Phagocytosis of IgG-coated particles via FcγR is accompanied by the generation of superoxide and inflammatory cytokines, which can cause collateral tissue damage in the absence of regulation. Molecular mechanisms regulating these phagocytosis-associated events are not known. SHIP is an inositol phosphatase that downregulates PI3K-mediated activation events. Here, we have examined the role of SHIP in FcγR-induced production of superoxide and inflammatory cytokines. We report that primary SHIP-deficient bone marrow macrophages produce elevated levels of superoxide upon FcγR clustering. Analysis of the molecular mechanism revealed that SHIP regulates upstream Rac-GTP binding, an obligatory event for superoxide production. Likewise, SHIP-deficient macrophages displayed enhanced IL-1β and IL-6 production in response to FcγR clustering. Interestingly, whereas IL-6 production required activation of both PI3K and Ras/Erk pathways, IL-1β production was dependent only on Ras/Erk activation, suggesting that SHIP may also regulate the Ras/Erk pathway in macrophages. Consistently, SHIP-deficient macrophages displayed enhanced activation of Erk upon FcγR clustering. Inhibition of Ras/Erk or PI3K suppressed the enhanced production of IL-6 in SHIP-deficient macrophages. In contrast, inhibition of Ras/Erk, but not PI3K, suppressed IL-1β production in these cells. Together, these data demonstrate that SHIP regulates phagocytosis-associated events through the inhibition of PI3K and Ras/Erk pathways.
Introduction
IgG-coated particles (immune complexes) engage FcγR on the surface of macrophages and monocytes and initiate a series of signaling events that result in the phagocytosis/destruction of the immune complex.1,2 This process is often accompanied by the generation of superoxide radicals and inflammatory cytokines, which are produced in an effort to clear the antibody-coated target. These phagocytosis-associated events can cause collateral tissue damage in the absence of regulation. Recent work has demonstrated that a similar macrophage response is elicited by antibody-coated tumor cells.3
Murine macrophages express both activating FcγR (FcγRI and FcγRIIIa) and inhibiting FcγR (FcγRII).4 Clustering of the activating FcγR by immune complexes results in phosphorylation of the receptor ITAMs (immunoreceptor tyrosine-based activation motifs) by membrane-associated Src kinases.5,6 The phosphorylated ITAMs serve as docking sites for SH2 domain–containing cytoplasmic enzymes and enzyme/adapter complexes. Thus, recruitment of the Shc/Grb2/Sos complex to the membrane activates the Ras/Erk pathway, and recruitment of PI3K via the p85 adapter subunit results in the generation of 3′ phosphorylated inositol phospholipids such as PtdIns3,4,5P3.7,8 PtdIns3,4,5P3 is an important second messenger that is necessary for the recruitment and activation of PH domain–containing enzymes such as Vav, the guanine nucleotide exchange factor for Rac, Btk, the tec family tyrosine kinase involved in calcium mobilization, and the serine/threonine kinase Akt.9 In addition to the activating enzymes recruited by the phosphorylated ITAM, negative regulatory phosphatases such as SHIP are recruited by both the phosphorylated ITAM as well as the phosphorylated ITIM of FcγRII(b).8,10
FcγR-mediated phagocytosis is a complex signaling cascade that involves several events, including rearrangement of cytoskeleton and production of superoxide.11,12 Extensive studies in the last few years have shown that the key molecule that plays a role in both these events is a small Rho family GTPase called Rac.13-17 Superoxide production is catalyzed by a multi-subunit enzyme, NADPH oxidase. Rac is essential for complete assembly of NADPH oxidase and its activation.12-15 It has been reported that previous studies have suggested a role for the Ras/Erk pathway in the induction of cytokine gene expression in response to FcγR clustering, precise signaling events involved in cytokine production are not known.2 Likewise, mechanisms that regulate these phagocytosis-associated events are not known.
SHIP is an inositol 5′ phosphatase expressed exclusively in hematopoietic cells, where it serves as a negative regulator of cell proliferation, activation, and survival.21 It is a multidomain cytosolic protein that has an N-terminal SH2 domain; central catalytic domain that hydrolyzes PtdIns3,4,5P3 to PtdIns3,4P2; 2 NPXY motifs; and a C-terminal proline-rich domain. Thus there are 2 aspects to SHIP's functional role: its catalytic activity and its interaction with other proteins. It has been shown that bone marrow–derived macrophages (BMMs) from SHIP–/– mice and cells expressing dominant-negative catalytic mutants of SHIP exhibit enhanced phagocytic ability compared with SHIP+/+ cells.10,22 Interestingly, studies in B cells demonstrated that the noncatalytic, interaction domains of SHIP are responsible for the regulation of the Ras/Erk pathway by virtue of their ability to interact with molecules such as p62dok and Shc.23-26 However, there are no studies to date examining the influence of SHIP on phagocytosis-associated events such as superoxide generation and inflammatory cytokine production.
In this study, we demonstrate that SHIP downregulates superoxide production when macrophages are stimulated with immune complexes and that SHIP negatively regulates upstream Rac activity. Likewise, SHIP has negative influence on FcγR-induced IL-1β and IL-6 production. Interestingly, our results indicated a differential requirement for the activation of the Ras/Erk and PI3K pathways in the generation of IL-1β and IL-6 in response to FcγR clustering. Analysis of the mechanism of SHIP regulation of FcγR-induced IL-1β and IL-6 production revealed that SHIP influences the production of these cytokines through the regulation of the Ras/Erk and the PI3K pathways. Based on these findings, we propose that SHIP is a key regulator of the FcγR-mediated inflammatory response.
Materials and methods
Cells, antibodies, and reagents
Raw 264.7 cells were obtained from ATCC (Manassas, VA) and maintained in RPMI supplemented with 5% fetal bovine serum. Rac antibody was purchased from Chemicon International (Temecula, CA). All phospho-specific antibodies were from Cell Signaling Technology (Beverly, MA). Anti–mouse CD16/32 (FcγRIII/II) was purchased from BD Pharmingen (San Diego, CA). Mouse antirat antibody was from Jackson ImmunoResearch (Bar Harbor, ME). Rabbit polyclonal SHIP antibody was a generous gift from Dr K. M. Coggeshall (Oklahoma Medical Research Foundation, Oklahoma City, OK). Akt antibody was from Santa Cruz Biotechnology (Santa Cruz, CA).
Culture of murine bone marrow macrophages
BMMs were derived as previously described.27 Briefly, bone marrow cells were cultured in RPMI containing 10% fetal bovine serum and supplemented with 10 ng/mL CSF-1 and 5 μg/mL polymixin B for 7 days. The cells obtained in this manner were greater than 99% positive for Mac-1, as determined by flow cytometry.
Generation of stable cell lines expressing SHIP
PINCO–wild-type (PINCO-wt) SHIP retroviral vector was kindly provided by Dr Martin Sattler (Dana-Farber Cancer Institute, Harvard University, Boston, MA).28-30 PINCO–catalytic-deficient D675A SHIP was generated as previously described.29 Retroviral infections of Raw 264.7 cells were performed following previously published standards.29 Briefly, infectious supernatants from PINCO (vector only), PINCO-wt SHIP, or PINCO–catalytic-deficient D675A SHIP-transfected Phoenix cells were collected 48 hours after transfection and used for 3 cycles of infection. Upon infection, Raw 264.7 cells were sorted (FACS Vantage; BD Biosciences, San Jose, CA) for green fluorescent protein (GFP) expression and stable lines were raised.
Preparation of heat-aggregated IgG
Heat-aggregated IgG was prepared according to methods described previously.27 In brief, Chromopure mouse IgG at a concentration of 750 μg/mL was heated at 62°C for 30 minutes, then cooled on ice immediately and used to stimulate cells.
Measurement of superoxide production in BMMs
Superoxide produced in cells was measured using the cell-permeable dye dihydroethidium (DHE; Sigma, St Louis, MO), which binds to nuclear DNA when oxidized by superoxide and emits red fluorescence.31 Briefly, BMMs were seeded in 6-well plates at a concentration of 1.5 million cells per well. The next day cells were washed once with PBS followed by addition of RPMI medium without phenol red. The cells were stimulated with heat-aggregated IgG for 2 hours, in the dark at 37°C, in the presence of 10 μM of fluorescent probe DHE (Sigma). After 2 hours the cells were washed with ice-cold PBS, scraped, and lysed by brief sonication. Lysates and supernatants were loaded in 96-well plates and the fluorescence was measured in a microplate fluorimeter (SpectraMax Gemini; Molecular Devices, Sunnyvale, CA) using excitation/emission filters 520/590 nm.
Rac activity assay
Glutathione agarose beads coated with GST-PAK1-PBD (p21 binding domain) were prepared as described in Benard et al32 and Benard and Bokoch.33 The p21 binding domain (PBD) of p21-activated kinase 1 (PAK1) binds and pulls down specifically the GTP-bound (active form) and not the GDP-bound form of Rac. Here, BMMs, or Raw 264.7 transfectants, were activated by clustering FcγR with anti–mouse FcγRIII/II 2.4G2 antibody followed by mouse antirat antibody for indicated time points. Cells were lysed in TN1 buffer (50 mM Tris, pH 8.0; 10 mM EDTA; 10 mM Na4P2O7; 10 mM NaF; 1% Triton-X 100; 125 mM NaCl; 10 mM Na3VO4; 10 μg/mL each aprotinin and leupeptin). Protein-matched cell lysates were incubated with GST-PAK1-PBD beads for 1 hour at 4°C. After 1 hour, beads were washed with TN1 and then boiled in 1 × SDS sample buffer (60 mM Tris, pH6.8; 2.3% SDS; 10% glycerol; 0.01% bromophenol blue; and 1% 2-ME) for 10 minutes. Proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membrane, probed with anti-Rac antibody, and developed by enhanced chemiluminescence (ECL).
Western blot data quantitation
The ECL signal was quantitated using an HP Scanjet 7400c scanner (Hewlett-Packard, Palo Alto, CA) and a densitometry program (Scion Image version 0.4.0.2; Scion, Frederick, MD). In experiments measuring phosphorylation, we first subtracted background, normalized the signal to the amount of actin or total target protein in the lysate, and plotted the values as fold increase over unstimulated samples, as previously described.27
Measurement of cytokines by ELISA
Cells were cultured for varying time points ranging from 2 hours to 8 hours, in the presence or absence of heat-aggregated IgG. Cell lysates and supernatants were harvested, centrifuged to remove dead cells, and analyzed by enzyme-linked immunosorbent assay (ELISA) using cytokine specific kits from R&D Systems (Minneapolis, MN). Data were analyzed using a paired t test, and a P value less than .05 was considered significant.
Results
Immune complex–induced production of superoxide is regulated by SHIP
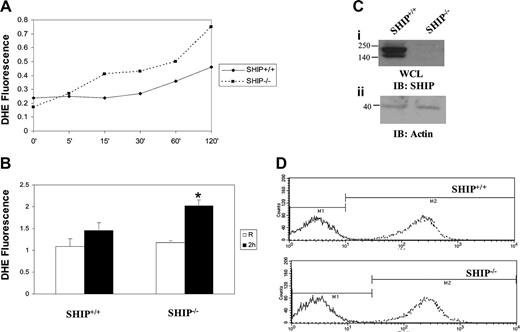
FcγR clustering activates the assembly of the NADPH oxidase complex and the generation of superoxide.12 Mechanisms of regulation of superoxide generation are not known. To examine whether SHIP regulates FcγR-induced superoxide production, BMMs from SHIP+/+ and SHIP–/– animals were stimulated with heat-aggregated IgG for varying time points ranging from 0 to 120 minutes in the presence of dihydroethidium. Results indicated that FcγR clustering induces superoxide production that is only detectable after one hour of stimulation in wild-type cells (Figure 1A). Superoxide production in SHIP–/– cells was enhanced over the SHIP+/+ cells at all time points tested after stimulation. For further analysis, the 120-minute time point was chosen, and 4 independent experiments were performed. Results confirmed that SHIP-deficient BMMs make significantly higher amounts of superoxide in response to immune-complex stimulation (Figure 1B). Figure 1C is a Western blot of protein-matched lysates demonstrating the presence and absence of SHIP in SHIP+/+ and SHIP–/– BMMs, respectively (Figure 1C). Further, flow cytometry following Mac-1 staining of SHIP+/+ and SHIP–/– BMMs demonstrated that all cells used were indeed macrophages.
SHIP regulates activation of upstream Rac
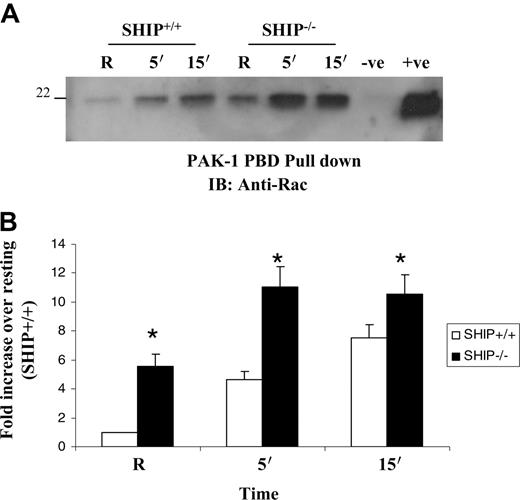
Activation of the low–molecular-weight GTP-binding protein Rac is essential for the assembly of NADPH oxidase complex and superoxide generation.14,15 Rac GTP binding is facilitated by the guanine nucleotide exchange factor Vav, which is activated by the products of PI3K.34,35 Since the enzyme activity of SHIP results in the hydrolysis of PI3K products and the subsequent downregulation of downstream enzymes that are dependent on PI3K products, we next asked whether SHIP influenced Rac activation. Here, SHIP+/+ and SHIP–/– BMMs were stimulated by clustering FcγR and protein-matched lysates were incubated with GST-PAK1-PBD as bait protein to capture GTP-bound Rac. The bound proteins were separated by SDS-PAGE and probed with anti-Rac antibody. Results indicated that FcγR clustering induces Rac GTP-binding (Figure 2). Rac activity was significantly higher in SHIP–/– BMMs compared with SHIP+/+ BMMs.
SHIP downregulates FcγR-induced superoxide production. (A) BMMs derived from SHIP+/+ and SHIP–/– mice were activated with heat-aggregated IgG for the time points indicated in the figure. Generation of superoxide was measured using 10 μM fluorescent probe DHE. DHE fluorescence intensity is plotted in the graph. (B) BMMs derived from SHIP+/+ and SHIP–/– mice were activated with heat-aggregated IgG for 2 hours. Generation of superoxide was measured using fluorescent probe DHE. Data represent mean and SEM of 4 independent experiments. Data were analyzed by Student t test. *P < .05. (C) Protein-matched whole-cell lysates (WCLs) were analyzed by Western blotting with SHIP antibody (i). The same membrane was reprobed with actin antibody (ii). IB indicates immunoblot. (D) Mac-1 expression on the SHIP+/+ and SHIP–/– BMMs was analyzed by flow cytometry. For this, the cells were labeled with APC-labeled Mac-1 antibody in the presence of the anti-FcγRII/III monoclonal antibody (mAb) 2.4G2 (to block Fcγ receptors; - - -). Cells were also labeled with APC-labeled isotype control antibody (—).
SHIP downregulates FcγR-induced superoxide production. (A) BMMs derived from SHIP+/+ and SHIP–/– mice were activated with heat-aggregated IgG for the time points indicated in the figure. Generation of superoxide was measured using 10 μM fluorescent probe DHE. DHE fluorescence intensity is plotted in the graph. (B) BMMs derived from SHIP+/+ and SHIP–/– mice were activated with heat-aggregated IgG for 2 hours. Generation of superoxide was measured using fluorescent probe DHE. Data represent mean and SEM of 4 independent experiments. Data were analyzed by Student t test. *P < .05. (C) Protein-matched whole-cell lysates (WCLs) were analyzed by Western blotting with SHIP antibody (i). The same membrane was reprobed with actin antibody (ii). IB indicates immunoblot. (D) Mac-1 expression on the SHIP+/+ and SHIP–/– BMMs was analyzed by flow cytometry. For this, the cells were labeled with APC-labeled Mac-1 antibody in the presence of the anti-FcγRII/III monoclonal antibody (mAb) 2.4G2 (to block Fcγ receptors; - - -). Cells were also labeled with APC-labeled isotype control antibody (—).
FcγR-induced Rac activation is enhanced in SHIP-deficient macrophages. (A) SHIP+/+ and SHIP–/– macrophages were activated by clustering Fcγ receptors for the indicated time points. GTP-bound Rac was captured with PAK-1 PBD beads as bait from protein-matched cell lysates and visualized by Western blotting with anti-Rac antibody. Unhydrolizable GTP and GDP analogs (obtained from Chemicon International) were used as positive and negative controls, respectively. (B) Fold induction of GTP Rac in the activated samples over resting (R). The graph represents mean and SEM of 3 independent experiments. Data were analyzed by Student t test (*P ≤ .05).
FcγR-induced Rac activation is enhanced in SHIP-deficient macrophages. (A) SHIP+/+ and SHIP–/– macrophages were activated by clustering Fcγ receptors for the indicated time points. GTP-bound Rac was captured with PAK-1 PBD beads as bait from protein-matched cell lysates and visualized by Western blotting with anti-Rac antibody. Unhydrolizable GTP and GDP analogs (obtained from Chemicon International) were used as positive and negative controls, respectively. (B) Fold induction of GTP Rac in the activated samples over resting (R). The graph represents mean and SEM of 3 independent experiments. Data were analyzed by Student t test (*P ≤ .05).
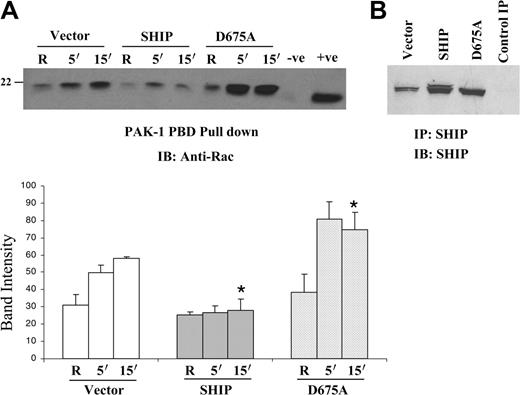
As a second approach to verify that the SHIP regulates activity of Rac, stable cell lines overexpressing wild-type SHIP, catalytic-deficient D675A SHIP, or vector alone were generated by retroviral infection of Raw 264.7 murine macrophage cells. These stable transfectants were stimulated by clustering FcγR and assayed for Rac GTP binding. The results shown in Figure 3A demonstrate that overexpression of wild-type SHIP downregulates Rac activation by FcγR clustering. The graph shown in the bottom panel (Figure 3A) represents results obtained from 3 independent experiments. To ensure that the SHIP constructs were indeed overexpressed in the transfectants, protein-matched lysates from transfected cells were subjected to immunoprecipitation with anti-SHIP antibody and analyzed by Western blotting with anti-SHIP antibody (Figure 3B). Taken together, these results demonstrate that SHIP negatively regulates Rac activity.
Overexpression of wild-type SHIP downregulates FcγR-induced Rac activation. (A) Raw 264.7 cells were retrovirally infected using vector alone, wild-type SHIP, or catalytic-deficient D675A SHIP. GTP-bound Rac was measured in these transfectants after FcγR clustering. The bottom panel represents the band intensity of the Rac GTP. Values obtained from 3 independent experiments are represented as mean and SEM. (B) The stable transfectants were analyzed for SHIP expression by immunoprecipitating SHIP with rabbit polyclonal SHIP antibody and immunoblotting with SHIP antibody. The last lane is a control immunoprecipitate (IP) with normal rabbit serum. Data were analyzed by the Student t test (*P ≤ .05).
Overexpression of wild-type SHIP downregulates FcγR-induced Rac activation. (A) Raw 264.7 cells were retrovirally infected using vector alone, wild-type SHIP, or catalytic-deficient D675A SHIP. GTP-bound Rac was measured in these transfectants after FcγR clustering. The bottom panel represents the band intensity of the Rac GTP. Values obtained from 3 independent experiments are represented as mean and SEM. (B) The stable transfectants were analyzed for SHIP expression by immunoprecipitating SHIP with rabbit polyclonal SHIP antibody and immunoblotting with SHIP antibody. The last lane is a control immunoprecipitate (IP) with normal rabbit serum. Data were analyzed by the Student t test (*P ≤ .05).
Immune complex–induced production of inflammatory cytokines is regulated by SHIP
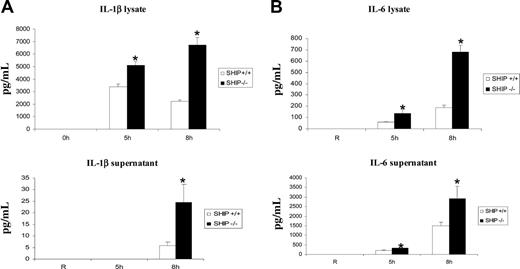
The phagocytic process is also accompanied by the production of inflammatory cytokines such as IL-1β and IL-6.2,19,20 While extensive analyses have been performed to understand mechanisms involved in the process of particle engulfment, little is known about signaling pathways involved in cytokine generation and its regulation in macrophages. Here, we first examined whether SHIP influenced FcγR-induced cytokine production. In these experiments, SHIP+/+ and SHIP–/– BMMs were stimulated with heat-aggregated IgG for the time points indicated in Figure 4. Cell lysates and supernatants were collected and assayed for the presence of IL-1β and IL-6 by ELISA (Figure 4A-B). Results from 3 independent experiments revealed that FcγR-induced production of IL-1β and IL-6 is significantly higher in SHIP–/– BMMs compared with SHIP+/+ BMMs. These results indicate that SHIP is a negative regulator of FcγR-induced IL-1β and IL-6 production. Of note, macrophages release minimal amounts of IL-1β into the supernatant (Figure 4B bottom panel), since this cytokine, unlike IL-6, requires posttranslational modification, which is reported to be dramatically impaired in macrophages compared with monocytes.36
SHIP downregulates FcγR-induced production of IL-1β and IL-6. BMMs obtained from SHIP+/+ and SHIP–/– mice were stimulated for the time points indicated in the figure with heat-aggregated IgG. (A) The levels of IL-1β and (B) IL-6 in lysates and supernatants were measured by ELISA. The graphs represent the mean and SEM of values obtained from 3 independent experiments. Data were analyzed by Student t test (*P ≤ .05).
SHIP downregulates FcγR-induced production of IL-1β and IL-6. BMMs obtained from SHIP+/+ and SHIP–/– mice were stimulated for the time points indicated in the figure with heat-aggregated IgG. (A) The levels of IL-1β and (B) IL-6 in lysates and supernatants were measured by ELISA. The graphs represent the mean and SEM of values obtained from 3 independent experiments. Data were analyzed by Student t test (*P ≤ .05).
In order to examine the mechanism by which SHIP influences cytokine production in macrophages, we analyzed the role of the Ras/Erk and PI3K pathways on FcγR-induced cytokine production. In B cells, in addition to its ability to hydrolyze PI3K products and downregulate downstream signaling, SHIP has been previously demonstrated to downregulate the Ras/Erk pathway by associating with the Ras GAP-binding protein p62dok and accelerating hydrolysis of Ras GTP and by competing with the Grb2-Shc adapter complex necessary for Ras activation. While the former function is mediated by SHIP's enzyme activity, the latter is a consequence of its noncatalytic domains. In these experiments BMMs were preincubated with either vehicle control or with inhibitors of the Ras/Erk pathway (U0126) or PI3K pathway (LY294002) and subsequently stimulated with heat-aggregated IgG. Production of IL-1β and IL-6 was measured by ELISA. As shown in Figure 5A, inhibition of the Ras/Erk pathway but not PI3K pathway suppressed the production of IL-1β. In contrast, inhibition of either the Ras/Erk pathway or the PI3K pathway resulted in partial downregulation of IL-6 production (Figure 5B). Simultaneous inhibition of both the PI3K and the Ras/Erk pathways resulted in further attenuation of IL-6 production. To ensure the efficacy/specificity of the inhibitors, protein-matched lysates from resting and stimulated cells were probed with antibodies to phospho-Erk and phospho-Akt (top panels of Figure 5C and D, respectively). The bottom panels (Figure 5C and D) are reprobes with Erk and Akt antibody, respectively, to ensure equal loading of protein in all lanes. Collectively, these results demonstrate several novel points: (a) that there is no cross talk between the Ras/Erk pathway and the PI3K pathway during FcγR signaling in BMMs; (b) that the 2 signaling pathways play differential roles in FcγR-induced cytokine production; (c) that SHIP may influence the Ras/Erk pathway as well as the PI3K pathway during FcγR signaling in BMMs, independently of each other.
Differential requirement for the PI3K and Ras/MAPK pathways in FcγR-induced IL-1β and IL-6 production. BMMs were treated with Me2SO (DMSO) or 10 μM LY294002 or 2.5 μM UO126 for 30 minutes at 37°C prior to stimulation with heat-aggregated IgG. (A) The levels of IL-1β and (B) IL-6 in cell lysates and supernatants were measured by ELISA. The graphs represent the mean and SEM of 3 independent experiments. Data were analyzed by Student t test. *P ≤ .05. (C) Protein-matched lysates from unstimulated and stimulated cells (stimulated for 7 minutes) were analyzed by Western blotting with phospho-Erk antibody. The bottom panel is a reprobe of the same membrane with anti-Erk antibody. (D) Parallel samples were probed with anti-phospho Serine Akt antibody, and the membrane was reprobed with anti-Akt antibody (bottom panel).
Differential requirement for the PI3K and Ras/MAPK pathways in FcγR-induced IL-1β and IL-6 production. BMMs were treated with Me2SO (DMSO) or 10 μM LY294002 or 2.5 μM UO126 for 30 minutes at 37°C prior to stimulation with heat-aggregated IgG. (A) The levels of IL-1β and (B) IL-6 in cell lysates and supernatants were measured by ELISA. The graphs represent the mean and SEM of 3 independent experiments. Data were analyzed by Student t test. *P ≤ .05. (C) Protein-matched lysates from unstimulated and stimulated cells (stimulated for 7 minutes) were analyzed by Western blotting with phospho-Erk antibody. The bottom panel is a reprobe of the same membrane with anti-Erk antibody. (D) Parallel samples were probed with anti-phospho Serine Akt antibody, and the membrane was reprobed with anti-Akt antibody (bottom panel).
FcγR-induced activation of the Ras/Erk pathway is downregulated by SHIP
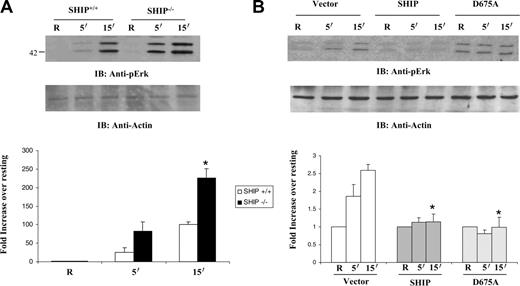
These experiments suggest that SHIP influences FcγR-induced activation of the Ras/Erk pathway in macrophages. To directly test this, SHIP+/+ and SHIP–/– BMMs were stimulated by clustering FcγR and assessed for the activity of the Ras signaling pathway by measuring phosphorylation of Erk. Protein-matched lysates from SHIP+/+ and SHIP–/– BMMs were subjected to Western blotting with phospho-specific antibodies to Erk. Results indicated Erk phosphorylation was significantly enhanced in SHIP–/– BMMs (Figure 6A). The graph shown in the bottom panel (Figure 6A) is a quantitative measure of Erk phosphorylation from 3 independent experiments.
As an additional approach, FcγR-induced Erk phosphorylation was assessed in the Raw 264.7 transfectants expressing vector alone, wild-type SHIP, or catalytic-deficient SHIP (Figure 6B). Results from 3 independent experiments are quantitated in the graph shown in the bottom panel (Figure 6B) and demonstrate that overexpression of either a wild-type SHIP or a catalytic-deficient SHIP inhibits the induction of Erk phosphorylation following FcγR clustering. These results demonstrate that SHIP downregulates activation of the Ras/Erk pathway by FcγR clustering, in a manner that is independent of SHIP's catalytic function.
SHIP downregulates FcγR-induced activation of Erk. (A) SHIP+/+ and SHIP–/– macrophages were activated by clustering Fcγ receptors for the indicated time points. Protein-matched lysates were probed with anti-phospho–ERK and reprobed with antibody against actin (bottom panel). The graph shown below is a quantitative estimate of Erk phosphorylation and represents the mean and SEM of values obtained from 3 independent experiments. Data were analyzed by Student t test. *P ≤ .05. □ indicates SHIP+/+; ▴, SHIP–/–. (B) Erk phosphorylation was likewise measured in stable transfectants overexpressing vector alone, wild-type SHIP, or catalytic-deficient D675A SHIP.
SHIP downregulates FcγR-induced activation of Erk. (A) SHIP+/+ and SHIP–/– macrophages were activated by clustering Fcγ receptors for the indicated time points. Protein-matched lysates were probed with anti-phospho–ERK and reprobed with antibody against actin (bottom panel). The graph shown below is a quantitative estimate of Erk phosphorylation and represents the mean and SEM of values obtained from 3 independent experiments. Data were analyzed by Student t test. *P ≤ .05. □ indicates SHIP+/+; ▴, SHIP–/–. (B) Erk phosphorylation was likewise measured in stable transfectants overexpressing vector alone, wild-type SHIP, or catalytic-deficient D675A SHIP.
SHIP downregulates FcγR-induced inflammatory cytokine production through the inhibition of the Ras/Erk and PI3K pathways
These experiments demonstrate that SHIP regulates FcγR-induced production of IL-1β and IL-6 and also that SHIP regulates the PI3K and the Ras/Erk pathways. In order to examine whether SHIP influences cytokine production through its influence on PI3K and the Ras/Erk pathways the following experiments were performed. Here, SHIP+/+ and SHIP–/– BMMs were preincubated with either vehicle alone (DMSO), the MEK inhibitor U0126, or the PI3K inhibitor LY294002. Cells were subsequently stimulated with heat-aggregated IgG for 5 hours. Cell lysates and supernatants were assayed for IL-1β and IL-6 by ELISA. The results shown in Figure 7 are representative of 3 independent experiments. As seen in Figure 7A, IL-1β production was enhanced in SHIP–/– BMMs in comparison to SHIP+/+ BMMs. Treatment with the MEK inhibitor significantly downregulated IL-1β production, even in cells deficient in SHIP, suggesting that SHIP influences IL-1β production through its influence on the Ras/Erk pathway. In contrast, inhibition of PI3K had no effect on IL-1β production (Figure 7A bottom panel). Figure 7B demonstrates that while SHIP–/– BMMs produce significantly elevated levels of IL-6, this production is downregulated in the presence of both the MEK inhibitor (top panel) and the PI3K inhibitor (bottom panel). These results demonstrate that SHIP influences FcγR-induced IL-6 production through its influence on both the PI3K and the Ras/Erk pathways. In all experiments, specificity of the inhibitors used was verified by Western blotting cell lysates with phospho-specific antibodies to Erk and Akt (Figure 7C).
SHIP downregulates FcγR-induced IL-1β and IL-6 production through the inhibition of PI3K and Ras/MAPK pathways. SHIP+/+ and SHIP–/– macrophages were treated with either Me2SO (DMSO), 10 μM LY294002, or 2.5 μM UO126 for 30 minutes at 37°C prior to stimulation with heat-aggregated IgG. (A) The levels of IL-1β in cells treated with DMSO or UO126 (i) and IL-1β in cells treated with DMSO or LY294002 (ii) were measured by ELISA. The graphs represent the mean and SEM of values obtained from 3 independent experiments. Data were analyzed by Student t test. *P ≤ .05. (B) The levels of IL-6 in supernatants of cells treated with DMSO or UO126 (i) and cells treated with DMSO or LY294002 (ii) were measured by ELISA. The graphs represent the mean and SEM of values obtained from 3 independent experiments. Data were analyzed by Student t test. *P ≤ .05. (C) Protein-matched lysates were analyzed by Western blotting with phospho-Erk antibody (top panels). Parallel samples were probed with antiphospho serine Akt antibody (middle panels), and the membrane was reprobed with anti-Akt antibody (bottom panels). (D) Raw 264.7 stable transfectants overexpressing vector alone, wild-type SHIP, or catalytic-deficient D675A SHIP were stimulated with heat-aggregated IgG. Production of IL-1β and IL-6 was analyzed by measuring lysates and supernatants, respectively, by ELISA at 5 hours and 8 hours after stimulation. Data were analyzed by Student t test. *P ≤ .05.
SHIP downregulates FcγR-induced IL-1β and IL-6 production through the inhibition of PI3K and Ras/MAPK pathways. SHIP+/+ and SHIP–/– macrophages were treated with either Me2SO (DMSO), 10 μM LY294002, or 2.5 μM UO126 for 30 minutes at 37°C prior to stimulation with heat-aggregated IgG. (A) The levels of IL-1β in cells treated with DMSO or UO126 (i) and IL-1β in cells treated with DMSO or LY294002 (ii) were measured by ELISA. The graphs represent the mean and SEM of values obtained from 3 independent experiments. Data were analyzed by Student t test. *P ≤ .05. (B) The levels of IL-6 in supernatants of cells treated with DMSO or UO126 (i) and cells treated with DMSO or LY294002 (ii) were measured by ELISA. The graphs represent the mean and SEM of values obtained from 3 independent experiments. Data were analyzed by Student t test. *P ≤ .05. (C) Protein-matched lysates were analyzed by Western blotting with phospho-Erk antibody (top panels). Parallel samples were probed with antiphospho serine Akt antibody (middle panels), and the membrane was reprobed with anti-Akt antibody (bottom panels). (D) Raw 264.7 stable transfectants overexpressing vector alone, wild-type SHIP, or catalytic-deficient D675A SHIP were stimulated with heat-aggregated IgG. Production of IL-1β and IL-6 was analyzed by measuring lysates and supernatants, respectively, by ELISA at 5 hours and 8 hours after stimulation. Data were analyzed by Student t test. *P ≤ .05.
As a second approach, IL-1β and IL-6 production was measured in Raw 264.7 transfectants overexpressing wild-type SHIP or catalytic-deficient SHIP. Results indicated that overexpression of either the wild-type or the catalytic-deficient version of SHIP significantly dampened the production of IL-1β production in response to FcγR clustering, indicating that the inhibition of IL-1β production is independent of the catalytic function of SHIP. These results are consistent with the findings that the influence of SHIP on the Ras/Erk pathway is independent of its catalytic function. In contrast, although IL-6 production was almost completely abrogated when wild-type SHIP was overexpressed, there was only partial inhibition of IL-6 when the catalytic mutant of SHIP was overexpressed. These results demonstrate that both the catalytic function and the noncatalytic domains of SHIP are necessary for the inhibition of IL-6 production.
Thus, we conclude that SHIP influences FcγR-induced production of IL-1β and IL-6 through the inhibition of the Ras/Erk and PI3K pathways.
Discussion
In this study we have analyzed for the first time the influence of SHIP on phagocytosis-associated events (ie, superoxide generation and inflammatory cytokine production). Our data reveal several novel aspects to the regulation of these phagocytosis-associated events. First, the expression of SHIP downregulates Rac activity and subsequent superoxide production. Interestingly, although we observed high basal Rac activity in SHIP–/– BMMs, there was no detectable production of superoxide in the absence of Fc receptor stimulation, suggesting that, while the high Rac activity may contribute to enhanced superoxide production, there are other FcγR-induced activation events that are necessary for generation of superoxide. Activation of Rac is critical for particle ingestion as well as for the assembly of NADPH oxidase complex and superoxide generation.16,17 While earlier reports have demonstrated that SHIP negatively regulates phagocytosis,10,22 the mechanism by which SHIP mediates its inhibitory effect was previously not known. As such, we believe these data represent a significant advance in our understanding of SHIP's influence on phagocytosis.
Although it is well established that FcγR clustering results in the production of inflammatory cytokines, there is surprisingly little known about signaling pathways involved in the induction of these cytokines. Our experiments demonstrate that the PI3K and Ras/Erk pathways are activated by FcγR clustering quite independently of one another. Thus, inhibition of the PI3K pathway has no effect on the activation of the Ras/Erk pathway and vice versa (Figure 5C-D). This is in contrast to the signaling events initiated by cytokines and growth factors, where PI3K has been shown to be both upstream and downstream of Ras.37,38
Interestingly, our current findings also indicate a differential requirement for the PI3K and Ras/Erk pathways in induction of the inflammatory cytokines IL-1β and IL-6. Thus, while activation of PI3K and Ras/Erk pathways was necessary for the induction of IL-6, activation of only Ras/Erk, but not PI3K, was sufficient for the induction FcγR-induced IL-1β production (Figure 5A-B). These observations are important because they allow for the distinction between the catalytic function of SHIP (which hydrolyzes PtdIns3,4,5P3 and downregulates the PI3K pathway) and the noncatalytic function of SHIP (which downregulates the Ras/Erk pathway).
SHIP is a cytosolic enzyme that requires activation-induced recruitment to the cell membrane where it encounters its lipid substrates. Extensive studies from our group and others have demonstrated that SHIP is recruited to the phosphorylated ITIM of FcγRII(b) via the SHIP SH2 domain.39,40 Interestingly, phosphorylated ITAMs of FcγR γ-subunit and human FcγRIIa can also recruit SHIP, albeit with much less efficiency than the ITIM of FcγRIIb.8,10,41,42 Association of SHIP with the phosphorylated ITAMs occurs both directly as well as through the adapter protein Shc.8,10,41 IgG immune complexes engage both ITAM-containing and ITIM-containing FcγR simultaneously, thus recruitment of SHIP to the plasma membrane, under these conditions, likely occurs through SHIP association with both types of receptors. Our data suggest that SHIP downregulates both the PI3K and the Ras/Erk pathways by virtue of its catalytic domain and its noncatalytic domains, respectively. While the catalytic function of SHIP is to hydrolyze 5′ phosphates from 3′ phosphorylated PI3K products, the noncatalytic domains interact with a number of cytoplasmic molecules to inhibit signaling pathways. The best characterized of these is the influence of SHIP on the Ras pathway. At least 2 models are proposed in this context in B cells: (i) the SHIP SH2 domain competes with Grb2/Sos complex for binding to phosphorylated Shc and thereby downregulates Ras activation25,26 ; and (ii) SHIP associates with p62dok, which results in hyperphosphorylation of dok, its association with RasGAP, and the subsequent hydrolysis of Ras-GTP.23 Future studies will tell whether there are other signaling pathways that are influenced by SHIP's association with additional cytoplasmic signaling molecules.
In summary, these findings demonstrate that phagocytosis-associated inflammatory responses are regulated by SHIP, in addition to the previously reported regulation of particle engulfment. Thus, the entire phagocytic process appears to be tightly regulated by the cumulative actions of the kinases and phosphatases in order to maintain homeostasis and prevent collateral tissue damage.
Prepublished online as Blood First Edition Paper, March 16, 2006; DOI 10.1182/blood-2005-09-3889.
Supported by National Institutes of Health (NIH)/National Cancer Institute (NCI) grants R01 AI059406 (S.T.) and P01 CA095426 (S.T., W.E.C., J.C.B., M.A.C.) and Leukemia and Lymphoma Society and D. Warren Brown Foundation grants (J.C.B.). V.K.K. was on sabbatical at Nizam's Institute of Medical Sciences (Hyderabad, India).
L.P.G. and T.J. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal