Abstract
We investigated the association of plasma thrombopoietin (TPO) and overall survival in 127 patients with previously treated and previously untreated chronic lymphocytic leukemia (CLL). Higher levels of TPO were associated with advanced Rai stage (P < .001), higher levels of β2-microglobulin (β2-M) (P < .001), and the absence of mutation in the immunoglobulin heavy chain variable region (IgVH) (P < .001), and were inversely correlated with platelet count (P = .002). We found that TPO correlated strongly in a continuous manner with overall survival in both previously treated and untreated patients. The univariate Cox proportional hazard model demonstrated that high TPO levels were associated with shorter survival (P < .001), and multiple variable Cox proportional hazards regression analysis demonstrated that this was independent of the IgVH mutation status, β2-M, and Rai stage. Recursive partitioning showed that a cutoff point of 639 pg/mL separated the CLL patients into 2 major survival groups (P < .001). The effects of β2-M were masked by the effects of TPO in the patients with TPO levels higher than 639 pg/mL, but in the remainder, patients with β2-M level higher than 4.95 mg/L had significantly shorter survival than those with lower values. Plasma TPO and β2-M may be useful for the prediction of clinical behavior in CLL and may replace the need for the determination of IgVH mutation status.
Introduction
Some patients with chronic lymphocytic leukemia (CLL) have aggressive disease that can adversely affect the quality of their lives, require early intervention, and lead to early mortality, whereas others have a more indolent course, are less symptomatic, and do not require intervention for many years, if at all.1-3 Because patients with indolent disease are unlikely to benefit from early intervention and because of the difficulty of predicting the course of the disease for many patients at the time of diagnosis, current guidelines recommend treatment only for patients with significant symptoms or demonstrated progression of disease.2 If it were possible to identify patients with aggressive disease at the early stage before the bulk of the disease became overwhelming, it might be possible to test interventions that may be effective in patients with low tumor burden and thus alter the natural course of the disease.3
Plasma β2-microglobulin (β2-M), the small subunit of the MHC class I molecule, is a good predictor of overall survival.4 The first easily available characteristic of CLL cells that was associated with relatively aggressive disease was the expression of surface CD38.5-8 Patients with elevated CD38 on average had shorter time to progression (time from diagnosis to initiation of therapy) and shorter overall survival. Subsequently, absence of somatic mutations in the rearranged immunoglobulin heavy chain variable region (IgVH) was found to be strongly associated with aggressive disease.9,10 In normal B lymphocytes, somatic mutation in these sequences is typical for antigen-stimulated cells.11 At the present time, sequence determination for rearranged variable regions is not routinely available in the clinical laboratory. Recently, it was proposed that expression of ZAP-70, an intracellular tyrosine kinase with a critical role in T-cell receptor signaling, was a surrogate for the mutational status of the immunoglobulin heavy chain variable region.12-14 ZAP-70 status can be determined by either immunohistochemistry or flow cytometry, and thus can be more easily determined in a clinical laboratory. A recent study suggested that ZAP-70 expression in CLL may actually be a stronger predictor of the need for treatment than the expression of an unmutated immunoglobulin heavy chain variable region.14
However, the overall clinical value for the ability of ZAP-70 to predict the need for therapy remains unknown and is in need of further study.
In both the Rai and Binet scoring systems, a platelet count lower than 100 000 × 109/L places a patient in the least favorable prognostic group for survival. In some cases, thrombocytopenia is immune mediated, but in the majority of patients with thrombocytopenia in CLL, the cause is not known. It has been proposed that thrombocytopenia may be due to tumor progression. Thrombopoietin (TPO) is the key growth factor for physiologic megakaryopoiesis and is essential for platelet production.15,16 TPO acts through its receptor, c-MPL, and the JAK-STAT signal transduction pathway.17 Normal TPO production is constitutive, and circulating TPO is regulated by c-MPL–mediated platelet binding, internalization, and catabolism. Plasma TPO levels are generally inversely proportional to total megakaryocyte and platelet mass.18 While it would be expected that late-stage patients with thrombocytopenia would have elevated plasma TPO levels, there would be no such expectation in relatively early stage patients with normal platelet counts.
We first evaluated plasma TPO levels in 64 patients with CLL and correlated these levels retrospectively with Rai stage, β2-M, CD38, and clinical outcome. After finding an association with survival in CLL patients, we enlarged our study group to 127 and determined immunoglobulin heavy chain variable region mutational status in 67 of these patients. Patient selection was based on whether a frozen plasma specimen or cell pellet was available for analysis. We examined whether the plasma level of TPO was a strong predictor of survival that correlates with the IgVH mutational status or other important parameters independent of IgVH.
Patients, materials, and methods
The general characteristics of the 127 patients studied are listed in Table 1. All samples were obtained before initiating any therapy at M. D. Anderson. All patients included in this study were off therapy for a minimum of 3 months. The outcome is from the time of collecting samples. Samples were collected at the first presentation to M. D. Anderson Cancer Center. All patients were off therapy at the time of collecting samples. All previously untreated patients were newly diagnosed. For previously treated patients, samples were obtained before therapy was initiated at M. D. Anderson. Some of these patients had one course of therapy; others had more than one. Patients were included whether or not they needed therapy. The stage was determined at the time of obtaining the sample for TPO measurement. All patient samples, as well as samples from 46 healthy controls, were collected under a protocol approved by the Internal Review Board of the M. D. Anderson Cancer Center (Houston, TX) and with written informed consent in accordance with the Declaration of Helsinki. Diagnosis was confirmed using peripheral blood and bone marrow samples. Blood counts, biochemical panel, flow cytometry for cell surface antigen expression, and β2-M were performed in the clinical laboratory. Peripheral blood samples at the time of presentation to M. D. Anderson Cancer Center were collected in EDTA. Plasma was separated by centrifuging samples at 800g for 10 minutes, and the samples were stored at –70°C. Complete clinical data were recorded from time of diagnosis at M. D. Anderson until the date of the study. Commercial enzyme-linked immunosorbent assay (ELISA) kits for the TPO measurements were purchased from R&D Systems (Quantikine; R&D Systems, Minneapolis, MN). All values for plasma TPO are reported in picogram per milliliter.
Patients' clinical characteristics
. | All patients . | IgVH patients only . | Previously untreated only . |
|---|---|---|---|
| No. patients | 127 | 67 | 85 |
| Age, y, median (range) | 61 (33-83) | 61 (33-83) | 59 (33-80) |
| Male, no. (%) | 75 (59) | 38 (56) | 48 (56) |
| WBC, × 109/L, median (range) | 47.5 (2-233.9) | 49.2 (2.4-233.9) | 48 (3.9-233.9) |
| Lymphocytes, % (range) | 82 (9-97) | 84 (17-97) | 85 (17-97) |
| Platelets, × 109/L (range) | 147 (7-342) | 149 (7-342) | 164 (45-342) |
| Hemoglobin, g/L (range) | 12.8 (6.2-17.8) | 12.6 (6.4-15.7) | 13.2 (9.4-16.3) |
| β2-M, mg/L | 3.4 (1.3-17.7) | 3.4 (1.3-11.7) | 2.8 (1.3-8.8) |
| Bone marrow cellularity, % (range) | 50 (10-95) | 47 (10-95) | 50 (15-95) |
| Bone marrow lymphocyte, % (range) | 61 (4-95) | 67 (12-95) | 64 (4-95) |
| Rai stage, no. (%) | |||
| 0 | 27 (21) | 15 (22) | 24 (28) |
| I | 40 (31) | 18 (27) | 30 (35) |
| II | 25 (20) | 13 (19) | 17 (20) |
| III | 7 (6) | 4 (6) | 3 (4) |
| IV | 28 (22) | 15 (23) | 11 (13) |
| Binet stage, no. (%) | |||
| A | 59 (46) | 31 (47) | 48 (57) |
| B | 34 (27) | 17 (26) | 22 (26) |
| C | 34 (27) | 18 (27) | 14 (16) |
| Lymph nodes involved, no. (%) | |||
| 0 to 1 | 58 (46) | 31 (47) | 43 (51) |
| 2 to 3 or more | 69 (54) | 36 (53) | 42 (49) |
| Previously treated, no. (%) | 42 (33) | 20 (30) | NA |
| Hepatomegaly, no. (%) | 24 (19) | 10 (15) | 9 (11) |
| Splenomegaly, no. (%) | 46 (36) | 21 (32) | 18 (21) |
. | All patients . | IgVH patients only . | Previously untreated only . |
|---|---|---|---|
| No. patients | 127 | 67 | 85 |
| Age, y, median (range) | 61 (33-83) | 61 (33-83) | 59 (33-80) |
| Male, no. (%) | 75 (59) | 38 (56) | 48 (56) |
| WBC, × 109/L, median (range) | 47.5 (2-233.9) | 49.2 (2.4-233.9) | 48 (3.9-233.9) |
| Lymphocytes, % (range) | 82 (9-97) | 84 (17-97) | 85 (17-97) |
| Platelets, × 109/L (range) | 147 (7-342) | 149 (7-342) | 164 (45-342) |
| Hemoglobin, g/L (range) | 12.8 (6.2-17.8) | 12.6 (6.4-15.7) | 13.2 (9.4-16.3) |
| β2-M, mg/L | 3.4 (1.3-17.7) | 3.4 (1.3-11.7) | 2.8 (1.3-8.8) |
| Bone marrow cellularity, % (range) | 50 (10-95) | 47 (10-95) | 50 (15-95) |
| Bone marrow lymphocyte, % (range) | 61 (4-95) | 67 (12-95) | 64 (4-95) |
| Rai stage, no. (%) | |||
| 0 | 27 (21) | 15 (22) | 24 (28) |
| I | 40 (31) | 18 (27) | 30 (35) |
| II | 25 (20) | 13 (19) | 17 (20) |
| III | 7 (6) | 4 (6) | 3 (4) |
| IV | 28 (22) | 15 (23) | 11 (13) |
| Binet stage, no. (%) | |||
| A | 59 (46) | 31 (47) | 48 (57) |
| B | 34 (27) | 17 (26) | 22 (26) |
| C | 34 (27) | 18 (27) | 14 (16) |
| Lymph nodes involved, no. (%) | |||
| 0 to 1 | 58 (46) | 31 (47) | 43 (51) |
| 2 to 3 or more | 69 (54) | 36 (53) | 42 (49) |
| Previously treated, no. (%) | 42 (33) | 20 (30) | NA |
| Hepatomegaly, no. (%) | 24 (19) | 10 (15) | 9 (11) |
| Splenomegaly, no. (%) | 46 (36) | 21 (32) | 18 (21) |
NA indicates not applicable.
Cytogenetic analysis
Bone marrow samples were cultured for 24, 48, and 72 hours at 37°C. Cells were also stimulated with lipopolysaccharide (GIBCO, Grand Island, NY) for 72 hours. Ham FIO tissue culture medium (GIBCO) supplemented with 10% fetal calf serum (FCS; Intergen, Purchase, NY) was used.
After the designated culture periods, the samples were harvested using established cytogenetic techniques, which included a 30-minute hypotonic treatment (0.057 M KCI) and a 30-minute fixation in 3:1 methano1–acetic acid fixative. The cell preparations were air-dried and the metaphases G-banded using a trypsin pretreatment and Gurr Giemsa stain (Biomedical Specialties, Santa Monica, CA). The slide preparations from all cultures were scored for analyzable metaphases. A maximum of 25 G-banded metaphases from the unstimulated and LPS-stimulated bone marrow was analyzed.
IgVH mutation status
IgVH mutation status was evaluated following the procedure by Hamblin et al.9 Briefly, the RNA was reverse transcribed using oligo(dT) primers, and the VH gene was amplified using polymerase chain reaction (PCR) with a mixture of 5′ primers specific for each of the leader sequences of the VH1 to VH6 families and 3′ primer for the germ-line JH region. The amplification product was isolated from a gel and sequenced using the 3′ primer and standard automated sequencing as recommended by the manufacturer (Applied Biosystems, Foster City, CA). The sequence was aligned to the V-gene database using both the GenBank19 and IMGT20 databases. Sequences with 3% or more mutations compared with the corresponding germ-line IgVH sequences were considered mutated.12 We have only one case with mutation rate between 2% and 3%.
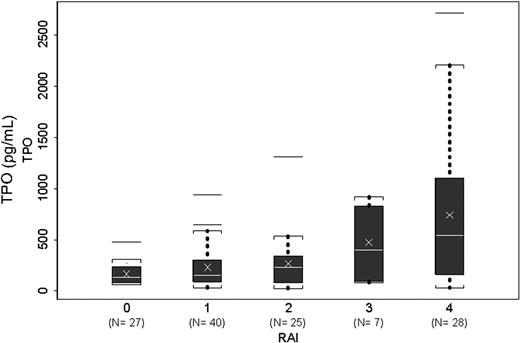
Association of Rai stage and plasma thrombopoietin levels. Box plots showing levels of TPO in various Rai stages. Number (N) of patients in each stage is shown in parenthesis. Brackets indicate the value nearest to the 1.5× interquartile range. Outliers are shown as horizontal lines.
Association of Rai stage and plasma thrombopoietin levels. Box plots showing levels of TPO in various Rai stages. Number (N) of patients in each stage is shown in parenthesis. Brackets indicate the value nearest to the 1.5× interquartile range. Outliers are shown as horizontal lines.
Statistical analysis
Descriptive statistics were analyzed and univariate analyses were performed using the chi-square or Kruskal-Wallis test for categoric data and t test for continuous data.21
Estimates of survival curves were calculated according to Kaplan-Meier product-limit method22 and were calculated from the time of referral to M. D. Anderson Cancer Center. Survival times were compared by means of the log-rank test.23 Clinical and biologic characteristics were analyzed for their association with survival using Cox proportional hazards models.24 Predictors used in the Cox proportional hazards regression model were reviewed to assess the need for transformation based on Martingale residual plots. Predictive variables with P values of less than .10 for the univariate Cox proportional hazards model were included in a multiple variable regression model. In this model, we used a backward elimination with P value cutoff of .05, then allowed any variable previously deleted to re-enter the final model if its P value was less than .05.25 Interactions between predictive variables in predicting survival were assessed using the Kaplan-Meier co-plot method of Thall and Estey.26 Recursive partitioning was used to search for appropriate cutoff points for continuous covariates and assesses the possibility of interactions among covariates.27,28 Recursive partitioning (via RPART) is performed using dividing rules based on the likelihood ratio test to examine all possible binary splits for the full group of patients and then to dichotomize the patients into groups based on the variable that maximally discriminates between those patients who survive and those patients who do not survive. In the context of this research, we were not using the recursive partitioning algorithm to search over all possible splits but used only the algorithm to classify patients into high- and low-risk groups using those variables that were found to be significant in our multiple variable Cox proportional hazards regression model. We searched for optimal cut point using dividing rules based on the P value from the log-rank test statistic. Because the resulting P value was obtained via a search algorithm, it overstates the association between TPO and survival and thus needs to be adjusted. To adjust the P value, we used a permutation test.29,30 This methodology is a computer-intensive algorithm that involves iteratively reassigning (permuting) patients' TPO values. At each iteration of the algorithm the optimal cut-point search is performed, and the resulting P value is recorded. This iterative process results in a distribution of P values. The adjusted P value is the P value obtained from comparing the P value obtained from the original data with the P values recorded via the permutation test. If the original P value is small relative to the P values obtained via permutation (ie, < 5% of the P values obtained from the permutation distribution are smaller) then there is evidence of an effect. The larger the P value, the smaller the support for an effect.
All computations were carried out using SAS (Cary, NC) and S-plus 2000 (Insightful, Seattle, WA).
Results
We studied TPO levels in 127 patients with CLL. Of these patients, 42 (33%) were previously treated. The characteristics of these patients are shown in Table 1. Of these patients, 22% were in Rai stage IV and 27% in Binet stage C. Thirty-six percent of the patients had splenomegaly. TPO levels in CLL patients varied between 26.4 and 2714.5 pg/mL, with a median of 232 pg/mL. In healthy controls, the median was 78.24 pg/mL, with a range from 27.49 to 370.39 pg/mL. Overall, the levels of TPO in patients with CLL were significantly higher than those in healthy controls (Kruskal-Wallis test, P = .006). The individuals in the control group were younger, with a median age of 35 years, compared with 61 years in the CLL patients. However, similar levels of TPO in control patients have been reported in older patients.31 Previously untreated CLL patients had significantly lower TPO (median, 163.98) compared with previously treated patients (median, 475.32) (P < .001). To study the reliability of the TPO assay, we first studied reproducibility. Upon repeating the assay 7 times using 3 samples, we demonstrate that the interassay variation (CV) for the samples with mean concentrations of 35.7 pg/mL, 83.3 pg/mL, and 230.4 pg/mL was 17.8%, 17.2%, and 13.4%, respectively. Furthermore, freezing and thawing for 3 cycles at –70°C of the 3 samples with the above-mentioned concentrations showed acceptable levels of TPO activity with CV less than 20%. None of our samples tested here was thawed more than once.
TPO levels correlate positively with β2-M (r = 0.43, P < .001), and negatively with hemoglobin (r =–0.43, P < .001) and platelet count (r =–0.26, P = .002). There was no correlation between TPO and white blood cell (WBC) count, lymphocyte count, bone marrow levels of infiltration by the leukemic cells, percent of CD38+ cells, or age.
There was a significant association between each Rai stage and plasma TPO levels in the study population (P < .001) (Figure 1). Patients with Binet stage A and B had no significant difference in TPO levels, but patients with Binet stage C had significantly higher levels of TPO compared with patients of stage A or B. Since an association between chromosomal 17p deletion and response to alemtuzumab, but not to fludarabine, has been reported,31,32 we evaluated the levels of TPO in patients with 17p. Using karyotyping, we found that 11 (14%) of the 77 patients, in whom karyotyping data were available, had 17p deletion. There was no statistically significant difference in TPO levels in the 17p deletion group. Similarly, when only the previously treated patients were considered, we found 7 (23%) of 31 patients with available cytogenetic data had 17p deletion, but no statistical difference in TPO levels between the 2 groups.
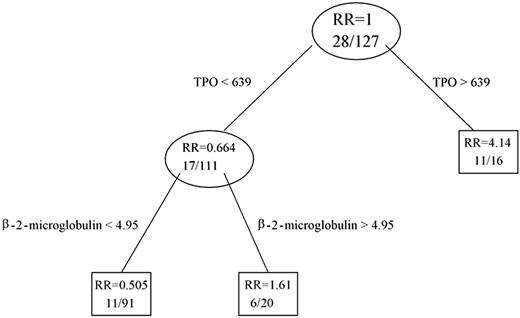
Classification tree for all 127 patients. The number of patients who died in each group is shown first, and then the total number of patients. RR indicates relative risk.
Classification tree for all 127 patients. The number of patients who died in each group is shown first, and then the total number of patients. RR indicates relative risk.
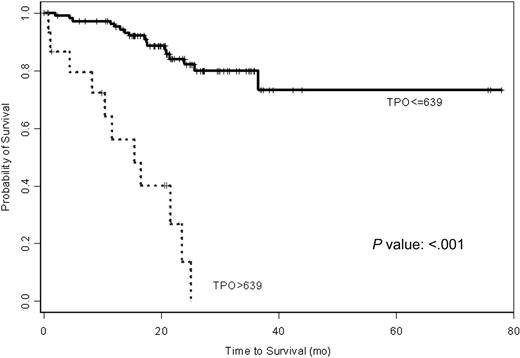
The univariate Cox proportional hazard analysis demonstrated that higher levels of TPO predict for shorter survival in a continuous fashion (P < .001). To ensure that the P value is not influenced by outliers, log transformation of TPO values was used and showed similar P value. As shown in Table 2, the hazard ratio is 1.002 with each 1-pg/mL increase in TPO level. As expected, Rai stage, β2-M, and the IgVH mutation status all predicted survival in univariate model (Table 2). The 47 patients with IgVH mutation had significantly lower levels of plasma TPO compared with the 20 patients without IgVH mutation (P = .001). The patients with available IgVH data are representative of all patients as shown in Table 1. The characteristics of this group of patients are shown side by side in Table 1. Using Martingale residual plot analysis and recursive partitioning, a cutoff point of 639 pg/mL separated the CLL patients into 2 major groups with significant difference in survival (P < .001) (Figure 2). Patients with levels of TPO of 639 pg/mL or lower had significantly longer (median not reached) survival than patients with TPO level higher than 639 pg/mL (median of 14 months). Only 17 of 111 patients died in the first group, while 11 of 16 patients died in the second group.
Univariate Cox proportional hazard regression for survival
Variable . | No. patients . | Parameter estimate . | Standard error . | P . | Hazard ratio . |
|---|---|---|---|---|---|
| Plasma TPO | 127 | 0.002 | 0.0004 | < .001 | 1.002 |
| β2-microglobulin | 127 | 0.306 | 0.07 | < .001 | 1.36 |
| Rai, III to IV vs 0 to II | 127 | 1.09 | 0.38 | .043 | 2.98 |
| IgVH mutation status, yes vs no | 67 | -1.89 | 0.59 | .001 | 0.15 |
Variable . | No. patients . | Parameter estimate . | Standard error . | P . | Hazard ratio . |
|---|---|---|---|---|---|
| Plasma TPO | 127 | 0.002 | 0.0004 | < .001 | 1.002 |
| β2-microglobulin | 127 | 0.306 | 0.07 | < .001 | 1.36 |
| Rai, III to IV vs 0 to II | 127 | 1.09 | 0.38 | .043 | 2.98 |
| IgVH mutation status, yes vs no | 67 | -1.89 | 0.59 | .001 | 0.15 |
We analyzed the prognostic value of TPO in the 85 patients who were previously untreated. In this group of patients, TPO levels did not correlate with Rai stage (P = .21), and there was no correlation between platelet count and TPO (r = 0.11, P = .34). However, in the previously untreated patients, TPO remained a strong predictor of survival in Cox proportional hazard model (Table 3).
Univariate Cox proportional hazard regression survival estimates in untreated CLL patients
Variable . | No. patients . | Parameter estimate . | Standard error . | P . | Hazard ratio . |
|---|---|---|---|---|---|
| Plasma TPO | 85 | 0.004 | 0.001 | .004 | 1.004 |
| β2-microglobulin | 85 | 0.35 | 0.15 | .017 | 1.42 |
| Rai, III to IV vs 0 to II | 85 | 0.93 | 0.60 | .12 | 2.54 |
| IgVH mutation status, yes vs no | 48 | -2.02 | 0.87 | .020 | 0.13 |
Variable . | No. patients . | Parameter estimate . | Standard error . | P . | Hazard ratio . |
|---|---|---|---|---|---|
| Plasma TPO | 85 | 0.004 | 0.001 | .004 | 1.004 |
| β2-microglobulin | 85 | 0.35 | 0.15 | .017 | 1.42 |
| Rai, III to IV vs 0 to II | 85 | 0.93 | 0.60 | .12 | 2.54 |
| IgVH mutation status, yes vs no | 48 | -2.02 | 0.87 | .020 | 0.13 |
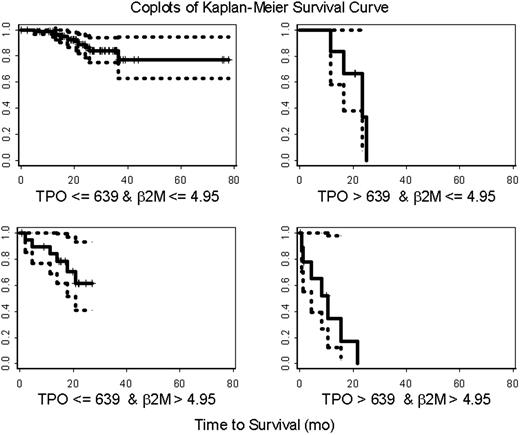
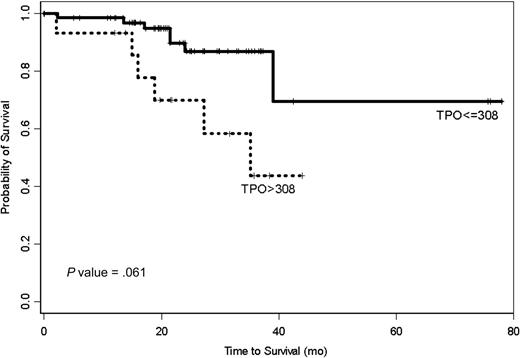
In a multiple variable Cox proportional hazards regression model incorporating Rai stage, β2-M, history of prior therapy, and TPO, only TPO and β2-M remained predictive of survival (Table 4). This did not change whether the Rai stages were grouped as 0 to II and III to IV or separated and used individually or with maintaining 0 to II as one group and separating III from IV. Using recursive partitioning (RPART), we were able to build a classification tree. Plasma TPO and β2-M were used to split the data into 3 groups (Figure 2). In addition to the recursive partitioning algorithm, we also used an optimal cut-point search to determine how to best split the TPO data. Both methods resulted in the same split point (ie, 639 pg/mL). According to the classification tree and optimal cut-point search, patients who had plasma TPO levels higher than 639 pg/mL (n = 16) had a higher risk of early death (69%) (Figure 3; optimal cut-point search, P value = .002). The majority of these patients (78%) was previously treated. Any effect of β2-M on survival was masked at plasma TPO level above this. In contrast, only 38% of the patients with TPO levels of 639 pg/mL or lower were previously treated, and the death rate was only 15% in this group of patients. However, β2-microglobulin and TPO had a joint effect on survival in this group of patients. A β2-microglobulin level higher than 4.95 mg/L was associated with significantly shorter survival, with a death rate of 30%. This is clearly shown in the co-plot of Kaplan-Meier curves (Figure 4). RPART and an optimal cut-point search were also applied to all patients without any prior treatment. Similar to the full data set, these 2 algorithms resulted in the same cut points in this patient population. According to this classification tree, untreated CLL patients had a much higher risk of early death if their TPO levels were higher than 308 (Figure 5; optimal cut-point search, P = .061). There were 7 deaths in the 70 previously untreated patients with TPO levels of 308 pg/mL or lower, while there were 6 deaths in the 15 previously untreated patients with TPO levels higher than 308 pg/mL. Median survival has not been reached in patients with TPO levels lower than 308 pg/mL, while it was 35 months in patients with TPO levels higher than 308 pg/mL.
Multivariable Cox proportional hazard regression for survival (127 patients)
Variable . | Parameter estimate . | Standard error . | P . | Hazard ratio . |
|---|---|---|---|---|
| Plasma TPO | 0.002 | 0.0004 | < .001 | 1.002 |
| β2-microglobulin | 0.18 | 0.078 | .023 | 1.19 |
| Prior treatment, more than 1 vs 1 or fewer | 0.16 | 0.48 | .74 | 1.17 |
| Rai, 3 to 4 vs 0 to 2 | 0.26 | 0.45 | .56 | 1.30 |
Variable . | Parameter estimate . | Standard error . | P . | Hazard ratio . |
|---|---|---|---|---|
| Plasma TPO | 0.002 | 0.0004 | < .001 | 1.002 |
| β2-microglobulin | 0.18 | 0.078 | .023 | 1.19 |
| Prior treatment, more than 1 vs 1 or fewer | 0.16 | 0.48 | .74 | 1.17 |
| Rai, 3 to 4 vs 0 to 2 | 0.26 | 0.45 | .56 | 1.30 |
Kaplan-Meier estimates of patients' time to death grouped by plasma TPO levels. Patients with TPO level higher than 639 pg/mL show significantly shorter survival.
Kaplan-Meier estimates of patients' time to death grouped by plasma TPO levels. Patients with TPO level higher than 639 pg/mL show significantly shorter survival.
Co-plots of Kaplan-Meier estimates of survival in CLL. One-hundred and twenty-seven patients were separated into 4 groups based on their TPO and β2-microglobulin (B2M) levels. The solid line represents the Kaplan-Meier estimates of overall survival for each group. The 95% confidence intervals of the survival curves are presented using the dotted lines. Each tick mark represents the time at which patients were censored.
Co-plots of Kaplan-Meier estimates of survival in CLL. One-hundred and twenty-seven patients were separated into 4 groups based on their TPO and β2-microglobulin (B2M) levels. The solid line represents the Kaplan-Meier estimates of overall survival for each group. The 95% confidence intervals of the survival curves are presented using the dotted lines. Each tick mark represents the time at which patients were censored.
We also performed subset analyses on those patients with available mutation status and previously untreated patients. A multiple variable Cox proportional hazards regression analysis on the subset of patients for whom mutation status was available showed that both TPO and mutation are independent predictors of survival (Table 5). This was also true when only previously untreated patients were considered (48 patients) (P = .02 for TPO and P = .01 for mutation status). The patients studied for the IgVH mutation were representative of the total group of patients as shown in Table 1, and there were 21 deaths in this group.
Exploratory multivariable Cox proportional hazard regression for a subgroup of 67 patients with IgVH data
Variable . | Parameter estimate . | Standard error . | P . | Hazard ratio . |
|---|---|---|---|---|
| Plasma TPO | 0.002 | 0.001 | .002 | 1.002 |
| IgVH mutation status, yes vs no | -1.54 | 0.62 | .013 | 0.22 |
Variable . | Parameter estimate . | Standard error . | P . | Hazard ratio . |
|---|---|---|---|---|
| Plasma TPO | 0.002 | 0.001 | .002 | 1.002 |
| IgVH mutation status, yes vs no | -1.54 | 0.62 | .013 | 0.22 |
Kaplan-Meier survival estimates for previously untreated CLL patients. Patients with TPO level higher than 308 pg/mL show significantly shorter survival.
Kaplan-Meier survival estimates for previously untreated CLL patients. Patients with TPO level higher than 308 pg/mL show significantly shorter survival.
Discussion
In this study of CLL, we found a strong association between plasma TPO, Rai stage, β2-M, IgVH, and overall survival in 127 patients with CLL. Moreover, for those patients who had mutation status available, we found a strong association between mutation status and overall survival. It was not surprising to find that plasma TPO levels correlated with Rai stage, since the most advanced Rai stage is defined by a platelet count lower than 100 000/mm3. However, the univariate Cox proportional hazard analysis demonstrated that higher levels of TPO predict for shorter survival in continuous fashion, and the multiple variable Cox proportional hazard analysis indicated that plasma TPO was independent of the Rai stage, β2-M, and IgVH mutation status. Plasma β2-M added further information in predicting overall survival in patients with relatively low plasma TPO. Based on a limited number of patients (67) once the combination of plasma TPO and β2-M was known, IgVH mutation status no longer had prognostic significance.
We found that the hazard ratio for survival for CLL patients with TPO levels higher than 640 pg/mL was 4.14. Measurements of TPO and β2-microglobulin are simple and reliable procedures, thus making them much more easily adapted to a clinical laboratory than sequence analysis of IgVH. These parameters are useful in trying to segregate patients earlier who will probably have an adverse course so that earlier interventions can be tested before tumor burden makes such interventions unlikely to succeed. We hypothesize that plasma TPO accurately reflects the extent in which the CLL clone may impose and suppress the normal hematopoiesis, even at an early stage of disease, and therefore is a key prognostic variable. The lack of correlation between TPO level and WBC count, percent of lymphocytes, or bone marrow cellularity suggests that TPO levels reflect the extent to which the tumor can suppress and disturb the normal environment in bone marrow rather than tumor bulk. It is true that some patients with CLL may develop immune thrombocytopenia purpura (ITP) as an independent process33,34 ; however, since most of the studies suggest that TPO levels in patients with ITP do not correlate with platelet counts and are not necessarily increased,35,36 the prognostic value of TPO in CLL patients is most likely not related to ITP. Our study suggests that plasma TPO along with β2-M can be adequately used to predict clinical behavior in patients with CLL and to stratify these patients for therapy; at the same time, the IgVH mutation status, when available, may still play a role in refining this model.
One potential limitation of this study is the fact that M. D. Anderson is a tertiary cancer center, and therefore some patients first visited M. D. Anderson at initial diagnosis, while others visited after diagnosis but prior to initiation of therapy. These data need to be validated in a large number of patients with samples and survival data collected in a more uniform fashion and with a more homogeneous measurement of survival.
Prepublished online as Blood First Edition Paper, March 21, 2006; DOI 10.1182/blood-2005-05-2110.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal