseased mice retain FL growth responsiveness ex vivo.
NUP98-HOX/Meis1 and NUP98-HOX/Flt3 bm cells show similar signal transduction properties
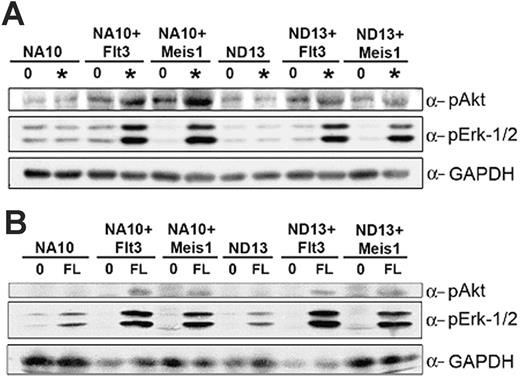
The similar ability to induce leukemic conversion of the NUP98-HOX preleukemic cells by overexpression of Meis1 or Flt3 prompted us to compare the phosphorylation status of Erk1/2 and Akt, known key downstream targets of Flt3, in these 2 settings. Cells were starved for 16 hours and then stimulated with nonsaturating concentrations of cytokines, including FL and with FL alone. As shown in Figure 6, overexpression of Flt3 or Meis1 enables these transduced cells to respond to low concentrations of growth factors that are not sufficient to induce a response in the parental, untransduced cells. By contrast, Erk1/2 are markedly phosphorylated upon growth factor stimulation only in the Meis1- and Flt3-transduced cells, and Akt shows modest activation in the same cells. Collectively, these results demonstrate that overexpression of Meis1 and wt-Flt3 in the NUP98-HOX cell lines induce similar growth factor dependent activation of normal Flt3 signal transduction pathways.
NUP98-HOX/Meis1 and NUP98-HOX/Flt3 bm cells show similar signal transduction properties. The indicated bm cells were starved for 16 hours in 0.5% FBS medium and then stimulated with starve media (labeled 0) or with nonsaturating concentrations (labeled *) of hIL-6, mIL-3, mSCF, and FL (A) or with FL alone (B) for 5 minutes at 37°C. Total-cell lysates were separated by SDS-PAGE, transferred to PVDF, and probed with antibodies for p-Akt ser 473, p-Erk1/2, or GAPDH as a loading control.
NUP98-HOX/Meis1 and NUP98-HOX/Flt3 bm cells show similar signal transduction properties. The indicated bm cells were starved for 16 hours in 0.5% FBS medium and then stimulated with starve media (labeled 0) or with nonsaturating concentrations (labeled *) of hIL-6, mIL-3, mSCF, and FL (A) or with FL alone (B) for 5 minutes at 37°C. Total-cell lysates were separated by SDS-PAGE, transferred to PVDF, and probed with antibodies for p-Akt ser 473, p-Erk1/2, or GAPDH as a loading control.
Discussion
The major finding of this study is the demonstrated collaboration between wt-Flt3 overexpression and NUP98-HOX fusions in the induction of AML. We demonstrate their synergism in freshly cotransduced bm cells, as well as the ability of wt-Flt3 overexpression to induce the leukemic progression of NUP98-HOX–derived myeloid progenitor cell lines. We also provide data supporting the recently reported finding that Meis1, in concert with HOXA9, positively influences the expression of Flt3 and extend this phenomenon of Meis1-induced up-regulation of Flt3 to the context of NUP98-HOX–induced leukemia.
The involvement of the FLT3 pathway in human leukemia is supported by the frequent expression of FLT3 in leukemic blast cells and the detection of activating FLT3 mutations as the most common genetic alterations in the disease. Moreover, wt-FLT3 has also been associated with leukemia based on its recurrent overexpression in leukemic patients with and without associated FLT3-activating mutations.15,22,23 In mice, FLT3-activating mutations have been shown to induce myeloproliferative disorder27 or lymphoid disease28 and to provide cytokine-independent growth of various cell lines.29 Furthermore, specific inhibition of FLT3 preferentially kills leukemia cells with high levels of FLT3 expression and/or with FLT3 internal tandem duplication (ITD) mutations.30,31 However, ectopic expression of wt-Flt3 does not result in myeloproliferative disease in murine models,27,28 thus raising the question of whether wt-FLT3 signaling plays a role in leukemogenesis. Importantly, in this context, our discovery of collaboration between wt-Flt3 and NUP98-HOX demonstrates, for the first time, a direct role of wt-Flt3 in the pathobiology of AML.
In human leukemia, coexpression of FLT3 and its ligand has been reported in a high percentage of patients with primary AML, suggesting that an autocrine or paracrine stimulatory loop may result in wt-FLT3 signaling contributing to the pathogenesis of AML.23 Moreover, constitutive activation of wt-FLT3 as a result of its increased levels has also been proposed.15 However, neither of these possibilities appears relevant to our model. Indeed, while expression of FL mRNA was detected in our NUP98-HOX cell lines, these cells failed to grow in the absence of added growth factors and showed a modest response to high concentrations of added FL. Furthermore, neither high levels of wt-Flt3 nor Meis1 increased FL expression or rendered the cells cytokine-independent, indicating that Flt3 activation requires exogenous FL, probably provided in vivo by bm stromal cells.
An emerging model of leukemogenesis argues that overt AML is the result of the synergism between at least 2 complementation groups of mutations. One group impairs hematopoietic differentiation and the other confers a proliferative and/or survival advantage to hematopoietic progenitors. In agreement with this multihit leukemogenic model, neither NUP98-HOX fusions7-9 nor activation of the Flt3 pathway27,28 are on their own able to induce rapid polyclonal leukemias in mouse models. However, collaboration between NUP98-HOX fusions with pathways involved in survival/proliferation has recently been demonstrated by the ability of NUP98-HOXA9 to cooperate with BCR/ABL in mouse models of chronic myeloid leukemia (CML) blast crisis.32,33 In addition, FLT3-activating mutations have been shown to induce leukemia in concert with fused transcription factors such as PML-RARα, MLL-SEPT6, and most recently AML-ETO.19-21 Thus, our results showing synergism between NUP98-HOX and elevated wt-Flt3 support and extend the notion of complementation between fused transcription factors and pathways involving tyrosine kinases in the induction of AML. Furthermore, the ability of wt-Flt3 to induce the leukemic progression of the NUP98-HOX–derived preleukemic cells provides experimental support for a 2-step model for NUP98-HOX/Flt3–mediated leukemia.
MEIS1, like FLT3, is abnormally expressed in both myeloid and lymphoid malignancies.34-36 Meis1 is not leukemogenic on its own; however, we and others have shown that Meis1 synergizes with native and NUP98-fused HOX to induce rapid development of AML in mice.7-9,37 Furthermore, we have recently provided evidence to suggest that the collaboration between Meis1 and NUP98-HOX fusions could be mediated by the ability of the former to induce the in vivo proliferation/self-renewal of progenitor cells blocked in differentiation by NUP98-HOX.24 Thus, our combined findings of the ability of wt-Flt3 to collaborate with NUP98-HOX, the increased levels of Flt3 in NUP98-HOX cells transduced with Meis1, and the ability of NUP98-HOX/Flt3 and NUP98-HOX/Meis1 to activate similar Flt3 signal transduction intermediates, implicate the FLT3 pathway as a putative mediator of the MEIS1-induced proliferative/self-renewal effect required for overt leukemia. It is of interest that the disease latency for NUP98-HOX/Flt3 (median between 107 and 185 days; Figures 1,5A) is longer than previously extensively documented for NUP98-HOX/Meis18,24 (median < 75 days), suggesting that Meis1 may trigger additional FLT3-independent pathways to accelerate leukemia development. Nevertheless, our data clearly demonstrate that Flt3 is sufficient to induce leukemia in collaboration with NUP98-HOX.
In conclusion, we provide new evidence that wt-Flt3 collaborates with NUP98-HOX fusions in the induction of AML and that disease progression induced by Meis1 is associated with up-regulation of Flt3 expression. Given the similarities in the leukemogenic role of native and NUP98-fused HOX genes, our results underscore the functional significance of the recurrent co-overexpression of wt-FLT3 and HOX in human AML and provide merit to further explore the effects of inhibiting the FLT3 pathway in these leukemias.
Prepublished online as Blood First Edition Paper, April 6, 2006; DOI 10.1182/blood-2005-12-007005.
Supported by grants from the National Cancer Institute of Canada with funds from the Terry Fox Foundation, Genome Canada/BC, and the Canadian NCE Stem Cell Network. L.P. was the recipient of a postdoctoral fellowship from the Swedish Cancer Society. B.A. is the recipient of a postdoctoral fellowship from the Leukemia Research Fund of Canada.
L.P. and B.A. have contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Patty Rosten for technical assistance, Dr Florian Kuchenbauer for reviewing the manuscript, and Colleen Mackinnon and Annette Bremen for help in preparing this manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal