Natural killer T (NKT) cells are CD1d-restricted glycolipid reactive innate lymphocytes that play an important role in protection from pathogens and tumors. Pharmacologic approaches to enhance NKT cell function will facilitate specific NKT targeting in the clinic. Here we show that lenalidomide (LEN), a novel thalidomide (Thal) analog, enhances antigen-specific expansion of NKT cells in response to the NKT ligand α-galactosylceramide (α-GalCer) in both healthy donors and patients with myeloma. NKT cells activated in the presence of LEN have greater ability to secrete interferon-γ. Antigen-dependent activation of NKT cells was greater in the presence of dexamethasone (DEX) plus LEN than with DEX alone. Therapy with LEN/Thal also led to an increase in NKT cells in vivo in patients with myeloma and del5q myelodysplastic syndrome. Together these data demonstrate that LEN and its analogues enhance CD1d-mediated presentation of glycolipid antigens and support combining these agents with NKT targeted approaches for protection against tumors.
Introduction
Natural killer T (NKT) cells are T lymphocytes bearing NK markers that recognize glycolipid ligands in the context of CD1d molecules.1 NKT cells play an important role in immune regulation and protection against tumors and pathogens.1,2 These cells mediate antitumor effects by several mechanisms, including direct effects on tumor cells, strong cytokine production, antiangiogenesis, and activation of other cells, particularly NK cells and dendritic cells.2
Thalidomide and its analog lenalidomide have shown clinical activity in myeloma and myelodysplastic syndrome (MDS).3,4 However, the mechanism of their observed antitumor effects remains unclear.5 Haslett et al6 demonstrated that thalidomide can costimulate human T cells, which led to the development of lenalidomide (LEN) as an immune-modulatory derivative.5,7,8 Initial studies of thalidomide in myeloma described an effect on NK cells.9 However, subsequent studies have suggested that the observed effects on NK cells were indirect.10 We hypothesized that innate CD1d-restricted NKT cells may be a proximal cellular target of LEN. Here we show that LEN greatly enhances ligand-dependent activation, proliferation, and cytokine production by human NKT cells, as well as enhance NKT cells in vivo.
Study design
Cells and materials
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated from buffy coats purchased from New York Blood Center, or from patients with multiple myeloma, following informed consent approved by the Rockefeller University (New York, NY), Memorial Sloan-Kettering Cancer Center (New York, NY), and St Vincent's Cancer Center (New York, NY) institutional review boards. Lenalidomide (LEN; Celgene, Warren, NJ) was dissolved in dimethyl sulfoxide (DMSO; Sigma, St Louis, MO) at 1 mM. NKT ligand α-galactosyl-ceramide (α-GalCer) was kindly provided by Kirin Breweries, Tokyo, Japan.
Dendritic cell–mediated NKT expansion
CD14 + monocytes were isolated from PBMCs using immunomagnetic bead selection with CD14 microbeads and cultured in the presence of granulocyte macrophage–colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4) to generate dendritic cells (DCs), as described.11,12 After day 5 to 6 of culture, DCs were matured by adding an inflammatory cytokine cocktail as described.12 To stimulate NKT cells, DCs were pulsed with α-GalCer (100 ng/mL) and cultured with CD14– cells at a DC-to-responder ratio of 1:10 to 1:20 with or without LEN (Celgene) at 1 μM, or as otherwise indicated.12,13 For some experiments, DEX (0.5 nM) was also added as indicated at the beginning of expansion. NKT expansion was moninored by flow cytometry based on the expression of invariant T-cell receptor (Vα24/Vβ11) and/or binding to CD1d-dimer loaded with α-GalCer, as described.12
Intracellular cytokine staining (ICS)
The ability of NKT cells to secrete cytokines following stimulation with NKT ligand (α-GalCer) was tested using both intracellular cytokine staining and TaqMan assays, as described.12,14 Freshly isolated PBMCs were stimulated with α-GalCer, with or without LEN (1 μM). Stimulation with PMA and ionomycin was used as a positive control. For the detection of cytokine production by expanded NKT cells, these cells were restimulated overnight with unpulsed or α-GalCer–pulsed DCs (DC-to-responder ratio 1:10-1:20), prior to ICS assay.
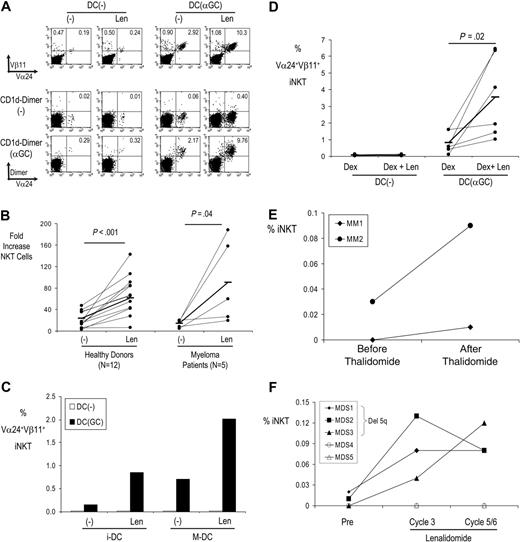
LEN/thalidomide-mediated enhancement of NKT cells in vitro and in vivo. (A) LEN boosts expansion of NKT cells from healthy donors by DCs pulsed with α-GalCer. T cells were expanded with DCs loaded with α-GalCer (or unpulsed DCs) in the presence or absence of LEN/DMSO control. After 2 weeks of culture, the presence of Vα24+Vβ11+ or CD1d-aGC dimer+ NKT cells was monitored by flow cytometry. (B) LEN boosts expansion of NKT cells by DCs pulsed with α-GalCer in both healthy donors and patients with myeloma. NKT cells were expanded with DCs loaded with α-GalCer (or unpulsed DCs) in the presence or absence of LEN/DMSO control. After 1 to 2 weeks of culture, the presence of Vα24+Vβ11+ NKT cells was monitored by flow cytometry. Data shown are fold increase in NKT cells for cultures with α-GalCer–loaded DCs. Horizontal bars represent mean NKT expansion. (C) Immature versus mature DCs. NKT cells were expanded as in panel A, with immature monocyte-derived DCs (iDCs), or after DC maturation with inflammatory cytokines (MDCs). After 2 weeks of culture, the presence of Vα24+Vβ11+ NKT cells was monitored by flow cytometry. Data are representative of 8 separate experiments. (D) Comparison of DEX versus DEX + LEN. NKT cells were expanded as in panel A, in the presence of DEX (0.5 nM) alone, or with LEN (1 μM). Data shown are percent iNKT cells. Horizontal bars represent mean NKT expansion. (E) Expansion of NKT cells in myeloma. Circulating NKT cells were monitored by flow cytometry before and 1 month after thalidomide therapy in patients with myeloma (n = 2). (F) NKT expansion in patients with MDS (n = 5) treated with LEN. iNKT cells were quantified by flow cytometry before and at the start of the third and fifth/sixth cycle of LEN therapy. Closed symbols represent patients with del5q.
LEN/thalidomide-mediated enhancement of NKT cells in vitro and in vivo. (A) LEN boosts expansion of NKT cells from healthy donors by DCs pulsed with α-GalCer. T cells were expanded with DCs loaded with α-GalCer (or unpulsed DCs) in the presence or absence of LEN/DMSO control. After 2 weeks of culture, the presence of Vα24+Vβ11+ or CD1d-aGC dimer+ NKT cells was monitored by flow cytometry. (B) LEN boosts expansion of NKT cells by DCs pulsed with α-GalCer in both healthy donors and patients with myeloma. NKT cells were expanded with DCs loaded with α-GalCer (or unpulsed DCs) in the presence or absence of LEN/DMSO control. After 1 to 2 weeks of culture, the presence of Vα24+Vβ11+ NKT cells was monitored by flow cytometry. Data shown are fold increase in NKT cells for cultures with α-GalCer–loaded DCs. Horizontal bars represent mean NKT expansion. (C) Immature versus mature DCs. NKT cells were expanded as in panel A, with immature monocyte-derived DCs (iDCs), or after DC maturation with inflammatory cytokines (MDCs). After 2 weeks of culture, the presence of Vα24+Vβ11+ NKT cells was monitored by flow cytometry. Data are representative of 8 separate experiments. (D) Comparison of DEX versus DEX + LEN. NKT cells were expanded as in panel A, in the presence of DEX (0.5 nM) alone, or with LEN (1 μM). Data shown are percent iNKT cells. Horizontal bars represent mean NKT expansion. (E) Expansion of NKT cells in myeloma. Circulating NKT cells were monitored by flow cytometry before and 1 month after thalidomide therapy in patients with myeloma (n = 2). (F) NKT expansion in patients with MDS (n = 5) treated with LEN. iNKT cells were quantified by flow cytometry before and at the start of the third and fifth/sixth cycle of LEN therapy. Closed symbols represent patients with del5q.
TaqMan RT-PCR quantitation of cytokine mRNA
For the TaqMan analysis, PBMCs were cultured with α-GalCer as described in ICS. Stimulated cells were pelleted and total RNA was isolated using the Qiagen RNeasy kit (Valencia, CA). The primers and probes for IFN-γ, IL-4, IL-10, and IL-13 were purchased from Applied Biosystems/Perkin Elmer (Shelton, CT). Reverse transcriptase–polymerase chain reactions (RT-PCRs) were performed in duplicate samples as described previously.12 mRNA levels for a housekeeping gene GAPDH were used to normalize gene expression from each sample.
Thal/Len-mediated effects in vivo
To analyze the effects of LEN on NKT cells in vivo, we obtained peripheral blood samples after institutional review board–approved informed consent from patients with MDS (n = 5) treated with single-agent LEN (10 mg/d in 28-day cycles), at baseline and at 3 to 6 cycles of therapy. Number of NKT cells was monitored by flow cytometry. Similarly, we also measured NKT cells in the blood of patients with myeloma (n = 2) before and 1 month after 50 mg/d to 100 mg/d thalidomide.
Results and discussion
Human monocyte–derived DCs loaded with NKT ligand α-GalCer are efficient at both activation and expansion of NKT cells in culture.13 However, the addition of LEN to these cocultures greatly increased NKT expansion, both in healthy donors and in patients with myeloma (Figure 1A-B and Table S1, which is available on the Blood website; see the Supplemental Table link at the top of the online article). Enhancement of number of NKT cells by LEN was ligand dependent and seen only in the α-GalCer–stimulated cultures. In prior studies, we have observed that mature DCs are more effective than immature DCs at mediating NKT expansion, presumably due to higher expression of costimulatory molecules.13 However, LEN-mediated enhancement of NKT cells was observed with both immature and mature DCs (Figure 1C). As LEN and Thal are sometimes used with DEX in the clinic, we tested whether the effect of LEN was maintained in the presence of DEX, which is thought to have a differential effect on T versus NKT cell lines.15 DEX alone did not lead to an increase in GalCer-mediated NKT expansion. Addition of LEN to DEX enhanced NKT expansion in response to α-GalCer–loaded DCs, compared with DEX alone (Figure 1D). Therefore, LEN boosts ligand-dependent expansion of NKT cells in vitro.
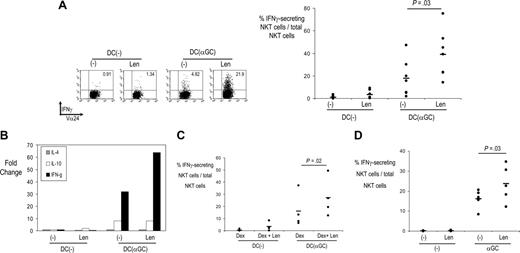
LEN-mediated enhancement of NKT effector function. (A) NKT cells expanded in the presence or absence of LEN, as in Figure 1A, were tested for the ability to secrete interferon-γ in response to unpulsed or α-GalCer–pulsed DCs, by intracellular cytokine secretion (ICS) assay. The right panel shows data from individual donors (n = 7). Horizontal bars represent mean percent NKT cells secreting interferon-γ. (B) TaqMan analysis showing changes in cytokine gene expression in NKT cells (expanded initially with α-GalCer–loaded DCs) and cultured overnight with unpulsed or α-GalCer–loaded DCs in the presence of absence of LEN. Data are representative of 2 separate experiments. (C) NKT cells expanded in the presence of DEX alone, or DEX + LEN, as in Figure 1D, were tested for the ability to secrete interferon-γ in response to unpulsed or α-GalCer–pulsed DCs, by ICS assay (n = 4). (D) NKT cells in freshly isolated PBMCs from healthy donors were tested for interferon-γ secretion in response to vehicle or α-GalCer–pulsed DCs, by ICS assay, as in panel A (n = 5).
LEN-mediated enhancement of NKT effector function. (A) NKT cells expanded in the presence or absence of LEN, as in Figure 1A, were tested for the ability to secrete interferon-γ in response to unpulsed or α-GalCer–pulsed DCs, by intracellular cytokine secretion (ICS) assay. The right panel shows data from individual donors (n = 7). Horizontal bars represent mean percent NKT cells secreting interferon-γ. (B) TaqMan analysis showing changes in cytokine gene expression in NKT cells (expanded initially with α-GalCer–loaded DCs) and cultured overnight with unpulsed or α-GalCer–loaded DCs in the presence of absence of LEN. Data are representative of 2 separate experiments. (C) NKT cells expanded in the presence of DEX alone, or DEX + LEN, as in Figure 1D, were tested for the ability to secrete interferon-γ in response to unpulsed or α-GalCer–pulsed DCs, by ICS assay (n = 4). (D) NKT cells in freshly isolated PBMCs from healthy donors were tested for interferon-γ secretion in response to vehicle or α-GalCer–pulsed DCs, by ICS assay, as in panel A (n = 5).
We have recently shown that injection of α-GalCer–loaded DCs leads to expansion of NKT cells in vivo in patients with advanced cancer.12 We treated 2 of these patients with low-dose (50 mg/d-100 mg/d) thalidomide, a parent analog of LEN, after NKT cells had returned back to baseline. In both patients, thalidomide led to an increase in circulating NKT cells at 1 month after therapy (Figure 1E). We also analyzed the number of NKT cells in patients with MDS treated with LEN, at baseline and at the third to sixth cycles of therapy. Increase in NKT cells was observed in 3 of 5 patients, which included all 3 of the patients with del5q MDS (Figure 1F).
Antitumor properties of NKT cells are linked to their ability to secrete interferon-γ.2 NKT cells expanded in the presence of LEN had greater ability to secrete IFN-γ (Figure 2A). Similar data were observed by real-time RT-PCR (TaqMan) analysis of these cells (Figure 2B). LEN-associated increase in interferon-γ production by NKT cells was detectable even in the presence of DEX, which blunted the cytokine response (Figure 2C). LEN also led to an increase in ligand-dependent induction of interferon-γ production by freshly isolated NKT cells in human PBMCs (Figure 2D). Therefore, LEN also leads to an increase in ligand-reactive interferon-γ secretion by human NKT cells in vitro.
In summary, these data show that LEN has a significant effect on the function of human CD1d-restricted NKT cells. LEN may therefore help improve clinical NKT targeting. NKT activation may also contribute to NK activation in vivo,16-18 and serve as an adjuvant for improving T-cell–based vaccines.19 These findings also have implications for understanding the mechanism of action of LEN in human studies. A major fraction of T cells in the human bone marrow are CD1d restricted.20 CD1d-restricted T cells in the bone marrow (including those lacking invariant T-cell receptor) may therefore be one of the proximal targets of LEN in both myeloma and MDS. It is of interest that recent studies have identified selective deficiency of NKT cells in patients with MDS.21,22 NKT cells may also play a role in regulating hematopoiesis in vivo.23 Combining NKT ligands with LEN may provide a simple rational approach to enhance the efficacy of either therapy against human cancer.
Prepublished online as Blood First Edition Paper, March 28, 2006; DOI 10.1182/blood-2005-10-4184.
Supported in part by funds from the National Institutes of Health (CA84 512, CA106 802, CA109 465, MO1-RR00 102), the Damon Runyon Cancer Research Fund, the Irene Diamond Foundation, the Dana Foundation, and the Irma T. Hirschl Foundation. D.H.C. is a Dana Foundation/Irvington Institute Human Immunology Fellow.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ralph M. Steinman for thoughtful critique and advice regarding this work; Arlene Hurley, RN, and Keren Osman, MD, for help with clinical aspects; Matthew Geller for technical assistance; and members of the Dhodapkar lab for many helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal