Triptolide, a diterpenoid isolated from the Chinese herb Tripterygium wilfordii Hook.f, has shown antitumor activities in a broad range of solid tumors. Here, we examined its effects on leukemic cells and found that, at 100 nM or less, it potently induced apoptosis in various leukemic cell lines and primary acute myeloid leukemia (AML) blasts. We then attempted to identify its mechanisms of action. Triptolide induced caspase-dependent cell death accompanied by a significant decrease in XIAP levels. Forced XIAP overexpression attenuated triptolide-induced cell death. Triptolide also decreased Mcl-1 but not Bcl-2 and Bcl-XL levels. Bcl-2 overexpression suppressed triptolide-induced apoptosis. Further, triptolide induced loss of the mitochondrial membrane potential and cytochrome C release. Caspase-9 knock-out cells were resistant, while caspase-8–deficient cells were sensitive to triptolide, suggesting criticality of the mitochondrial but not the death receptor pathway for triptolide-induced apoptosis. Triptolide also enhanced cell death induced by other anticancer agents. Collectively, our results demonstrate that triptolide decreases XIAP and potently induces caspase-dependent apoptosis in leukemic cells mediated through the mitochondrial pathway at low nanomolar concentrations. The potent antileukemic activity of triptolide in vitro warrants further investigation of this compound for the treatment of leukemias and other malignancies.
Introduction
Tripterygium wilfordii Hook.f, a member of the Celastraceae family of plants, has been used in Chinese medicine for centuries. Triptolide, a diterpenoid, was first isolated from the plant and structurally characterized in 19721 and has been used for the treatment of a variety of autoimmune diseases and as an immunosuppressant in patients with organ and tissue transplantations.1-4 Recently, triptolide was shown to have antitumor properties by suppressing the growth and inducing apoptosis of a broad range of human tumor cells.5-9 Triptolide was also shown to sensitize cells to death induced by various agents, such as Apo2/Trail, TNF-α, and different chemotherapeutic agents.10-12 However, despite the recognized potent antitumor activity of triptolide, our knowledge regarding its mechanism of action is still limited. So far it is known only that triptolide blocks TNF-α–mediated induction of c-IAP1 and c-IAP2, and the activation of NFκB,10,13,14 and induces caspase activation.15,16
Acute myeloid leukemia (AML) is an aggressive hematologic malignancy. Despite major efforts during the past 30 years, limited progress has been made in the treatment of AML. The current primary treatment for AML is chemotherapy, which induces cell death mainly by apoptosis mediated by either the intrinsic mitochondrial or the extrinsic death receptor pathway, both of which lead to caspase activation and cell disintegration. The development of innovative therapies and identification of more effective drugs, therefore, remain high priorities for leukemia research. Because of its antitumor properties, triptolide holds promise as a treatment for leukemia. However, other than recent reports that triptolide induced apoptosis in U937 cells by activating caspase-3 and down-regulating XIAP,16 and that it down-regulated Bcr-Abl expression and induced apoptosis in K562 cells,17 virtually no work has been done to elucidate the activity and mechanisms of triptolide in leukemia. Through an understanding of the molecular mechanisms that mediate the proapoptotic activity of triptolide, it will be possible to better understand its antileukemic effects and to determine whether it is a candidate for clinical use. In addition, knowing the molecular targets of triptolide and its mechanism of action will enable us to design rational combination therapies that more efficiently eradicate leukemic cells. In the study described here, we examined the effects of triptolide on various leukemic cell lines and primary AML blasts and showed that triptolide at low nanomolar concentrations potently induced apoptosis in various leukemic cell lines and primary AML blasts. We also investigated the mechanisms of triptolide-induced apoptosis in leukemic cells. Of note, a water-soluble derivative of the drug is in clinical trials in Europe.
Materials and methods
Cells and cell cultures
OCI-AML3, OCI-AML3vec, OCI-AML3Bcl-2, U937, U937neo, U937XIAP, Jurkat, JurkatI2.1, KG1, HL-60, and K562 cells were cultured in RPMI 1640 medium; KBM5 cells, in Iscoves modified Dulbecco medium (Gibco-BRL, Gaithersburg, MD); and mouse embryonic fibroblasts (MEFs) and MEF-caspase-9–/– cells, in α-MEM medium. All 3 media contained 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. U937, Jurkat, JurkatI2.1 (a Jurkat clone deficient in caspase-818 ), K562, KG1, and HL-60 cells were purchased from the American Type Culture Collection (Manassas, VA). OCI-AML3 cells were kindly provided by Dr M. Minden (Ontario Cancer Institute, Toronto, ON). Bcl-2–overexpressing OCI-AML3 cells (OCI-AML3Bcl-2) and the vector control cells (OCI-AML3vec) were obtained by electroporating (solution V, program T19; Amaxa Biosystem, Cologne, Germany) OCI-AML3 cells with Bcl-2 cDNA and the control vector. OCI-AML3Bcl-2 and OCI-AML3vec cells were selected in the presence of 0.8 mg/mL G418 (Gibco-BRL). The Bcl-2–expressing vector was kindly provided by Dr P. Ruvolo (The University of Texas Health Science Center, Houston, TX). U937 cells overexpressing XIAP (U937XIAP) and the vector control cells (U937neo) were kindly provided by Dr D. Kufe (Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA).19 KBM5 cells,20 derived from a Ph+ chronic myelogenous leukemia (CML) patient in blast crisis, were a kind gift of Dr M. Beran of The University of Texas M. D. Anderson Cancer Center (Houston, TX). Caspase-9 knock-out MEFs (MEF-caspase-9–/–) were obtained from Dr R. Flavell (Yale University School of Medicine, New Haven, CT).21 Fresh primary AML patient samples with high blast counts (≥ 64%) and normal bone marrow specimens were acquired after informed consent was obtained according to institutional guidelines set forth by The University of Texas M. D. Anderson Cancer Center and the Declaration of Helsinki. Mononuclear cells were purified by Ficoll-Hypaque (Sigma Chemical, St Louis, MO) density-gradient centrifugation and cultured in RPMI 1640 medium supplemented with 10% FCS.
Treatment of cells
Exponentially growing cells (0.2 × 106/mL) or mononuclear cells from AML and normal bone marrow samples (1 × 106/mL) were treated with various concentrations of triptolide (Alexis Biochemicals, San Diego, CA) for 24 and 48 hours. An appropriate amount of DMSO was used as the control. To block caspase activation or proteasome degradation, cells were pretreated with 20 μM IDN-1965, a general caspase inhibitor22 (kindly provided by IDUN Pharmaceuticals, San Diego, CA), or 0.2 μM MG132, a proteasome inhibitor (Calbiochem, San Diego, CA), 1 hour before triptolide was added. For studies of combination treatment, exponentially growing cells (0.2 × 106/mL) or mononuclear cells from AML samples (1 × 106/mL) were treated with increasing concentrations of triptolide and the selected compound at a constant ratio for 48 hours. Cell death was analyzed by annexin V staining, as described in “Cell viability assay.”
Cell viability assay
Cell viability was determined by trypan blue exclusion using a Vi-Cell XR Cell Counter (Beckman Coulter, Fullerton, CA). Apoptosis was estimated by both Western blot analysis of caspase-3 activation and flow cytometry measurements of phosphatidyl serine23 with the Annexin-V-FLUOS Staining Kit (Roche Diagnostics, Indianapolis, IN). Membrane integrity was simultaneously assessed by propidium iodide (PI) exclusion in the annexin V–stained cells. To measure changes in the mitochondrial membrane potential (MMP), cells were loaded with CMXRos (300 nM) and MitoTracker Green (100 μM) (both from Molecular Probes, Eugene, OR) for 1 hour at 37°C. The loss of MMP was then assessed by measuring CMXRos retention (red fluorescence) while simultaneously adjusting for mitochondrial mass (green fluorescence).
Cell cycle distribution
Cells were fixed with 70% ice-cold ethanol and stained with PI solution (25 μg/mL PI, 180 U/mL RNase, 0.1% Triton X-100, and 30 mg/mL polyethylene glycol in 4 mM citrate buffer, pH 7.8; Sigma Chemical). The DNA content was determined using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). The cell cycle distribution was analyzed using ModFit LT software (Verity Software House, Topsham, ME).
Western blot analysis
Western blot analysis to determine the levels of various proteins was done as described previously.24 Briefly, treated cells were washed with PBS and lysed in protein lysis buffer (0.25 M Tris-HCl, 2% SDS, 4% β-mercaptoethanol, 10% glycerol, and 0.02% bromophenol blue). An equal amount of cell lysate was loaded onto a 12% SDS-PAGE gel (Bio Rad, Hercules, CA). To measure the cytochrome C released from the mitochondria into the cytosol, cells were lysed with ice-cold lysis buffer (25 mM Tris-HCl and 5 mM MgCl2 [pH 7.4]). Supernatant was collected by a microfuge, analyzed by Western blot, and probed with the antibody against cytochrome C (PharMingen, San Diego, CA). After incubating with the second antibody, the membranes were reacted with ECL solution (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Signals were detected by a PhosphorImager (Storm 860 Version 4.0; Molecular Dynamics, Sunnyvale, CA). β-Actin was included as a loading control.
TaqMan reverse-transcriptase–polymerase chain reaction (RT-PCR)
RNA was isolated from triptolide-treated OCI-AML3 cells with Trizol solution (Life Technologies, Grand Island, NY). RNA was reverse-transcribed by avian myeloblastosis virus (AMV) reverse transcriptase (Roche Diagnostics) at 42°C for 1 hour. PCR amplification reaction mixtures (25 μL) contained cDNA, XIAP forward primer (5′-CCCAAATTGCAGATTTATCAACG-3′), XIAP reverse primer (5′-TGCATGTGTCTCAGATGGCC-3′), XIAP probe (5′-ATCTGGGAAGCAGAGATCATTTTGCCTTAGAC-3′), and TaqMan Universal PCR Master Mix (PE Applied Biosystems, Foster City, CA). β2-Microglobulin (β2-m) coamplified with XIAP was included as an internal control for normalization of the variable content of cDNA in each sample (forward primer, 5′-AGCTGTGCTCGCGCTACTCT-3′; reverse primer, 5′-TTGACTTTCCATTCTCTGCTGG-3′; and probe, 5′-TCTTTCTGGCCTGGAGGGCATCC-3′). Thermal cycling conditions included holding the reactions at 50°C for 2 minutes and at 95°C for 10 minutes and cycling for 40 cycles between 95°C for 15 seconds and 60°C for 1 minute. Results were collected and analyzed with an ABI Prism 7700 Sequence Detection System (PE Applied Biosystems) as follows: the PCR cycle number that generated the first fluorescence signal above the threshold (threshold cycle, CT, 10 standard deviations above the mean fluorescence generated during the baseline cycles) was determined, and a comparative CT method was then used to measure relative gene expression. The following formula was used to calculate the relative amount of the transcript of interest in the treated sample (X) and the control sample (Y), both normalized to an endogenous reference (β2-m):2–ΔΔCT, where ΔCT is the difference in CT between the gene of interest and β2-m, and ΔΔCT for sample X =ΔCT (X) –ΔCT(Y).25
Colony-formation assay
The colony-formation assay was performed as previously described.26 Briefly, 1 × 105 mononuclear cells from AML patient bone marrow were plated in 0.8% methylcellulose in Iscoves modified Dulbecco medium supplemented with 10% FCS and 15 ng/mL recombinant human granulocyte-macrophage–colony-stimulating factor (hGM-CSF). Triptolide was added at the initiation of cultures at concentrations of 10 to 100 nM. AML blast colonies were evaluated under a microscope on day 7 of culture in duplicate dishes. Cells from normal bone marrow were plated in 0.8% methylcellulose in Iscoves modified Dulbecco medium, 1 unit/mL human erythropoietin (Amgen, Thousand Oaks, CA), and 50 ng/mL recombinant hGM-CSF. Triptolide was added at concentrations of 10 to 100 nM. All cultures were analyzed after 14 days to determine the number of colony-forming units: colony-forming unit granulocyte-macrophage (CFU-GM) colonies and burst-forming unit-erythroid (BFU-E) colonies.
Statistics
All experiments were carried out 3 times or more and results were expressed as the mean plus or minus standard error (SE), unless otherwise stated. The concentration of triptolide that induced annexin V positivity in 50% of cells was calculated using Calcusyn software (Biosoft, Ferguson, MO). The combination index (CI) was determined by the Chou-Talalay method27 and Calcusyn software and was expressed as the mean plus or minus SE of the CI values obtained at the ED50,ED75, and ED90. A CI of less than 1 was considered to indicate a synergistic effect, and a CI of 1 was considered to have an additive effect.
Results
Triptolide inhibits cell growth and induces cell death of various leukemic cells
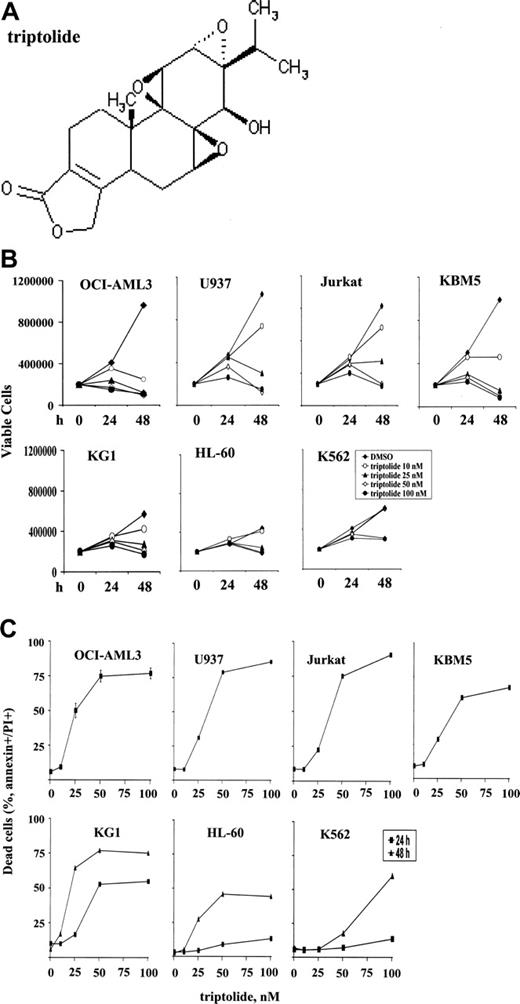
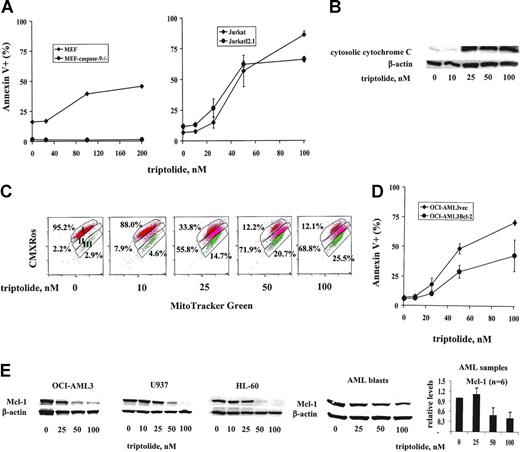
To investigate the effects of triptolide (see Figure 1A for the structure) on growth and survival of leukemic cells, we treated various leukemia cell lines, including OCI-AML3, U937, Jurkat, KBM5, KG1, HL-60, and K562 cells, with increasing concentrations of triptolide. As shown in Figure 1, triptolide in nanomolar concentrations induced significant cell growth arrest (Figure 1B) and cell death (Figure 1C) in all leukemic cells tested. However, OCI-AML3, U937, Jurkat, and KBM5 cells were more sensitive to triptolide than were KG1, HL-60, and K562 cells. The concentration of triptolide that caused 50% of OCI-AML3 cells to be annexin V positive was 34.5 ± 4.2 nM at 24 hours, as determined by Calcusyn software. No significant change in cell cycle distribution was observed in OCI-AML3, U937, and HL-60 cells at 24 hours (results not shown), suggesting that cell death is the primary cause of cell growth inhibition by triptolide in these leukemic cells.
Triptolide induced significant cell growth arrest and cell death in leukemic cells. Cells at a density of 0.2 × 106/mL were treated with various concentrations of triptolide. (A) Structure of triptolide. (B) Viable leukemic cells treated with triptolide, as determined by trypan blue exclusion after 24 and 48 hours. (C) Cell death induced by triptolide in various leukemic cells, as determined by annexin V staining with PI after 24 or 48 hours of treatment.
Triptolide induced significant cell growth arrest and cell death in leukemic cells. Cells at a density of 0.2 × 106/mL were treated with various concentrations of triptolide. (A) Structure of triptolide. (B) Viable leukemic cells treated with triptolide, as determined by trypan blue exclusion after 24 and 48 hours. (C) Cell death induced by triptolide in various leukemic cells, as determined by annexin V staining with PI after 24 or 48 hours of treatment.
Triptolide induces cell death in primary AML samples
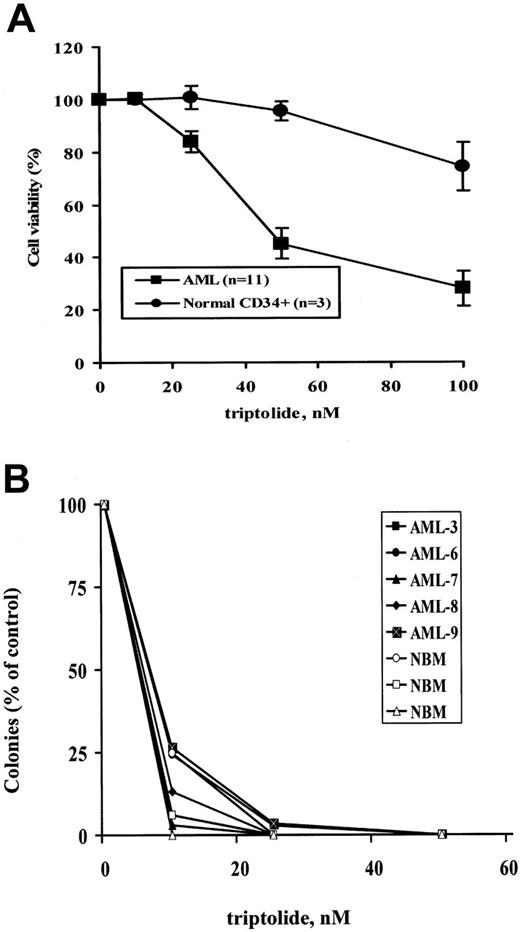
To assess the potential of triptolide for the treatment of AML, we tested the effects of triptolide on the short- and long-term survival of primary AML blasts. Characteristics of the patient samples used are summarized in Table 1. Eleven primary AML patient samples (3 bone marrow and 8 peripheral blood) and 3 normal bone marrow samples were treated with triptolide in vitro for 24 hours. Cell death was determined by annexin V staining. In normal bone marrows, cell death was measured in the CD34+ population after triptolide-treated cells were labeled with mouse anti-CD34 antibody conjugated with allophycocyanin (CD34-APC; Becton Dickinson). As shown in Figure 2A, triptolide at low nanomolar concentrations potently induced cell death in all 11 AML blast samples tested, independent of patient sample source, cytogenetics, previous treatments, and the clinical responses to these therapies. In contrast, significantly less cell death was induced in normal bone marrow CD34+ cells (Figure 2A). At 100 nM triptolide, more than 70% of AML cells but only about 25% of CD34+ cells from normal bone marrow samples lost their viability (P = .01) (Figure 2A).
Characteristics of AML patient samples
Sample . | Source . | Assay . | Blasts, % . | Cytogenetics . | Previous treatments and responses . |
|---|---|---|---|---|---|
| AML-1 | PB | Annexin V | 82 | 46,XY, diploid, poor | Resistant to Ara-C + daunorubicin, topotecan + Ara-C + mitoxantrone, and gemtuzumab ozogamicin |
| AML-2 | PB | Annexin V | 85 | Diploid | Untreated |
| AML-3 | BM | Annexin V, colony | 99 | Poor | Resistant to topotecan + Ara-C + gemtuzumab ozogamicin; achieved CR with mitoxantrone + VP-16; then relapsed; resistant to SAHA |
| AML-5 | PB | Annexin V | 90 | Poor | Resistant to Ara-C + mitoxantrone, Bryo + Ara-C, and DAC + VPA; achieved CR with Ara-C, CI × 7 d + mitoxantrone d 5-7 + FA twice daily × 5 d; then relapsed |
| AML-6 | BM | Annexin V, colony | 81 | Good | Untreated |
| AML-7 | BM | Colony | 85 | Poor | Achieved CR with FAMP + HDAC; then relapsed |
| AML-8 | BM | Colony | 67 | Diploid | Resistant to Ara-C + Ida, HDAC, cyclophosphamide + VP-16, and rosiglitazone + bexarotene |
| AML-9 | BM | Colony | 90 | Poor | Achieved CR with Ida + Ara-C and allotransplantation; then relapsed; resistant to mitoxantrone + VP-16, FA twice daily × 5 d, and DAC + VPA |
| AML-10 | PB | Annexin V, WB | 93 | 46,XX, diploid | Resistant to CLOFA + LDAC, XL119, AVE9633, and IA |
| AML-11 | PB | Annexin V, WB, combination | 98 | 46,XY, diploid | Achieved CR with IA; resistant to mitoxantrone, etoposide + cytarabine + oral CEP-701, and L-001281814 |
| AML-12 | BM | Annexin V, WB | 94 | t(9;11) etc, poor | Refractory AML with unknown treatment history |
| AML-13 | PB | Annexin V, WB, combination | 96 | inv(7) etc | Resistant to IA, Ara-C, L-001281814, and FA twice daily × 5 d + gemtuzumab ozogamicin |
| AML-14 | PB | Annexin V, WB, combination | 95 | 48,XX, +6, +8[7] | Resistant to IA, gemtuzumab ozogamicin, and BCH |
| AML-15 | PB | Annexin V, WB | 64 | 46,XY[20], diploid | Untreated |
| AML-16 | PB | Combination | 98 | 46,XX[28] | Resistant to IA, fludarabine + Ara-C, mitoxantrone + Ara-C + VP-16, and XKR69R |
Sample . | Source . | Assay . | Blasts, % . | Cytogenetics . | Previous treatments and responses . |
|---|---|---|---|---|---|
| AML-1 | PB | Annexin V | 82 | 46,XY, diploid, poor | Resistant to Ara-C + daunorubicin, topotecan + Ara-C + mitoxantrone, and gemtuzumab ozogamicin |
| AML-2 | PB | Annexin V | 85 | Diploid | Untreated |
| AML-3 | BM | Annexin V, colony | 99 | Poor | Resistant to topotecan + Ara-C + gemtuzumab ozogamicin; achieved CR with mitoxantrone + VP-16; then relapsed; resistant to SAHA |
| AML-5 | PB | Annexin V | 90 | Poor | Resistant to Ara-C + mitoxantrone, Bryo + Ara-C, and DAC + VPA; achieved CR with Ara-C, CI × 7 d + mitoxantrone d 5-7 + FA twice daily × 5 d; then relapsed |
| AML-6 | BM | Annexin V, colony | 81 | Good | Untreated |
| AML-7 | BM | Colony | 85 | Poor | Achieved CR with FAMP + HDAC; then relapsed |
| AML-8 | BM | Colony | 67 | Diploid | Resistant to Ara-C + Ida, HDAC, cyclophosphamide + VP-16, and rosiglitazone + bexarotene |
| AML-9 | BM | Colony | 90 | Poor | Achieved CR with Ida + Ara-C and allotransplantation; then relapsed; resistant to mitoxantrone + VP-16, FA twice daily × 5 d, and DAC + VPA |
| AML-10 | PB | Annexin V, WB | 93 | 46,XX, diploid | Resistant to CLOFA + LDAC, XL119, AVE9633, and IA |
| AML-11 | PB | Annexin V, WB, combination | 98 | 46,XY, diploid | Achieved CR with IA; resistant to mitoxantrone, etoposide + cytarabine + oral CEP-701, and L-001281814 |
| AML-12 | BM | Annexin V, WB | 94 | t(9;11) etc, poor | Refractory AML with unknown treatment history |
| AML-13 | PB | Annexin V, WB, combination | 96 | inv(7) etc | Resistant to IA, Ara-C, L-001281814, and FA twice daily × 5 d + gemtuzumab ozogamicin |
| AML-14 | PB | Annexin V, WB, combination | 95 | 48,XX, +6, +8[7] | Resistant to IA, gemtuzumab ozogamicin, and BCH |
| AML-15 | PB | Annexin V, WB | 64 | 46,XY[20], diploid | Untreated |
| AML-16 | PB | Combination | 98 | 46,XX[28] | Resistant to IA, fludarabine + Ara-C, mitoxantrone + Ara-C + VP-16, and XKR69R |
WB, XIAP, Mcl-1, and caspase-3 protein levels were determined by Western blot after cells were treated with triptolide for 24 hours; and combination, cells were treated in vitro with triptolide in combination with doxorubicin, gemtuzumab ozogamicin, or Ara-C.
PB indicates peripheral blood; BM, bone marrow; SAHA, suberoylanilide hydroxamic acid; Bryo, bryostatin; DAC, dacitabine; VPA, valproic acid; FA, fludarabine + Ara-C; FAMP, fludarabine; HDAC, high-dose Ara-C; Ida, idarubicin; CLOFA, clofarabine; LDAC, low-dose Ara-C; IA, Ida—Ara-C; etc, other cytogenetic abnormalities; and BCH, troxacitabine.
Since triptolide induced cell death in clinically drug-resistant primary AML cells, we tested the ability of triptolide to overcome drug resistance. We treated blasts from 2 clinically arabinoside C (Ara-C)–resistant patients with AML (patients nos. 13 and 16 in Table 1) with a low concentration of triptolide in combination with Ara-C for 48 hours. These 2 samples were extremely resistant to Ara-C in vitro even at micromolar concentrations (not shown). Of interest, for patient 13, in the presence of 15.5 nM triptolide, the IC50 for Ara-C became 0.93 μM and for patient 16, in the presence of 47.8 nM triptolide, the IC50 was lowered to 2.87 μM, suggesting the potential of triptolide in overcoming resistance to Ara-C in AML.
Five bone marrow samples from primary patients with AML (Table 1) and 3 from healthy donors were treated with triptolide, and their long-term survival was analyzed in colony-formation assays. As shown in Figure 2B, triptolide inhibited clonogenic survival in all AML samples tested independent of cytogenetics, treatment, and patients' responses to treatments (Table 1). At less than 30 nM triptolide, almost no colonies were recovered in any cases. However, triptolide also inhibited normal progenitor cell growth (Figure 2B). Both CFU-GM and BFU-E colonies were equally reduced.
Triptolide inhibits XIAP and induces caspase-dependent cell death in leukemic cells
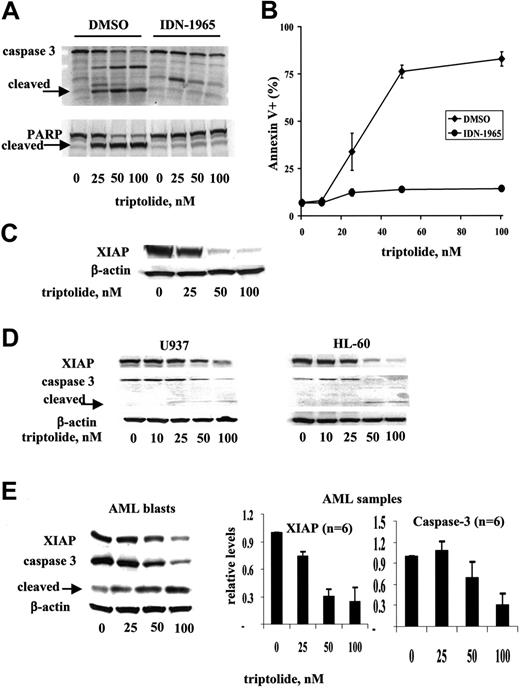
To understand the mechanisms of triptolide-induced cell death in leukemic cells, we examined the involvement of caspases in cell death. We treated OCI-AML3 cells with triptolide in the absence or presence of the general caspase inhibitor IDN-1965. At 20 μM, IDN-1965 efficiently inhibited caspase-3 activation and the cleavage of PARP, a substrate of caspase-3, without cell toxicity (Figure 3A). At this concentration, cell death induced by triptolide was also inhibited, clearly indicating that triptolide-induced cell death is caspase dependent in OCI-AML3 cells (Figure 3B).
Since triptolide-induced cell death seems to be caspase dependent and XIAP is the most potent natural cellular inhibitor of caspases, we examined the effect of triptolide on XIAP protein levels. As shown in Figure 3C, XIAP protein levels were significantly decreased in triptolide-treated OCI-AML3 cells. To ensure that decrease of XIAP and activation of caspase-3 are a common mechanism of triptolide-induced cell death in leukemic cells, we also analyzed XIAP and caspase-3 protein levels in triptolide-treated U937 and HL-60 cells and in cells from AML samples (n = 6). We found that triptolide induced decreases of XIAP protein levels and activation of caspase-3 in all cell lines and primary samples examined (Figure 3D and E, respectively).
Triptolide induced significant cell death in AML samples. (A) Comparison of triptolide-induced cell death in AML samples with that in normal bone marrow samples. Cells from 11 AML blasts and 3 normal bone marrow samples at a density of 1 × 106/mL were treated with various concentrations of triptolide. Cell death was determined by annexin V staining with PI after 24 hours of treatment. Normal bone marrows were stained with CD34-APC after triptolide treatment, and PS/annexin V was determined in the CD34+ population. (B) Results of colony-formation assays in blasts from 5 AML samples and cells from 3 normal bone marrow samples treated with the indicated concentrations of triptolide.
Triptolide induced significant cell death in AML samples. (A) Comparison of triptolide-induced cell death in AML samples with that in normal bone marrow samples. Cells from 11 AML blasts and 3 normal bone marrow samples at a density of 1 × 106/mL were treated with various concentrations of triptolide. Cell death was determined by annexin V staining with PI after 24 hours of treatment. Normal bone marrows were stained with CD34-APC after triptolide treatment, and PS/annexin V was determined in the CD34+ population. (B) Results of colony-formation assays in blasts from 5 AML samples and cells from 3 normal bone marrow samples treated with the indicated concentrations of triptolide.
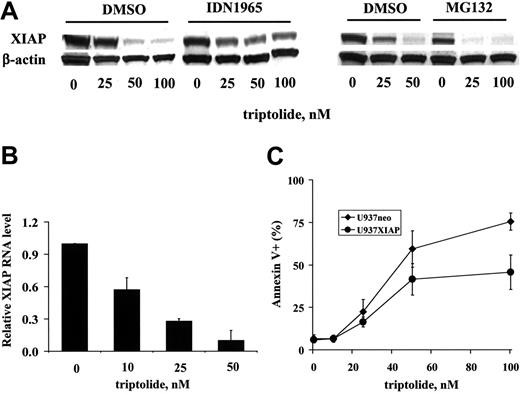
To elucidate the mechanism of XIAP inhibition, we analyzed the XIAP protein levels in the presence of the caspase inhibitor IDN-1965 and the proteasome inhibitor MG132 in OCI-AML3 cells treated with triptolide. It is well known that XIAP is a caspase substrate.22,28 Thus, when caspases are activated, XIAP is cleaved, resulting in decreased XIAP protein levels. As shown in Figure 4A, in the presence of 20 μM IDN-1965, the XIAP reduction induced by triptolide was partially blocked, indicating that caspases contributed to the decreased XIAP levels observed. However, MG132 (at a concentration of 0.2 μM) did not prevent a decrease in the XIAP protein level, suggesting that the decrease in XIAP was not due to proteasome degradation. To elucidate the mechanism of XIAP decrease not derived from caspase degradation, we examined the XIAP RNA levels in triptolide-treated OCI-AML3 cells by quantitative TaqMan RT-PCR. As demonstrated in Figure 4B, triptolide significantly decreased the XIAP RNA level, indicating that triptolide potently inhibits XIAP transcription.
If triptolide-induced cell death is indeed mediated through XIAP, then, logically, XIAP level will determine the extent to which triptolide induces cell death. To test this, we treated U937neo cells and U937XIAP cells with triptolide. As shown in Figure 4C, although the potent triptolide still induced significant cell death in U937XIAP cells, cell death was attenuated in the presence of XIAP overexpression.
Triptolide reduced the XIAP level and induced caspase-dependent cell death in leukemic cells. OCI-AML3 cells at a density of 0.2 × 106/mL were treated for 24 hours with various concentrations of triptolide in the absence or presence of the general caspase inhibitor IDN-1965 (20 μM). (A) Caspase-3 activation and PARP cleavage were analyzed by Western blot. (B) Cell viability was determined by annexin V staining with PI. (C) XIAP levels were analyzed by Western blot. (D) U937 and HL60 cells at a density of 0.2 × 106/mL and (E) AML blasts (n = 6) at a density of 1 × 106/mL were treated for 24 hours with various concentrations of triptolide. XIAP and caspase-3 protein levels were determined by Western blot.
Triptolide reduced the XIAP level and induced caspase-dependent cell death in leukemic cells. OCI-AML3 cells at a density of 0.2 × 106/mL were treated for 24 hours with various concentrations of triptolide in the absence or presence of the general caspase inhibitor IDN-1965 (20 μM). (A) Caspase-3 activation and PARP cleavage were analyzed by Western blot. (B) Cell viability was determined by annexin V staining with PI. (C) XIAP levels were analyzed by Western blot. (D) U937 and HL60 cells at a density of 0.2 × 106/mL and (E) AML blasts (n = 6) at a density of 1 × 106/mL were treated for 24 hours with various concentrations of triptolide. XIAP and caspase-3 protein levels were determined by Western blot.
Mitochondrial pathway–mediated caspase activation is essential for triptolide-induced cell death
To determine which of the 2 apoptotic signaling pathways is essential for triptolide-induced caspase-dependent cell death, we examined the requirement for caspase-9, which is activated through the mitochondrial signaling pathway, and caspase-8, which is activated through the death receptor signaling pathway, for triptolide-induced cell death. MEFs deficient in caspase-9 (MEF-caspase-9–/–)21 and Jurkat cells deficient in caspase-8 (JurkatI2.1),18 as well as their respective control cells, were treated with triptolide for 24 hours. As shown in Figure 5A, caspase-9 knock-out MEFs were resistant but caspase-8–deficient Jurkat cells were sensitive to triptolide-induced cell death, suggesting that caspase-9, but not caspase-8, is essential for triptolide-induced cell death and thus that apoptosis induced by triptolide is induced via the mitochondrial pathway. To further show that the mitochondrial pathway participates in triptolide-induced cell death, we examined cytochrome C release, changes in MMP, and the protein levels of some common Bcl-2 family proteins in leukemic cells treated with triptolide for 24 hours. Triptolide induced significant cytosolic release of cytochrome C (Figure 5B) and loss of MMP in OCI-AML3 cells (Figure 5C). A significant decrease of Mcl-1 was detected in OCI-AML3, U937, and HL-60 cells and cells from AML blasts (n = 6) (Figure 5E). There were no decreases of antiapoptotic proteins Bcl-2 and Bcl-XL (results not shown). Furthermore, OCI-AML3 cells overexpressing Bcl-2 were more resistant to triptolide than the control OCI-AML3vec cells (Figure 5D). Taken together, our data demonstrated conclusively that the mitochondrial pathway plays a critical role in triptolide-induced cell death.
Triptolide synergistically enhances doxorubicin- and gemtuzumab ozogamicin–induced cell death
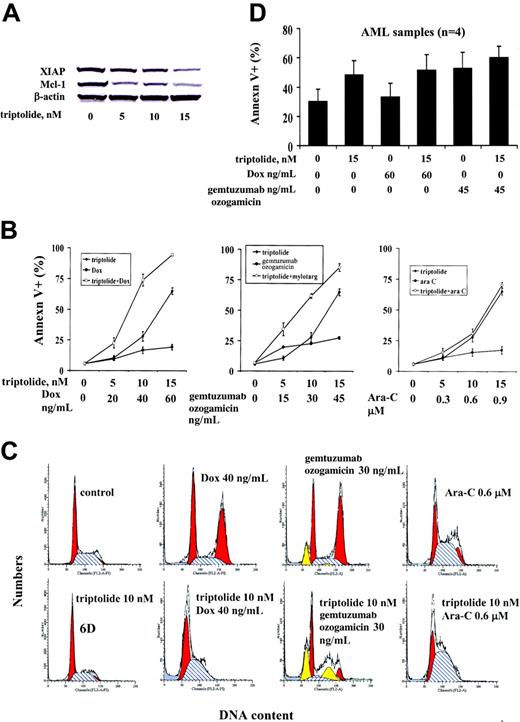
Doxorubicin (Dox), an anthracycline derivative, is one of the most active anticancer agents, with a broad spectrum of activity against solid tumors and hematologic malignancies.29 Dox is a DNA-damaging agent and induces G2 cell cycle block and cell death.30,31 Gemtuzumab ozogamicin is an antibody-targeted chemotherapeutic agent consisting of the humanized anti-CD33 antibody linked to calicheamicin, a potent enediyne antitumor antibiotic.32 It is currently used in the treatment of patients with AML.33 It induces a G2 cell cycle block and cell death in various leukemic cells34 (B.Z.C., M. Milella, M.A., unpublished observation, March 2001). Ara-C is the most commonly used chemotherapeutic agent in patients with AML and induces S-phase arrest and cell death. Since triptolide decreases the antiapoptotic proteins XIAP and Mcl-1, we hypothesized that triptolide might enhance the cell death induced by other anticancer agents. We therefore treated OCI-AML3 cells with low nanomolar concentrations of triptolide in combination with Dox, gemtuzumab ozogamicin, or Ara-C. We observed reduction of XIAP and Mcl-1 protein levels in response to low nanomolar concentrations of triptolide after 48 hours of treatment (Figure 6A). As shown in Figure 6B, Dox, gemtuzumab ozogamicin, and Ara-C had a minimal effect on cell death at the concentrations used. However, cell death induced by Dox and gemtuzumab ozogamicin in combination with triptolide was significantly enhanced. In particular, when used alone, triptolide at 10 nM induced 27.94% ± 3.85% annexin V+ cells, Dox at 40 ng/mL induced 16.8% ± 2.59% annexin V+ cells, and gemtuzumab ozogamicin at 30 ng/mL induced 22.67% ± 0.66% annexin V+ cells. However, when 10 nM triptolide was combined with 40 ng/mL Dox or 30 ng/mL gemtuzumab ozogamicin, the annexin V+ cells increased to 73.83% ± 4.47% and 61.82% ± 1.98%, respectively. The CIs were 0.52 ± 0.10 for triptolide and Dox cotreatment and 0.61 ± 0.01 for triptolide and gemtuzumab ozogamicin cotreatment (ie, highly synergistic). Triptolide did not, however, sensitize OCI-AML3 cells to Ara-C. Cell cycle analysis showed that, after 48 hours of treatment, 40 ng/mL Dox and 30 ng/mL gemtuzumab ozogamicin induced significant G2 block and 0.6 μM Ara-C induced S-phase block, in agreement with findings reported in the literature, while 10 nM triptolide did not significantly affect the cell cycle distribution (Figure 6C). However, when triptolide was combined with Dox or gemtuzumab ozogamicin, the G2 block was disrupted and cell death was greatly enhanced. This and the finding that the triptolide/Ara-C combination had minimal effects on either the S-phase block induced by Ara-C or the enhanced cell death suggest that triptolide disrupts the Dox- and gemtuzumab ozogamicin–induced G2 cell cycle block to promote cell death.
Triptolide-induced cell death is mediated, at least in part, through XIAP down-regulation. (A) OCI-AML3 cells at a density of 0.2 × 106/mL were treated with various concentrations of triptolide for 24 hours in the presence or absence of the caspase inhibitor IDN-1965 (20 μM) or the proteasome inhibitor MG132 (0.2 μM). The XIAP protein level was determined by Western blot. (B) OCI-AML3 cells at a density of 0.2 × 106/mL were treated for 24 hours with various concentrations of triptolide. RNA was isolated, and the XIAP RNA level was determined by TaqMan RT-PCR. (C) U937neo and U937XIAP cells at a density of 0.2 × 106/mL were treated for 24 hours with various concentrations of triptolide. Cell death was determined by annexin V staining with PI.
Triptolide-induced cell death is mediated, at least in part, through XIAP down-regulation. (A) OCI-AML3 cells at a density of 0.2 × 106/mL were treated with various concentrations of triptolide for 24 hours in the presence or absence of the caspase inhibitor IDN-1965 (20 μM) or the proteasome inhibitor MG132 (0.2 μM). The XIAP protein level was determined by Western blot. (B) OCI-AML3 cells at a density of 0.2 × 106/mL were treated for 24 hours with various concentrations of triptolide. RNA was isolated, and the XIAP RNA level was determined by TaqMan RT-PCR. (C) U937neo and U937XIAP cells at a density of 0.2 × 106/mL were treated for 24 hours with various concentrations of triptolide. Cell death was determined by annexin V staining with PI.
Mitochondria- not death receptor–mediated caspase activation is essential for triptolide-induced cell death. (A) MEFs, MEFs deficient in caspase-9 (MEF-caspase-9–/–), Jurkat cells, and Jurkat cells deficient in caspase-8 (JurkatI2.1) were treated with the indicated concentrations of triptolide for 24 hours. Cell death was determined by annexin V staining with PI. (B) Measurement of cytosolic cytochrome C by Western blot of OCI-AML3 cells treated with triptolide for 24 hours. (C) Determination of MMP by CMXRos-MitoTracker Green staining and flow cytometry analysis of OCI-AML3 cells treated with triptolide for 24 hours. Area I represents the cells with intact mitochondria; area II; cells with partial loss of MMP; and area III, complete loss of MMP. (D) OCI-AML3vec and OCI-AML3Bcl-2 cells at a density of 0.2 × 106/mL were treated for 24 hours with various concentrations of triptolide. Cell death was determined by annexin V staining with PI. (E) Mcl-1 levels in OCI-AML3, U937, and HL-60 cells and cells from patients with AML (n = 6) treated with triptolide for 24 hours determined by Western blot.
Mitochondria- not death receptor–mediated caspase activation is essential for triptolide-induced cell death. (A) MEFs, MEFs deficient in caspase-9 (MEF-caspase-9–/–), Jurkat cells, and Jurkat cells deficient in caspase-8 (JurkatI2.1) were treated with the indicated concentrations of triptolide for 24 hours. Cell death was determined by annexin V staining with PI. (B) Measurement of cytosolic cytochrome C by Western blot of OCI-AML3 cells treated with triptolide for 24 hours. (C) Determination of MMP by CMXRos-MitoTracker Green staining and flow cytometry analysis of OCI-AML3 cells treated with triptolide for 24 hours. Area I represents the cells with intact mitochondria; area II; cells with partial loss of MMP; and area III, complete loss of MMP. (D) OCI-AML3vec and OCI-AML3Bcl-2 cells at a density of 0.2 × 106/mL were treated for 24 hours with various concentrations of triptolide. Cell death was determined by annexin V staining with PI. (E) Mcl-1 levels in OCI-AML3, U937, and HL-60 cells and cells from patients with AML (n = 6) treated with triptolide for 24 hours determined by Western blot.
To further investigate the synergistic effects of triptolide plus Dox or triptolide plus gemtuzumab ozogamicin on leukemic cell death, we treated U937, HL-60, and 4 AML patient samples (Table 1) with triptolide in combination with Dox or gemtuzumab ozogamicin. Triptolide plus Dox and triptolide plus gemtuzumab ozogamicin synergistically induced cell death in HL-60 cells with CIs of 0.17 ± 0.05 and 0.25 ± 0.08, respectively. The combination also induced synergistic cell death in U937 cells but to a lesser degree, with CIs of triptolide plus Dox of 0.63 ± 0.057 and triptolide plus gemtuzumab ozogamicin of 0.92 ± 0.003. However, when blasts from patients with AML (n = 4) were treated with triptolide plus Dox or triptolide plus gemtuzumab ozogamicin, no synergistic effect on cell killing was observed (Figure 6D), probably due to the fact that AML blasts have a relatively low proliferative rate and therefore no G2 block was observed.
Discussion
We conclude from our findings that triptolide at low nanomolar concentrations down-regulates XIAP and Mcl-1, potently inhibits cell growth, and promotes cell death through the mitochondrial pathway in various leukemic cell lines. It also induced significant cell death in leukemic blasts isolated from the bone marrow and peripheral blood of patients with AML. The responses do not seem to depend on cytogenetics or previous treatments and responses. Additional data may identify patient groups with increased/decreased sensitivity to triptolide, but the data presented here do not identify such groups. Although triptolide decreased XIAP and Mcl-1 levels, and induced activation of caspase-3 and cell death in all leukemic cells tested, some cell lines are less sensitive than others. The reason for this is not clear at this point. However, it seems that the faster-growing cells are more responsive than the slower-growing cells.
Triptolide-induced cell death in AML patient samples was observed in both short-term in vitro cultures, as shown by annexin V staining, and long-term cultures, as shown by colony-forming assays. After 24 hours of treatment, blasts from patients with AML were much more sensitive to triptolide than were CD34+ cells from normal bone marrow (Figure 2A). However, triptolide indistinguishably decreased clonogenic survival in AML blasts and normal bone marrow samples. Nonetheless, even though it is preferable for therapeutic agents to eliminate or reduce the colony formation of leukemic blasts and spare that of normal bone marrow progenitor cells, this cannot be the single determinant of the potential clinical applicability of a drug. In fact, Ara-C, the most commonly used chemotherapeutic agent in the treatment of AML, also shows little or no selectivity for malignant cells over normal cells in colony-forming assays.35 It was reported that PG490-88, a water-soluble derivative of triptolide, at 0.25 mg/kg markedly decreased tumor growth and at 0.5- and 0.75-mg/kg doses caused profound tumor regression without apparent toxicities in nude mouse human tumor xenograft models.36 A decrease in white blood cells was reported in clinical trials with triptolide or extracts of Tripterygium wilfordii.37,38 A phase 1 clinical trial with a water-soluble derivative of triptolide on solid tumors is presently ongoing in Europe.
In terms of its mechanism, triptolide was shown here to effectively inhibit XIAP expression at both the protein and RNA levels (Figure 4A and 4B, respectively). In addition, we found that the decrease in XIAP protein levels caused by triptolide was mediated through both caspase degradation of XIAP protein and transcriptional inhibition of XIAP mRNA, but not through proteasome degradation (Figure 4A-B). NFκB39-41 and both the phosphatidylinositol-3 kinase (PI3K) and the mitogen-activated protein kinase kinase/extracellular-signal regulated kinase (MEK/ERK) pathways24,42,43 have been shown to regulate XIAP expression. Since triptolide has been shown to inhibit NFκB,13,14 this finding suggests that the transcriptional down-regulation of XIAP by triptolide is mediated, at least in part, through NFκB inhibition. We did not, however, detect any changes in AKT and ERK phosphorylation up to 4 hours in OCI-AML3 and Jurkat cells treated with triptolide (data not shown). The notion that triptolide induces cell death through XIAP inhibition is further supported by the finding that forced XIAP overexpression attenuated triptolide-induced cell death (Figure 4C).
Effect of triptolide in combination with Dox, gemtuzumab ozogamicin, or Ara-C on cell death in AML cells. OCI-AML3 cells (0.2 × 106/mL) were treated with low concentrations of triptolide, Dox, gemtuzumab ozogamicin, Ara-C, or their combinations, as indicated, for 48 hours. (A) XIAP and Mcl-1 protein levels in OCI-AML3 cells treated with low concentrations of triptolide at 48 hours. (B) Cell viability determined by annexin V staining in OCI-AML3 cells treated with triptolide, Dox, gemtuzumab ozogamicin, Ara-C, or the combinations for 48 hours. (C) Cell cycle distribution of OCI-AML3 cells treated with triptolide, Dox, gemtuzumab ozogamicin, Ara-C, or their combinations at 48 hours, as shown by PI staining. (D) Blasts (1 × 106/mL) from patients with AML (n = 4) were treated with low concentrations of triptolide, Dox, gemtuzumab ozogamicin, or their combinations for 48 hours. Cell viability was determined by annexin V staining.
Effect of triptolide in combination with Dox, gemtuzumab ozogamicin, or Ara-C on cell death in AML cells. OCI-AML3 cells (0.2 × 106/mL) were treated with low concentrations of triptolide, Dox, gemtuzumab ozogamicin, Ara-C, or their combinations, as indicated, for 48 hours. (A) XIAP and Mcl-1 protein levels in OCI-AML3 cells treated with low concentrations of triptolide at 48 hours. (B) Cell viability determined by annexin V staining in OCI-AML3 cells treated with triptolide, Dox, gemtuzumab ozogamicin, Ara-C, or the combinations for 48 hours. (C) Cell cycle distribution of OCI-AML3 cells treated with triptolide, Dox, gemtuzumab ozogamicin, Ara-C, or their combinations at 48 hours, as shown by PI staining. (D) Blasts (1 × 106/mL) from patients with AML (n = 4) were treated with low concentrations of triptolide, Dox, gemtuzumab ozogamicin, or their combinations for 48 hours. Cell viability was determined by annexin V staining.
The inhibition of caspases by the broad-spectrum caspase inhibitor IDN-1965 blocked cell death induced by triptolide, suggesting that it was caspase-dependent. On the other hand, MEFs deficient in caspase-9 were resistant to triptolide, indicating that mitochondrial pathway–mediated caspase-9 activation leading to effector caspase-3 cleavage is critical to triptolide's action. This was further supported by the finding that triptolide decreased Mcl-1 levels, enhanced cytochrome C release, and induced changes in MMP and that overexpression of Bcl-2 suppressed the cell death induced by triptolide. Mcl-1 is a member of the antiapoptotic Bcl-2 family of proteins that inhibits cell death at the mitochondrial level, and XIAP is a potent caspase-9 and caspase-3 inhibitor that blocks cell death, both upstream and downstream of the apoptotic cell death cascade. Since Mcl-1 and XIAP are frequently overexpressed in leukemias,22,44,45 the ability of triptolide to reduce the levels of both Mcl-1 and XIAP makes it a powerful inducer of apoptosis.
Even though triptolide was very effective as a single agent in killing various leukemic cell lines and blasts from patients with AML, its benefits would be even greater if it could enhance the efficacy of various drugs currently used clinically in treating hematologic malignancies or solid tumors. In this regard, others and we have shown that triptolide is a potent XIAP inhibitor. Here, we demonstrate for the first time that triptolide also decreased Mcl-1 protein level. By decreasing both XIAP, an IAP family protein, and Mcl-1, an antiapoptotic Bcl-2 family protein, triptolide can lower the apoptotic threshold and thereby enhance cell death induced by chemotherapeutic agents. However, the combination of triptolide and Ara-C in OCI-AML3 cells did not increase cell death over that induced by Ara-C alone. This may be due to the fact that since OCI-AML3 cells are relatively resistant to Ara-C, the decrease in XIAP and Mcl-1 induced by triptolide at low nanomolar concentration (≤ 10 nM) did not lower the apoptotic threshold enough to overcome cellular resistance to Ara-C. Starting at a concentration of 15 nM, triptolide by itself can induce cell death in OCI-AML3 cells after 48 hours. Nevertheless, we observed sensitization by triptolide of Ara-C–induced cell death in 2 samples from Ara-C–resistant patients with AML. Triptolide at low concentrations was found to synergistically induce cell death in cell lines but not in AML blasts when combined with Dox or gemtuzumab ozogamicin. The mechanism of this effect is not clear. It may be important to point out that triptolide also decreased levels of survivin and cdc2 (not shown). Survivin is an IAP that plays important roles in both cell proliferation and cell death,46,47 and its expression is cell-cycle dependent, with the highest level in G2M.48 Cdc2, a cyclin B1–dependent kinase (Cdk1), is essential for the progression from the G2 phase to mitosis.49,50 Studies have shown that survivin is phosphorylated by cdc2–cyclin B1. Therefore, the inhibition of cyclin-dependent kinase decreases survivin levels and induces cell death.51-53 Triptolide's inhibition of cdc2, resulting in a decrease in survivin, could, at least in part, explain the synergistic effects on cell survival of triptolide in combination with G2 blockers such as Dox and gemtuzumab ozogamicin. The lack of synergistic effects of triptolide and Dox or gemtuzumab ozogamicin on primary AML blasts is probably due to the low proliferative rate of primary leukemic cells in vitro. Therefore, when testing the efficacy of therapeutic agents in vitro, we need to consider that leukemic cells may respond differently to treatment outside their bone marrow microenvironment.54
The potent effects of triptolide in leukemic cells reported in this study warrant further investigations of this compound for the treatment of leukemia. As noted, a phase 1 clinical trial of a water-soluble derivative of the drug is ongoing.
Prepublished online as Blood First Edition Paper, March 23, 2006; DOI 10.1182/blood-2005-09-3898.
Supported in part by grants from the National Institutes of Health (PO1 CA49639, PO1 CA55164, and CA16672) to M.A.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Rosemarie Lauzon for assistance in the preparation of the paper, Dr Christian Bailly from Pierre-Fabre for valuable comments, and Wenjing Chen for help in collecting patient information.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal