The Hoxa9 and Meis1 genes represent important oncogenic collaborators activated in a significant proportion of human leukemias with genetic alterations in the MLL gene. In this study, we show that the transforming property of Meis1 is modulated by 3 conserved domains, namely the Pbx interaction motif (PIM), the homeodomain, and the C-terminal region recently described to possess transactivating properties. Meis1 and Pbx1 interaction domain-swapping mutants are dysfunctional separately, but restore the full oncogenic activity of Meis1 when cotransduced in primary cells engineered to overexpress Hoxa9, thus implying a modular nature for PIM in Meis1-accelerated transformation. Moreover, we show that the transactivating domain of VP16 can restore, and even enhance, the oncogenic potential of the Meis1 mutant lacking the C-terminal 49 amino acids. In contrast to Meis1, the fusion VP16-Meis1 is spontaneously oncogenic, and all leukemias harbor genetic activation of endogenous Hoxa9 and/or Hoxa7, suggesting that Hoxa gene activation represents a key event required for the oncogenic activity of VP16-Meis1.
Introduction
To date, numerous studies have implicated Hox genes in human and murine leukemias. These include (1) the development of leukemias in mice that received a transplant of bone marrow cells engineered to overexpress retrovirus-transduced HOXB3,1 HOXB6,2 HOXB8,3 HOXA9,4 or HOXA105 ; (2) the rearrangements between the NUP98 gene (on chromosome 11p15) and several members of the Hox gene family, including HOXA9,6,7 HOXD13,8 HOXA13 and HOXA11,9 and HOXC11 and HOXC13,10 which generate fusion genes (eg, NUP98-HOXA9) that characterize myeloid leukemias in human and mice; (3) the overexpression of HOXA7 and HOXA9 in several MLL-induced human leukemias11,12 ; (4) the distinctly high correlation between high levels of HOXA9 expression and poor prognosis in human acute myeloid leukemia (AML)13 ; and (5) coexpression of BCR-ABL and NUP98-HOXA9 in a significant number of human leukemic specimens,14 and a recent proof of their genetic interaction.15 Together, these studies establish a direct and indirect role for multiple Hox genes in human leukemias, and highlight the importance of revealing the molecular bases of Hox-induced transformation.
Some insight into the molecular mechanisms of Hox-induced transformation may be gained from studies of Hox cofactors, members of the TALE (for 3-amino-acid loop extension) family of homeodomain proteins. Of interest, founders of 2 subgroups within this family, namely PBX116,17 and Meis1,18 were identified based on their participation in human and mouse leukemia, respectively.
Hox, Pbx, and Meis participate in a multiprotein interaction network. The cooperative interaction between Pbx and Hox proteins enhances the DNA binding affinity and specificity of Hox proteins19 and is essential for at least some of the Hox-dependent developmental programs.20-22 In contrast, a functional role for a dimeric Hox-Meis complex has so far not been established.23 Members of the Meis family can form complexes with Pbx in DNA-dependent and independent manners.24-26 Interaction with Meis induces nuclear localization of Pbx by preventing its nuclear export27,28 and promoting nuclear import.29,30 Indirect interaction between Hox and Meis proteins was established by the identification of Hox-Pbx-Meis heterotrimeric complexes.26
PBX1 is involved as part of the fusion E2a-PBX1 oncoprotein in a high proportion of human pre-B leukemias.16,17 We recently generated a mouse model of E2a-PBX1–induced pre–B-cell leukemia, and showed that the Hoxa locus was targeted by murine Moloney leukemia virus (MMLV) in the majority of the leukemias analyzed.31 These leukemias were characterized by aberrant expression of Hoxa genes, and all expressed high levels of Hoxa7, thus pointing to a genetic interaction between E2a-PBX1 and Hoxa genes. In support of these findings, we have previously reported a strong genetic interaction between Hoxa9 and E2a-PBX1 in mouse AML.32
Meis1 is frequently activated by insertion of endogenous provirus in BXH2 mouse model of AML18 and appears to represent a key target gene to some MLL fusion proteins.11,33,34 In AML, Meis1 genetically interacts with HOXB3,1 HOXA7, HOXA9,4,7,18,35,36 and HOXB4 (N.M. and G.S., unpublished observations, February 2002; and Pineault et al37 ). In contrast to Meis1, the highly related Meis family member PREP1 fails to accelerate Hoxa9-induced leukemia.1 PREP1 and Meis1 share similarities in their domains required for interaction with Pbx (the Pbx-interacting motif [PIM]) and have similar homeodomains (HDs), whereas their sequences between the PIM and C-terminus diverged considerably. This divergent region may be dispensable for the oncogenic function of Meis1.38 The distinct C-terminal domain of Meis1, which is absent in PREP1, appears to be critical for transcriptional activity39 and transformation (Wang et al38 and this paper).
Experiments performed with Drosophila support the model predicting a transactivating role for Meis1. Fly gene HTH (Meis1 ortholog), fused to the repression domain of Engrailed, phenocopied loss of HTH function, such as the appearance of ectopic eyes in the ventral head region, antenna-to-leg transformation, fusion of proximal leg segments, and deformations in proximal wing structures.40,41 Conversely, fusion of the transactivating domain of VP16 to HTH generated gain-of-function phenotypes (loss of eyes, loss of aristae, and abnormal distal leg development40,41 ). Together, these studies highlighted a possible inherent transactivating function of Meis1 that, at least in part, maps to the C-terminal region of the protein.39
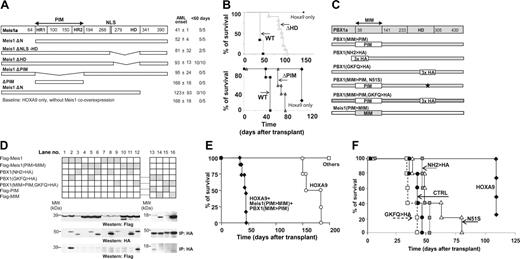
Identification of Meis1 domains implicated in acceleration of HOXA9-induced leukemia. (A) Schematic representation of Meis1 mutants (left), and average onset of AML in recipients of bone marrow cells transduced with the indicated mutants, and HOXA9 (right). (B, top) Survival curves of mice that received a transplant of HOXA9-transduced, HOXA9 plus Meis1–transduced, or HOXA9 plus Meis1ΔHD–transduced bone marrow cells. (Bottom) Survival curves of mice that received a transplant of HOXA9-transduced, HOXA9 plus Meis1–transduced, or HOXA9 plus Meis1ΔPIM–transduced bone marrow cells. (C) Schematic representation of Meis1a and PBX1 domain-swapping mutants. (D) In vitro analysis of interactions between the domain-swapping mutants. (Top blots) Western blot analysis of FLAG-tagged Meis1 and Meis1(PIM>MIM) expression in lysates of 293 cells transfected with vectors expressing the indicated Meis1 and PBX1 mutants. Lysates of cells transfected with vectors indicated at the left of the panel were subjected to immunoprecipitation with anti-HA antibodies. The amount of HA-tagged PBX1 mutants was determined by Western blot analysis with HA antibody (middle blots), and interaction of these proteins with Meis1 was determined using anti-FLAG antibody (bottom blots). (E) Survival curves of mice that received a transplant of HOXA9 or of HOXA9 plus Meis(PIM>MIM) plus PBX1(MIM>PIM) cells. Mice that received cells expressing HOXA9 plus individual swapping mutant (others) survived past the time when majority of HOXA9 recipients succumbed to AML. (F) Survival curves of mice that received a transplant of triply transduced bone marrow cells. □ indicates HOXA9 plus FLAG-Meis1(PIM>MIM) plus PBX1(MIM>PIM, GKFQ>HA); •, HOXA9 plus Meis1(PIM>MIM) plus PBX1(MIM>PIM); ▦, HOXA9 plus FLAG-Meis1(PIM>MIM) plus PBX1(MIM>PIM, NH2>HA); and ▵, HOXA9 plus Meis1(PIM>MIM) plus PBX1(MIM>PIM, N51S).
Identification of Meis1 domains implicated in acceleration of HOXA9-induced leukemia. (A) Schematic representation of Meis1 mutants (left), and average onset of AML in recipients of bone marrow cells transduced with the indicated mutants, and HOXA9 (right). (B, top) Survival curves of mice that received a transplant of HOXA9-transduced, HOXA9 plus Meis1–transduced, or HOXA9 plus Meis1ΔHD–transduced bone marrow cells. (Bottom) Survival curves of mice that received a transplant of HOXA9-transduced, HOXA9 plus Meis1–transduced, or HOXA9 plus Meis1ΔPIM–transduced bone marrow cells. (C) Schematic representation of Meis1a and PBX1 domain-swapping mutants. (D) In vitro analysis of interactions between the domain-swapping mutants. (Top blots) Western blot analysis of FLAG-tagged Meis1 and Meis1(PIM>MIM) expression in lysates of 293 cells transfected with vectors expressing the indicated Meis1 and PBX1 mutants. Lysates of cells transfected with vectors indicated at the left of the panel were subjected to immunoprecipitation with anti-HA antibodies. The amount of HA-tagged PBX1 mutants was determined by Western blot analysis with HA antibody (middle blots), and interaction of these proteins with Meis1 was determined using anti-FLAG antibody (bottom blots). (E) Survival curves of mice that received a transplant of HOXA9 or of HOXA9 plus Meis(PIM>MIM) plus PBX1(MIM>PIM) cells. Mice that received cells expressing HOXA9 plus individual swapping mutant (others) survived past the time when majority of HOXA9 recipients succumbed to AML. (F) Survival curves of mice that received a transplant of triply transduced bone marrow cells. □ indicates HOXA9 plus FLAG-Meis1(PIM>MIM) plus PBX1(MIM>PIM, GKFQ>HA); •, HOXA9 plus Meis1(PIM>MIM) plus PBX1(MIM>PIM); ▦, HOXA9 plus FLAG-Meis1(PIM>MIM) plus PBX1(MIM>PIM, NH2>HA); and ▵, HOXA9 plus Meis1(PIM>MIM) plus PBX1(MIM>PIM, N51S).
Using a strategy similar to that described for the Drosophila HTH protein, and in vivo–based leukemia assays, we now provide genetic evidence linking the oncogenic activity of Meis1 to its C-terminal transactivating domain, and show that this function could be complemented in cis by the addition of VP16. We also exploit the generation of dysfunctional Meis1-Pbx1 swapping mutants to document the nucleating function of PIM in Meis1 oncogenic activity. Finally, we demonstrate that in contrast to Meis1, the fusion VP16-Meis1 is spontaneously oncogenic, and all leukemias harbor genetic activation of endogenous Hoxa9 and/or Hoxa7, thus revealing a key function of Hoxa gene activation for the oncogenic activity of VP16-Meis1.
Materials and methods
cDNA constructs and retroviral vectors
cDNAs encoding Meis1a lacking amino acids (aa's) 2 to 63 (Meis1ΔN, Figure 1), aa's 70 to 97 (Meis1ΔHR1, Figure 1A), aa's 107 to 187 (Meis1ΔHR2, Figure 1A), aa's 65 to 193 (Meis1ΔPIM, Figure 1A), aa's 295 to 334 (Meis1ΔHD, Figure 1A), or aa's 270 to 336 (Meis1ΔNLS-HD, Figure 1A), and the swapping mutants Meis(PIM>MIM) and Pbx1(MIM>PIM) were generated by polymerase chain reaction (PCR) amplifications and verified by sequencing. The PBX1(N51S) and PBX1(GKFQ > HA) mutants, and cDNAs encoding Meis1a lacking the N-terminal 56 aa (Meis1Δ334), VP16-Meis1a, or VP16-MeisΔ334 (Figure 2A) were previously described.25,42 The wild-type and the mutant Meis1 cDNAs were introduced into the murine stem cell virus (MSCV)–based vector that encodes green fluorescent protein marker gene (MSCV-GFP), the PBX1 mutants in MSCV-based vector encoding red fluorescent protein (MSCV-RFP), and Hoxa9 in MSCV-PGK-neor retroviral vector as previously described.4,43
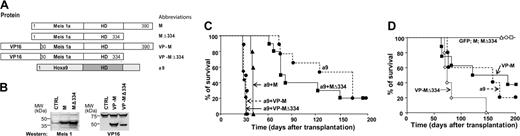
VP16-Meis is independently oncogenic. (A) Schematic representation of Meis1 mutants. (B) Western blot analysis of MEIS1 levels in the transduced GP + E cells. MEIS1 antibody recognizes N-terminal 30 amino acids of MEIS1 (left). VP16 fusion proteins were detected using anti-VP16 antibody (right panel). Nonspecific bands at approximately 70 kDa and approximately 75 kDa on the left and the right panel, respectively, serve as loading controls. (C) Survival curves of mice that received a transplant of HOXA9, HOXA9 plus Meis1, HOXA9 plus Meis1Δ334, HOXA9 plus VP16-Meis1, or HOXA9 plus VP16-Meis1Δ334 cells. (D) Survival curves of mice that received a transplant of HOXA9, VP16-Meis1, or VP16-Meis1Δ334 cells. Note that control HOXA9 mice are the same as in Figure 1C.
VP16-Meis is independently oncogenic. (A) Schematic representation of Meis1 mutants. (B) Western blot analysis of MEIS1 levels in the transduced GP + E cells. MEIS1 antibody recognizes N-terminal 30 amino acids of MEIS1 (left). VP16 fusion proteins were detected using anti-VP16 antibody (right panel). Nonspecific bands at approximately 70 kDa and approximately 75 kDa on the left and the right panel, respectively, serve as loading controls. (C) Survival curves of mice that received a transplant of HOXA9, HOXA9 plus Meis1, HOXA9 plus Meis1Δ334, HOXA9 plus VP16-Meis1, or HOXA9 plus VP16-Meis1Δ334 cells. (D) Survival curves of mice that received a transplant of HOXA9, VP16-Meis1, or VP16-Meis1Δ334 cells. Note that control HOXA9 mice are the same as in Figure 1C.
Animals
Ly5.1+ bone marrow and fetal liver donors were (C57BL/6Ly-Pep3b × C3H/HeJ)F1 or (C57Bl6/Pep3b), and recipients were Ly5.2+(C57BL/6J × C3H/HeJ)F1 or (C57Bl/6J) mice. Fetal liver cells were isolated at 14.5 dpc. Animals were housed and handled in accordance with the guidelines of the animal care and use committee at the IRIC.
Retroviral infection and transplantation of primary hematopoietic cells
Generation of recombinant ecotropic retrovirus-producing GP + E86 cells and infections of primary hematopoietic cells were performed as described.43,44 Recipients were irradiated (850 cGy, 160 cGy/min, 137Cs
Immunoprecipitation and Western blot analyses
Total cell lysis, immunoprecipitation, and Western blot analyses were performed as described.45,46 Primary antibodies used were anti-HA (Boehringer Mannheim, Mannheim, Germany; Roche Molecular Biochemicals, Indianapolis, IN), anti-FLAG (Stratagene, La Jolla, CA), anti-Meis1a(NT),25 and anti-VP16 (BD Bioscience, San Jose, CA; Pharmingen, San Diego, CA). Secondary antirabbit and antimouse horseradish peroxidase–conjugated antibodies were from Santa Cruz Biotech (Santa Cruz, CA).
Quantitative reverse transcription–polymerase chain reaction (RT-PCR)
Quantitative PCR (Q-PCR) was carried out using TaqMan probe-based chemistry (Applera, Foster City, CA) as described.31 Oligonucleotides for murine Hox and TALE genes were designed against nucleotide sequences deposited in murine genome databases (GenBank,47 RefSeq,48 and EMBL49 ) using Primer Express (Applera). Reactions, analysis, and validation of the amplicons were carried out as previously described.50
Inverse PCR and identification of retroviral insertion sites
DNA isolated from leukemic bone marrow was digested with XhoI. Two consecutive rounds of PCR reactions were performed using Klentaq LA-16 (AB Peptides, St Louis, MO) as described.31,51 The second round of amplification was done using nested set of primers, and the melting temperature was increased to 65°C. Primers used for first amplification were as follows: 5′-AGGGCATCGACTTCAAGGAGGACGGCAACATC-3′ and 5′-CTCGCCCTCGCCGGACACGCTGAACTTGTG-3′; and the nested pair was as follows: 5′-TCCGCCCTGAGCAAAGACCCCAACGAGAAG-3′ and 5′-GCCGCCTACAGGTGGATGTGGAATGTGTG-3′.
The amplified fragments were then subcloned in pBluescript KS+ and sequenced at the Genomic Platform of IRIC. Retroviral insertion sites were identified using BLAT alignment tool from UCSC Genome Browser v120 (University of California, Santa Cruz).
Morphologic classification of leukemias
Morphologic features of leukemic cell populations were examined under 100-fold magnification of Wright stain–dyed cytospin or touch preparations of bone marrow, spleen, liver, lung, and kidney of diseased mice. Images were acquired using a Leica DMIRB microscope with an HCXPL FluotarL 40 ×/0.6 numeric aperture objective (Leica, Wetzlar, Germany) and a Retiga EKI camera (Q-Imaging, Burnaby, BC, Canada). Images were transformed directly into TIFF files using Adobe Photoshop version 6.0 (Adobe Systems Canada, Ottawa, ON, Canada). The first grouping of leukemias was based on the proportions of nondifferentiated blasts: group I, more than 80% nondifferentiated myeloid blasts; group II, less than 80% nondifferentiated blasts. Each group was then subdivided into subgroups with distinct morphologic characteristics: group 1: A, 2 distinct blast populations; B, myeloblasts with fine granules; and C, myeloblasts with coarse granules; and group II: A, 20% or more monocytes and differentiated myeloid cells; B, 20% or more differentiated myeloid cells and less than 20% monocytes.
Results
A modular nature of the PBX-interacting motif of Meis1 in leukemic transformation
To identify domains of Meis1 that are required for its leukemogenic potential, we created several deletion mutants spanning the conserved motifs (Figure 1A) and analyzed their capacity to accelerate Hoxa9-induced leukemia in transplantation assays. The first series of experiments exploited high proliferation potential of fetal liver–derived cells that enabled reconstitutions of recipients with inocula comprising only 1000 to 1500 Hoxa9 + Meis1–transduced myeloid progenitors. Results from these studies suggested a critical function for the homeodomain (Figure 1B top panel) and the C-terminal region of Meis1 (Figure 1B bottom panel), and indicated that the Pbx-interacting motif (PIM) of Meis1 contributes significantly to its oncogenic function. Although our observations largely confirmed findings reported by Pineault et al52 and Wang et al,38 they also suggested that both mutants retained some oncogenic activity (Figure 1B).
The Meis1-Pbx interaction is mediated through the PIM domain of Meis1 and the MIM (Meis1-interaction-motif) region of Pbx proteins (Figure 1C). To further examine the role of PIM in the oncogenic activity of Meis1, several PIM and MIM domain-swapping mutants of Meis1a and Pbx1A were generated (Figure 1C). These mutants have lost the capacity to interact with their usual partner, that is, Meis1(PIM>MIM) does not interact with PBX1, and PBX1(MIM>PIM) does not interact with Meis1 (Figure 1D). The coimmunoprecipitation of Meis1(PIM>MIM) with PBX1(MIM>PIM) demonstrated, however, that the swapped PIM and MIM domains still mediated interaction between the mutants (Figure 1D lane 12). Meis1(PIM>MIM), moreover, retained a certain capacity for nuclear translocation (Figure S1 [row 5], available on the Blood website; see the Supplemental Materials link at the top of the online article) and appeared to enhance nuclear import of PBX(MIM>PIM) (Figure S1 row 7) in a subpopulation of examined cells.
Fetal liver cells were first transduced with Hoxa9 alone, or in combination with Meis1(PIM>MIM), with or without PBX1(MIM>PIM), or with a combination of both genes. Meis1(PIM>MIM)– and PBX1(MIM>PIM)–transduced cells did not induce leukemia in the absence of Hoxa9 (Figure 1E), and could not accelerate the onset of Hoxa9-induced AML, as all mice from these 2 groups survived beyond the time at which recipients of Hoxa9-transduced cells died of acute myeloid leukemia (AML) (Figure 1E). Of importance, co-overexpression of both Meis1(PIM>MIM) and PBX1(MIM>PIM) together with Hoxa9 resulted in a rapid onset of AML in all mice that underwent transplantation (Figure 1E). Southern blot analyses of genomic DNA extracted from multiple tissues of several leukemic animals showed that leukemias were monoclonal or oligoclonal, and that all 3 recombinant proviruses were present and intact (data not shown).
This triple transduction/transplantation model enabled additional analyses of structural requirements for Pbx1 in Meis1/HOXA9 leukemic collaboration. First, we examined whether in this context the DNA-binding capacity of Pbx1 is required for reconstitution of the transforming capacity of Meis1(PIM>MIM). Specifically, the N51S homeodomain (HD) mutation affects a critical residue that directly contacts DNA,25,53 while in the GKFQ > HA mutant, a 13-aa hemmaglutinin tag inserted immediately C-terminal to the Pbx1 HD (aa's 296-308) prevents the Pbx-DNA interactions.42 None of the DNA-binding mutations markedly affected the capacity of Pbx1(MIM>PIM) to rescue the transforming potential of Meis1(PIM>MIM) (Figure 1F), suggesting that the DNA-binding activity of PBX1(MIM>PIM) mutants is not required for leukemia induction. Together, our observations suggest that while Meis1(PIM>MIM) might enable the nuclear translocation of PBX1(MIM>PIM), the PIM domain, presented within a heterologous context (ie, the Pbx protein), likely interacts with essential proteins involved in Hoxa9/Meis-induced leukemia.
VP16 rescues the oncogenic potential of Meis1Δ334
The C-terminal domain of Meis1, necessary for the acceleration of Hoxa9-induced leukemia (Figure 1A), is also critical for its transcriptional activation function.39 To verify correlation between the transforming and transactivating functions of Meis1, we examined the leukemogenic potential of chimeric proteins comprising wild-type Meis1, or Meis1 lacking the C-terminal domain (Meis1Δ334), fused to the heterologous Herpes simplex–derived VP16-transactivating domain (Figure 2A). Cells transduced with each construct produced proteins of appropriate size well in excess of the endogenous protein (Figure 2B).
We first investigated the capacity of Meis1 chimeric proteins to accelerate Hoxa9-induced leukemia. Initial experiments showed that the source (ie, bone marrow vs fetal liver) of primary cells did not affect the onset or the phenotype of the myeloid leukemias that developed in these mice, therefore only leukemias in recipients of transduced bone marrow cells were further analyzed.
In agreement with our previously reported studies,1,4 recipients of Hoxa9 + Meis1–transduced cells developed AML within 4 to 5 weeks after transplantation (Figure 2C), and similar latency characterized recipients of Hoxa9 + VP16-Meis1–transduced cells. We next examined the ability of the C-terminal deletion mutant (Meis1Δ334) to collaborate with Hoxa9 in leukemia acceleration. The leukemia onset in this group was noticeably delayed compared with recipients of Hoxa9 + Meis1–transduced cells (Figure 2C), and was similar to that seen in mice that received a transplant of only Hoxa9-transduced cells (Figure 2C). We next tested whether exogenous, VP16-derived transactivating domain could rescue the oncogenic function of Meis1Δ334, and found that recipients of Hoxa9 + VP16-Meis1Δ334–transduced cells died of leukemia with latency comparable with that of Hoxa9 + VP16-Meis1 or Hoxa9 + Meis1 mice (Figure 2C).
Taken together, these data confirm the important role of the C-terminal domain of Meis1 in accelerating Hoxa9-induced AML, and demonstrate that the heterologous VP16 transactivation domain could restore the oncogenic function of Meis1Δ334.
VP16-Meis1 is independently oncogenic
Although Meis1 accelerates Hox-induced leukemia, its overexpression alone fails to induce leukemic transformation.1,4 A series of experiments was conducted to determine whether VP16-Meis1 fusion proteins gained the capacity to induce leukemia. Of interest, in recipients of VP16-Meis1 or VP16-Meis1Δ334–transduced cells, AML development was observed within the same time frame as in recipients of Hoxa9-transduced cells, that is, within 4 to 6 months after transplantation (Figure 2D). Variation in cell doses probably explains the apparently shorter leukemia latency determined for VP16-Meis1Δ334 recipients, since no such differences were found in experiments where transplantation inocula comprised similar numbers of transduced cells (data not shown).
Taken together, these data demonstrate that the heterologous VP16 transactivation domain dramatically enhanced the “oncogenic potency” of Meis1, which became as efficient as Hoxa9 in leukemia induction, and rescued the ability of the C-terminal Meis1Δ334 mutant to accelerate Hoxa9-induced leukemia.
Variations in leukemia phenotypes are not determined by Meis1 mutants
We previously reported that Meis1 accelerates the occurrence of Hox-induced myeloid leukemia, and that leukemia phenotype mainly depended on the Hox gene that initiated disease. For example, Hoxa9-induced leukemias were characterized by much higher blast content than leukemias caused by Hoxb3 overexpression.1 To determine if the newly generated VP16-Meis mutants affected the phenotype of the leukemia they induced, independently or in collaboration with Hoxa9, expression of cell surface markers was analyzed on a large proportion of these leukemias (n ≥ 30; data not shown). Except for one specimen, all leukemic cells expressed moderate to high levels of the myeloid marker Mac-1 (mean ± SD: 82% ± 25%), and a wide range of c-Kit levels (from 4% to 92%), while none expressed the granulocyte marker Gr-1, or noticeable levels (≥ 10%) of the B-cell marker CD19, or the stem cell marker Sca1, suggesting that the majority of leukemic populations comprised solely immature myeloid cells.
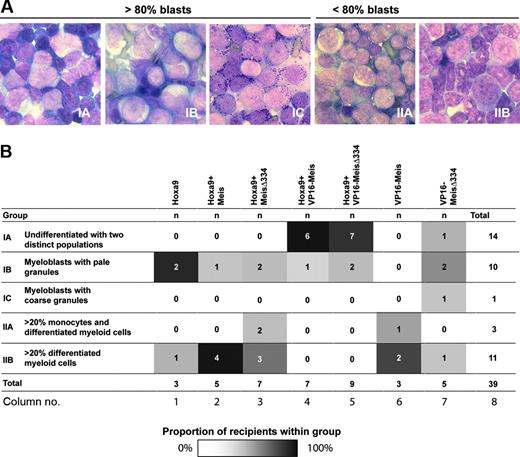
A complementary assessment of these leukemias was performed by morphologic evaluation of bone marrow preparations, which was then used to categorize leukemias into 5 subgroups based on blast content (≥ 80 vs < 80% for group I vs II, Figure 3A), and evidence of differentiation (granules, evidence of granulocytic or monocytic differentiation, etc; Figure 3A-B). Correlation analysis failed to reveal any strong link between the transduced oncogenes (eg, VP16-Meis1) and morphologic characteristics of the resulting leukemias, except for the clustering of Hoxa9 + VP16-Meis1 and Hoxa9+VP16-MeisΔ334 leukemias in group 1A (Figure 3B columns 4-5). Leukemias generated by VP16-Meis1 or VP16-Meis1Δ334 (Figure 3B columns 6-7) were scattered through all 5 groups, indicating that the VP16-Meis fusions alone had no deterministic role in the phenotype of the myeloid leukemias generated in this in vivo model.
VP16-Meis leukemias express high levels of Hoxa genes
We surveyed the expression of all Hox and TALE genes in 26 leukemias spanning all genotypes and morphologic groups listed in Figure 3B. The results of this comprehensive analysis are shown in Figure 4.
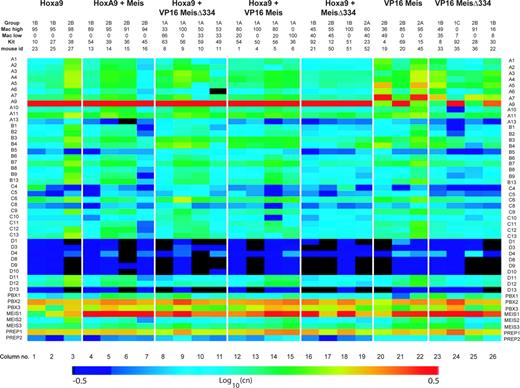
The magnitude of Hoxa9 overexpression driven by the MSCV retrovirus established the upper limit of values to which levels of Hox expression in leukemias with other genotypes could be compared. Levels of Hoxa9 were between 2 × 106-7 copies per 25 ng RNA (mean ± SD: 6 ± 4 × 106) in all leukemias derived from Hoxa9-transduced cells (Figure 4 columns 1-19), whereas levels as low as 224 or 312 copies were observed in leukemias that developed in the absence of engineered Hoxa9 overexpression (Figure 4, columns 20, 22, 23, 25). Of interest, 3 leukemias with the VP16-Meis1 or VP16-Meis1Δ334 genotype (Figure 4, columns 21, 24, and 26) also expressed very high levels of Hoxa9. For example, leukemia no. 22 (column 26) with 1B phenotype expressed 3 × 104 copies of Hoxa9 per 25 ng RNA, whereas leukemia no. 36 (column 25), with the same genotype and phenotype, barely expressed this gene. Five (nos. 19, 45, 33, 36, and 22) of 7 VP16-Meis1 or VP16-Meis1Δ334 leukemias expressed very high levels of Hoxa7 (∼ 0.4-7 × 104 copies per 25 ng RNA), which was otherwise poorly expressed. Within the cohort of VP16-Meis1 or VP16-Meis1Δ334 leukemias (Figure 4 columns 20-26), there is a clear tendency for aberrant activation of either Hoxa7 or Hoxa9, but not both together. These results suggest that VP16-Meis1 and VP16-Meis1Δ334 require activation of selected Hoxa genes, such as Hoxa7 or Hoxa9, for leukemia development.
Morphologic characterization of leukemias. (A) Representative samples of Wright-stained bone marrow preparations, × 100 magnification. Group I, more than 80% blasts: IA, 2 distinct blast populations; IB, myeloblasts with pale granules; IC, myeloblasts with coarse granules. Group II, less than 80% blasts: IIA, 20% or more monocytes and differentiated myeloid cells; IIB, 20% or more differentiated myeloid cells and less than 20% monocytes. (B) Distribution of leukemias within 5 subgroups defined by index cases presented in panel A. Genotypes of leukemias are listed at the top, subgroup number and its major characteristics are listed to the left, and column numbers are listed at the bottom. Different intensities of shading within the subgroups defined by genotype listed on top of table illustrate proportions of mice within each group with the particular characteristics. White cells indicate 0% of recipients; black cells, 100% of recipients.
Morphologic characterization of leukemias. (A) Representative samples of Wright-stained bone marrow preparations, × 100 magnification. Group I, more than 80% blasts: IA, 2 distinct blast populations; IB, myeloblasts with pale granules; IC, myeloblasts with coarse granules. Group II, less than 80% blasts: IIA, 20% or more monocytes and differentiated myeloid cells; IIB, 20% or more differentiated myeloid cells and less than 20% monocytes. (B) Distribution of leukemias within 5 subgroups defined by index cases presented in panel A. Genotypes of leukemias are listed at the top, subgroup number and its major characteristics are listed to the left, and column numbers are listed at the bottom. Different intensities of shading within the subgroups defined by genotype listed on top of table illustrate proportions of mice within each group with the particular characteristics. White cells indicate 0% of recipients; black cells, 100% of recipients.
Analysis of Hox cofactor gene expression is presented in the bottom 8 rows of Figure 4. A majority of leukemias derived from Meis1-transduced cells expressed high levels of this gene (columns 4-26). Of interest, Hoxa9 leukemia no. 23 (column 1) expressed very low levels of Meis1 and did not show up-regulation of other Meis family members, indicating that high levels of Meis1 are not obligatory for Hoxa9-induced transformation. Levels of Meis2 or Meis3 were either low or undetectable in all leukemias. Prep1 was highly expressed in all samples, while Prep2 levels clustered at or below the threshold of detection. Pbx1 was also expressed at very low levels, but the majority of samples expressed high to very high levels of Pbx2 and Pbx3.
Expression levels of Hoxb and Hoxc cluster genes varied between the different leukemias, but remained generally low to very low, except in leukemias no. 27 (Hoxa9) and no. 45 (VP16-Meis). Hoxd cluster genes, with the exception of Hoxd11 and Hoxd12 (Figure 4), were not expressed in our leukemias.
High levels of Hoxa gene expression detected in VP16-Meis and VP16-MeisΔ334 leukemias could reflect the VP16-Meis–promoted transcriptional activation, or alternatively, insertional activation of Hox genes by retrovirus(es) integrated into, or in the proximity of, the Hoxa cluster.
Hox and TALE gene expression in leukemia specimens. Expression of Hox and TALE genes in BM cells from leukemic mice as evaluated by absolute quantitative RT-PCR. Mice are grouped according to the inducing oncogene listed on the top. Morphologic classification group and expression levels of Mac-1 and c-Kit are provided for each mouse. CT values were normalized for 18S expression levels determined in 25 ng total RNA (CT = 9). CT values from all genes were then converted into copy numbers (cn) based on the formula cn = 2(38–ct). Visualization of expression levels was enhanced by color gradient based on a logarithmic scale of cn values.
Hox and TALE gene expression in leukemia specimens. Expression of Hox and TALE genes in BM cells from leukemic mice as evaluated by absolute quantitative RT-PCR. Mice are grouped according to the inducing oncogene listed on the top. Morphologic classification group and expression levels of Mac-1 and c-Kit are provided for each mouse. CT values were normalized for 18S expression levels determined in 25 ng total RNA (CT = 9). CT values from all genes were then converted into copy numbers (cn) based on the formula cn = 2(38–ct). Visualization of expression levels was enhanced by color gradient based on a logarithmic scale of cn values.
To examine retroviral insertion sites (RISs), inverse PCR was performed on genomic DNA isolated from VP16-Meis (n = 2) and VP16-MeisΔ334 (n = 4) leukemias. Among 19 RISs identified, there was one insertion in the intergenic region between Hoxa7 and Hoxa9 (Table 1, leukemia no. 33, 6qB3 locus). All other RISs were either separated from Hoxa locus by a considerable distance (> 15 Mb) or integrated elsewhere in the genome, suggesting a limited role for retroviral integrations in activation of Hoxa genes in our leukemias. Of interest, VP16-Meis1Δ334 leukemias nos. 22, 33, and 35 shared identical RIS at 6qC1 locus (Table 1), 140-Kb upstream of the DNA damage–inducible Gadd45a gene.54 This observation, together with clonal analysis of proviral integrations (Figure S2), suggests that following insertion into 6qC1, the ancestral cell underwent at least 2 self-renewal divisions during which its progeny acquired additional integration events.
Retroviral integration sites in VP16-Meis and VP16-MeisΔ334 leukemias
. | Hox expression levels, no. of copies . | . | . | . | ||
|---|---|---|---|---|---|---|
| Genotype, mouse ID no. . | Hoxa5 . | Hoxa7 . | Hoxa9 . | Integration (distance to Hox locus, Mb) . | ||
| VP16-Meis1 | ||||||
| 20 | 460 | 880 | 100 000 | 3qF1 | ||
| 45 | 8979 | 50 000 | 1 330 | 4qD3, 5qF, 5qG2 | ||
| VP16-MeisΔ334 | ||||||
| 22 | 1200 | 2 100 | 30 000 | 5qG2, 6qC1 (+15*) | ||
| 33 | 220 | 6 900 | 2 980 | 6qG1 (+85), 4qE2, 1qB, 6qB3 (0†), 8qE1, 6qC1 (+15*), 11qB3 (+41) | ||
| 35 | 350 | 120 | 400 000 | 3qE1, 8qE1, 1qC5, 6qC1 (+15*) | ||
| 36 | 300 | 4 360 | 420 | 19qB, 10qC2, 11qB3 (-33), 4qE2, 11qA1 (-46) | ||
. | Hox expression levels, no. of copies . | . | . | . | ||
|---|---|---|---|---|---|---|
| Genotype, mouse ID no. . | Hoxa5 . | Hoxa7 . | Hoxa9 . | Integration (distance to Hox locus, Mb) . | ||
| VP16-Meis1 | ||||||
| 20 | 460 | 880 | 100 000 | 3qF1 | ||
| 45 | 8979 | 50 000 | 1 330 | 4qD3, 5qF, 5qG2 | ||
| VP16-MeisΔ334 | ||||||
| 22 | 1200 | 2 100 | 30 000 | 5qG2, 6qC1 (+15*) | ||
| 33 | 220 | 6 900 | 2 980 | 6qG1 (+85), 4qE2, 1qB, 6qB3 (0†), 8qE1, 6qC1 (+15*), 11qB3 (+41) | ||
| 35 | 350 | 120 | 400 000 | 3qE1, 8qE1, 1qC5, 6qC1 (+15*) | ||
| 36 | 300 | 4 360 | 420 | 19qB, 10qC2, 11qB3 (-33), 4qE2, 11qA1 (-46) | ||
+ and - refer to integration of provirus centromeric versus telomeric, respectively, from the Hox locus.
Identical RIS.
Integration at 2.1 kb downstream of Hoxa7 and 4.4 kb upstream of the Hoxa9 gene.
Collectively, our results provide a molecular dissection of the complex oncogenic activities of Meis1 and its unique link to Hoxa gene activation for leukemia development. They also provide a unique analysis of the relationship between Hox and Meis gene activation in leukemia.
Discussion
In these studies, we have used mutational analysis and fusion constructs to identify 2 important domains essential for the transforming activity of Meis1. One of these domains is located in the C-terminal region of the protein and could be functionally substituted by VP16 in the VP16-MeisΔ334 mutants, thereby conferring a new attribute to Meis1: oncogenic independency. Of interest, and not unlike what has been suggested for Meis1, the oncogenic role of these fusion proteins appeared limited to myeloid cells and to involve obligatory activation of Hoxa cluster genes, with prominent up-regulation of Hoxa7 or Hoxa9. Functional validation of the genetic activation of Hoxa9 as a collaborating event in VP16-Meis1 leukemia was determined within the setup of our studies.
The mechanism leading to the activation of Hoxa7 or Hoxa9 (and other Hoxa genes) in our VP16-Meis1 leukemias remains to be determined, but the lack of consistency in Hoxa gene activation in the different leukemias (ie, the last 4 groups in Figure 4) strongly argues against direct transcriptional activation. In agreement with this, our initial analysis of chromatin immunoprecipitation using FLAG-Meis1 failed to reveal any sequences derived from the Hoxa gene loci (N.B. and G.S., unpublished observations, September 2004). Our brief survey of retroviral insertion sites in VP16-Meis1 leukemias also suggested a limited role for retroviral integrations in activation of Hoxa genes. One possible target of Meis1 in leukemia induction might be Flt3, as levels of this gene expression correlate with the presence of overexpressed Meis1.38
The unidirectional interaction between VP16-Meis → Hoxa gene activation suggests that VP16-Meis1 uses transformation pathways similar to those used by Meis1. The restrictive collaboration between these 2 sets of genes was first suggested by the studies presented by Copeland and collaborators (Nakamura et al55 ) showing that insertional activations of Meis1 in BXH2 model of mouse AML were mostly detected together with those in the Hox gene clusters, whereas analyses of leukemias developing in (NUP98-Hoxa9 × BXH2) mice suggest that the converse situation is not necessarily true.56 From this perspective, our findings reinforce an emerging model in cancer genetics predicting that oncogenic activation follows a given order of events that is at least in part dictated by the complementation group to which the oncogene belongs. In this scenario, and from a genetic perspective, the promiscuity of possible oncogenic partners and the availability of a locus for oncogenic events would determine the probability leading to a sequential acquisition of genetic alterations leading to cancer.
The second distinct domain of Meis1, critical for its oncogenic potency, was the PIM (or PBX1-interacting motif), which, in one experiment, was swapped with its known interacting motif in PBX1 (also known as MIM or Meis1-interaction motif). Within these studies, it was possible to create 2 dysfunctional proteins, each of them incapable of genetic interaction with HOXA9 for acceleration of leukemia onset in vivo. These 2 dysfunctional proteins were also incapable of spontaneously generating leukemia, even during long-term observation (data not shown), thus indicating that the inability of Meis1 to spontaneously induce leukemic transformation is not due to the limiting amounts of Pbx proteins. Results of our TALE gene expression survey (Figure 4) reinforce this argument, showing that although PBX1 levels in our leukemias were low, the PBX2 and PBX3 levels were high in most of our specimens. The combined introduction of the 2 dysfunctional mutants, PBX1(MIM>PIM) and Meis(PIM>MIM), together with HOXA9 resulted in leukemias that were very aggressive in vivo, and highly proliferative in vitro (not shown). This highly aggressive nature suggested that our PBX1(MIM>PIM) mutant encompassed elements in addition to PIM that are limiting in wild-type PBX1. One of these limitations could be MIM itself (now in Meis1(PIM>MIM)), but not the DNA-binding elements in the homeodomain of PBX1, since both our PBX1(MIM>PIM) mutants lacking the DNA-binding activity25 complemented the oncogenic potential of Meis1(PIM>MIM) as efficiently as the “wild-type” PBX1(MIM>PIM) (Figure 1E). Thus, providing that it is associated with Meis1, PIM retains its critical function even upon transfer into a heterologous protein, suggesting a context-independent nucleating activity for this domain. The participation of PBX1, or more likely PBX2 or PBX3 (Figure 4), in such putative protein complexes remains unclear. This hypothesis can now be further tested with different leukemias described in this paper and may represent a critical strategy for eradication of Meis1-induced leukemias.
Another interesting finding from our studies is the preferential activation of the Hoxa locus in the context of VP16-Meis1–induced leukemias. We and others have shown experimentally that Hoxb cluster genes1,2 (N.M. and G.S., unpublished observations, February 2002) and likely all Hox genes57 have an inherent capacity to induce cellular transformation, and are likely to collaborate with Meis1. Preferential activation of Hoxa cluster genes in mouse and human leukemias may thus reflect a greater availability of this locus, since our studies (this report, Bijl et al,31 and Sauvageau et al58 ) have consistently shown the dominant expression of Hoxa cluster genes in the primitive hematopoietic cells.
Prepublished online as Blood First Edition Paper, February 9, 2006; DOI 10.1182/blood-2005-06-2244.
Supported by a grant from the National Cancer Institute of Canada to G.S. and from Génome Canada. A.M. was the recipient of a Canadian Institute of Health Research studentship, and A.T. was supported by the fellowship from the American Cancer Society for Beginning Investigators (ACSBI) under the administration of Union Internationale Contre le Cancer (UICC). G.S. is a scholar of the Leukemia and Lymphoma Society of America and a recipient of a Canadian Research Chair in Molecular Genetics of Normal and Cancer Stem Cells.
A.M., J.K., and E.K. contributed equally to this study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Special thanks are owed to Dr Josée Hébert, head of the Leukemia Cell Bank of Québec, for help with morphologic analysis and to Melanie Frechette for assistance with the animal care and transplantation experiments. We also wish to thank Danièle Gagné from the flow cytometry service of IRIC for sorting and assistance in fluorescence-activated cell sorter (FACS) analysis, and Christian Charbonneau from the Imaging service for the capture and editing of images. Sylvie Provost and Claude Belisle are also acknowledged for their help with Q-PCR.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal