Interferon consensus sequence-binding protein (ICSBP)/interferon regulatory factor 8 (IRF-8) is a transcription factor that plays critical roles in the differentiation of defined dendritic-cell (DC) populations and in the immune response to many pathogens. In this study, we show that splenic DCs (s-DCs) from ICSBP–/– mice are markedly defective in their ability to capture and to present exogenous antigens (Ags) to naive CD4+ T lymphocytes. We found that CD8α+ DCs and, to a lesser extent, CD8α– DCs from ICSBP–/– mice are impaired at internalizing Ags, either through a receptor-mediated pathway or by macropinocytosis, in spite of having a more immature phenotype than their wild-type (WT) counterparts. These features reflected a greatly impaired ability of ICSBP–/– s-DCs to present injected soluble ovalbumin (OVA) to OVA-specific CD4+ T cells in vivo. Conversely, bone marrow (BM)–derived DCs from ICSBP–/– mice, in keeping with their immature phenotype, exhibited higher endocytic activity than WT cells. However, Ag-loaded ICSBP–/– BM-DCs were defective in priming Ag-specific CD4+ T lymphocytes and failed to induce a contact hypersensitivity (CHS) response when injected into competent WT hosts. Together, these results indicate that, throughout the developmental program of DCs, ICSBP differentially controls Ag uptake and MHC class II (MHC-II) presentation affecting both functions only in differentiated peripheral DCs.
Introduction
Dendritic cells (DCs) are the most powerful antigen-presenting cells (APCs), with unique features in initiating antigen (Ag)–specific immune responses, activating both naive CD4+ and CD8+ T lymphocytes.1 To do so, DCs undergo a series of developmental changes in a process known as “maturation.” Immature DCs are endowed with potent ability to capture Ags but are inefficient at presenting the processed Ags on their MHC class II (MHC-II) molecules, and thus are poor T-cell stimulators. Upon contact with microbial products or inflammatory cytokines, DCs are induced to mature and become highly efficient at presenting Ags and activating T cells while exhibiting reduced capacity for uptake and processing of newly encountered antigens.2 Immature DCs are tuned to sample their environment and to capture any foreign particle. To accomplish this task, DCs use at least 2 mechanisms to internalize soluble proteins, fluid-phase macropinocytosis or receptor-mediated endocytosis by members of different receptor families.3 The most prominent member of these membrane-bound proteins is the mannose receptor (MR). The MR acts as a pattern recognition molecule that enables efficient capture of mannosylated or fucosylated antigens, like dextran (DX), and is the main effector of receptor-mediated endocytosis.4-6 In this regard, MR-mediated uptake of Ags has been shown to potently enhance MHC-II–restricted Ag presentation in both human and mouse DCs.7,8
In mouse spleen, distinct DC subtypes, including CD8α+ and CD8α– DCs, can be identified with diverse localization, ontogeny, and nature of immune response induced, but with a common ability to capture, process, and present soluble antigens.9 Lymphoid organ–resident DCs have been generally considered as mature DCs based on high-level expression of costimulatory molecules as well as for their exceptional ability to stimulate both allogeneic T cells and Ag-specific naive T cells.9,10 However, a number of reports have shown that spleen DC (s-DCs) can efficiently endocytose Ags, both in vitro and in vivo, suggesting that these cells may also exhibit features of immature DCs.11-15 A recent study has clarified this controversy by showing that most subsets of lymphoid organ DCs, including CD8α+ and CD8α– s-DCs, are phenotypically and functionally immature in the steady state and are induced to mature only upon stimulation in vitro or in vivo.16,17 This immature feature of s-DCs is particularly relevant for the maintenance of peripheral tolerance and allows DCs to promptly capture blood-circulating antigens and to mature in situ.18
Interferon consensus sequence-binding protein (ICSBP), also known as interferon regulatory factor 8 (IRF-8), is a transcription factor that plays a prominent role in myeloid cell development as well as in the immune response to various infectious agents.19,20 We and others have recently demonstrated that ICSBP is critically required for the normal development of defined DC populations. ICSBP–/– mice lack plasmacytoid DCs and display a selective reduction of CD8α+ DCs and epidermal Langerhans cells as well as dermal DCs.21-25 In addition to controlling the differentiation of some DC subsets, it appears that ICSBP also affects the functional maturation of DCs. In fact, the few CD8α+ DCs arising in ICSBP–/– mice exhibit very low levels of costimulatory molecules and the CD8α– DCs display reduced expression of MHC-II.21,23 In addition, bone marrow (BM)–derived DCs from ICSBP–/– mice display an immature phenotype, altered chemotactic behavior, and defective responses to inflammatory stimuli.24,25 Finally, ICSBP–/– mice show impaired trafficking of skin DCs in vivo that results in failure to initiate T-cell–mediated responses.25 Together, these findings indicate that ICSBP affects both the differentiation and the functional maturation of DCs and suggest that this factor may regulate many aspects of DC-mediated T-cell immunity.
In the present work, we provide evidence that ICSBP plays a role on the ability of DCs to capture and to present exogenous Ags to MHC-II–restricted CD4+ T lymphocytes. We show that s-DCs in ICSBP–/– mice, especially the CD8α+ subset, display a marked defect in Ag internalization as well as a poor capacity to stimulate Ag-specific naive CD4+ T cells. Moreover, although BM-DCs from ICSBP–/– mice exhibit high endocytic activity, they are much less able to stimulate CD4+ T cells than their wild-type (WT) counterparts. Altogether, our data demonstrate that ICSBP affects the capacity of DCs for both Ag uptake and MHC-II presentation and suggest that this factor may control these 2 processes independently and at different stages of DC development.
Materials and methods
Mice
ICSBP-deficient mice were generated and bred as described.20,21 ovalbumin (OVA)–specific MHC-II–restricted, T-cell receptor (TCR)–transgenic mice (OT-II mice)26 were kindly provided by Dr D. F. Tough (The Edward Jenner Institute, Compton, United Kingdom). All mice were kept under specific pathogen–free conditions. Animal procedures were performed in conformance with institutional guidelines.
Reagents
Dextran-FITC (70 000 kDa) was purchased from Molecular Probes (Leiden, The Netherlands). OVA-FITC was obtained by conjugation of OVA–Chicken Egg (Sigma-Aldrich, St Louis, MO) with FITC, using the FluoroTag FITC-conjugation kit (Sigma-Aldrich). Horseradish peroxidase (HRP) was purchased from Amersham Biosciences (Little Chalfont, United Kingdom). Granulocyte-macrophage colony-stimulating factor (GM-CSF) was obtained from Peprotech (London, United Kingdom). All monoclonal antibodies (mAbs) used for flow cytometry were from Pharmingen (BD Biosciences, San Jose, CA). Biotinylated mAbs were detected with streptavidin-Tricolor (Caltag Laboratories, Burlingame, CA). Stained cells were analyzed on a FACSsort flow cytometer (BD Biosciences).
Dendritic-cell isolation
The procedure of DC isolation from spleens has been described in detail elsewhere.21,27 Briefly, spleen fragments were digested in collagenase/DNase, followed by density-gradient centrifugation. The low-density fraction was positively selected using anti-CD11c Microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). The cells obtained were routinely more than 97% CD11c+. In some experiments, DCs were cultured overnight in Iscove modified Dulbecco medium (IMDM; Cambrex, East Rutherford, NJ) containing 10% fetal calf serum (FCS; Euroclone, Pero, Italy), with or without 0.5 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich). BM-DCs were generated with GM-CSF.25 Cells were used for experiments at day 7 of culture. Where indicated, BM-DCs were replated with or without LPS (0.5 μg/mL) for 1 day before use.
Assays for Ag uptake in vitro
For flow cytometric analysis, 105 DCs were incubated for various times with 1 mg/mL DX-FITC or with 0.2 mg/mL OVA-FITC at 37°C. To calculate background fluorescence, control cells were incubated with Ag at 4°C. Cells were washed in cold phosphate-buffered saline (PBS), stained with anti-CD8a–PE mAb, and analyzed by fluorescence-activated cell sorting (FACS). For confocal laser scanning microscopy (CLSM), 3 × 105 DCs were incubated for 40 minutes with 2 mg/mL DX-FITC or with 0.3 mg/mL OVA-FITC at 37°C. Cells were then washed in cold PBS and left to adhere on Superfrost Plus glass slides (Menzel Glasser, Braunschweig, Germany) for 1 hour at 4°C. Slides were stained with biotin anti-CD8a for 20 minutes at 4°C, followed by Streptavidin–Alexa 594 (Molecular Probes), washed and fixed in acetone for 20 minutes at –20°C. Cover glasses were then mounted on microscope slides with antifade Vectashield reagent (Vector Laboratories, Burlingame, CA). CLSM observations were performed using a Leica TCS SP2 AOBS apparatus (Leica Lasertechnik, Heidelberg, Germany), using excitation spectral laser lines 488 and 594 nm properly tuned by Acousto-optic tunable filters (AOTF; Leica LaserFechnik). Image acquisition and processing were conducted by using the Leica confocal software (Leica Lasertechnik). Signals from different fluorescent probes were taken in parallel. For immunohistochemistry, 2 × 105 DCs were incubated in RPMI in presence of 1 mg/mL HRP at 37°C for 10 minutes, washed in cold PBS, and cytospun for 5 minutes at 500g. Slides were fixed in 4% paraformaldehyde and washed in Tris-NaCl (TN) buffer; the enzymatic reaction was developed by diaminobenzidine (DAB; SigmaAldrich) reaction. Slides were counterstained with hematoxylin. Slides were observed by using a Zeiss Axioplan microscope (Zeiss, Göttingen, Germany). Images were captured with a Canon Powershot G5 camera (Canon, Tokyo, Japan) and were processed with Photoshop software (Adobe Systems, Mountain View, CA).
Ag uptake in vivo
Mice were injected intravenously with 1 mg OVA-FITC or PBS. Two hours later, mice were killed and spleens were removed. Splenic DCs were isolated, double-stained with anti-CD11c and anti-CD8, and analyzed by FACS to assess the uptake of OVA-FITC.
Proliferative responses of OVA-specific CD4+ T cells in vitro
OVA-specific CD4+ T cells were magnetically sorted from lymph nodes (LNs) and spleens of OT-II mice by using anti-CD4 Microbeads (Miltenyi Biotech). T cells (50 000) were cultured with graded numbers of OVA-loaded s-DCs or BM-DCs from ICSBP–/– and WT mice. OVA loading of BM-DCs was performed by incubating cells with 0.2 mg/mL OVA for 40 minutes at 37°C. OVA loading of s-DCs was performed in vivo by intravenous injection with 1 mg OVA or PBS; DCs were isolated 2 hours later. In some experiments, OVA-loaded DCs were cultured for 18 hours with or without LPS (0.5 μg/mL) and then assayed for their ability to prime OVA-specific CD4+ T cells. Cell cultures were incubated in RPMI containing 10% FCS for 3 days at 37°C in 5% CO2 and then pulsed with 3H-thymidine (1 μCi/well [0.037 MBq/well]) for 16 hours. Incorporation of 3H-thymidine was analyzed by liquid scintillation counting.
Proliferative responses of OVA-specific CD4+ T cells in vivo
CD4+ T cells purified from OT-II mice were incubated with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) for 10 minutes at 37°C, then washed in cold PBS.28 5 × 106 CFSE-labeled OT-II cells were transferred intravenously into WT recipients. One day later, recipients were injected in the hind footpads with 5 × 105 OVA-loaded s-DCs or BM-DCs from ICSBP–/– and WT mice. PBS-injected mice served as controls. Three days after immunization, popliteal LNs were harvested and cell suspensions were stained with PE-labeled anti-CD4. OVA-specific proliferation of OT-II cells was measured by cytofluorometric analysis of CFSE dilution.29
CHS response to FITC
To assess the capacity of DCs to induce Ag-specific T-cell sensitization in vivo, BM-DCs from ICSBP–/– or WT mice were incubated with 12.5 μg/mL FITC for 20 minutes at 37°C.30 FITC-loaded DCs (5 × 105) were then injected in 1 footpad of WT recipients. Six days later, mice were challenged by painting 1 ear with 10 μL FITC. Contact hypersensitivity (CHS) response was determined 24 to 72 hours after challenge by measuring ear thickness using a dial thickness micrometer. Ear swelling was calculated by subtracting the thickness of the ear not treated with FITC.
qRT-PCR
Total RNA was extracted from 2-5 × 106 splenic DC or BM-DC and reverse-transcribed as previously described.25 Quantitative real-time reverse transcriptase–polymerase chain reaction (qRT-PCR) was performed using QuantiTect SYBR kit (Applied Biosystems, Epson, United Kingdom). Forward and reverse primers at a final concentration of 0.3 mM and 2 μL of each cDNA sample were also used. The conditions of PCR reaction were as follows: 15 seconds at 95°C, 30 seconds at 60°C, and 30 seconds at 72°C (50 cycles). PCR products were continuously measured by means of an ABI PRISM 7700 detection system (PerkinElmer/Cetus, Norwalk, CT). Triplicates were performed for each experimental point. Threshold cycle (CT) values were determined automatically by the Sequence Detection System software (PerkinElmer/Cetus). These values were used to calculate relative expression of mannose receptor (Mrc1) mRNA expression with respect to β-actin expression using the ΔΔCT method.31 The primer pairs used were as follows: Mrc1: 5′-ATGCCAAGTGGGAAAATCTG-3′ (forward), 5′-TGTAGCAGTGGCCTGCATAG-3′ (reverse); β-actin: 5′-AGAGGGAAATCGTGCGTGAC-3′ (forward), 5′-CAATAGTGATGACCTGGCCGT-3′ (reverse).32
Results
Impaired Ag uptake in splenic DCs from ICSBP–/– mice
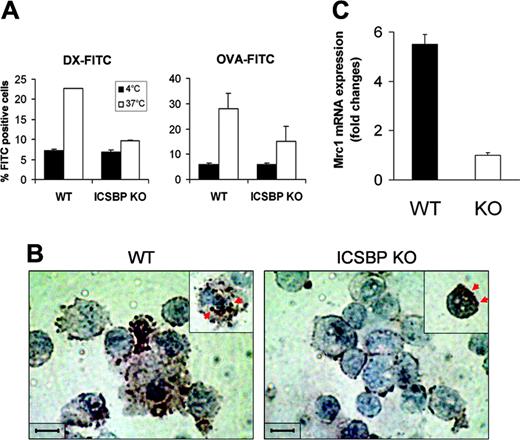
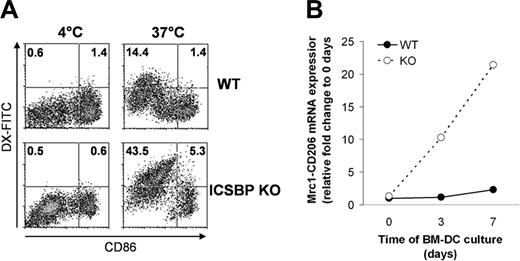
Spleen-resident DCs in the steady state display a relatively immature phenotype and therefore retain considerable capacity to internalize soluble Ags both in vitro and in vivo.16 Previous work has shown that s-DCs from ICSBP–/– mice tested in vitro display reduced expression of costimulatory as well as MHC molecules, indicative of a poorly differentiated phenotype with respect to their WT counterparts.21,23 Therefore, we asked whether the more immature features of s-DCs in ICSBP–/– mice could be associated with a higher potential for Ag uptake. To this end, we used different kinds of Ag internalized either through the MR-mediated pathway or by macropinocytosis, namely DX and OVA.5,33,34 As shown in Figure 1A, s-DCs from WT mice exhibited considerable endocytic activity for both DX-FITC and OVA-FITC, as revealed by 3-fold and 5-fold increases, respectively, of FITC positivity in cells incubated with Ag at 37°C over those incubated at 4°C. Surprisingly, CD11c+ s-DCs from ICSBP–/– mice exhibited almost undetectable uptake of DX-FITC and low uptake of OVA (3-fold increase of FITC-positive cells over control; Figure 1A). As an alternative approach to visualizing Ag internalization, we used HRP as the Ag. Like DX-FITC, HRP is known to be internalized through the MR-mediated pathway.5,35 Morphologic analysis revealed that while macropinosomes containing HRP could be detected in many WT CD11c+ DCs, few particles were found in ICSBP–/– DCs (Figure 1B). Interestingly, in ICSBP–/– s-DCs, the HRP spots were observed to be primarily on the surface membrane rather than in macropinosomal vesicles, suggesting a defective ability to internalize the Ag. To better understand the role of ICSBP in the MR-dependent pathway of Ag internalization, we analyzed the expression of Mrc1 mRNA in ICSBP–/– and WT s-DCs. As shown by qRT-PCR, Mrc1 transcripts were 5.5-fold higher in s-DCs of WT mice than those of ICSBP–/– mice (Figure 1C).
Impaired Ag uptake in splenic DC from ICSBP–/– mice. CD11c+ s-DCs were purified from ICSBP–/– and WT mice by magnetic cell sorting. (A) Cells were incubated for 40 minutes with 1 mg/mL DX-FITC or with 0.2 mg/mL OVA-FITC, then washed and analyzed by FACS. Histograms represent the uptake of FITC-conjugated antigens, expressed as percentage of FITC+ cells, in samples incubated with the Ag at 37°C and control cells incubated with Ag at 4°C. (B) Splenic DCs were incubated with 1 mg/mL HRP for 10 minutes at 37°C, then washed in cold PBS, cytospun on glass slides, and fixed; the enzymatic reaction was then developed. Slides were counterstained with hematoxylin. Brown spots (indicated by red arrows) reflect the presence of HRP in macropynosomal vesicles. Images were observed through an Achroplan 100 ×/1.25 oil-immersion objective lens. Scale bar represents 50 μm. (C) qRT-PCR analysis of mannose receptor (Mrc1) mRNA in ex vivo s-DCs. Total RNA from ICSBP–/– and WT s-DCs was used to generate cDNA that was analyzed by qRT-PCR. The cDNA concentrations were normalized to β-actin expression. Fold change of Mrc1 transcripts in WT s-DCs (▪) are expressed relative to those from ICSBP–/– mice (□) using the ΔΔCT method (“Materials and methods”). Results are represented as the mean ± SD of 3 separate experiments.
Impaired Ag uptake in splenic DC from ICSBP–/– mice. CD11c+ s-DCs were purified from ICSBP–/– and WT mice by magnetic cell sorting. (A) Cells were incubated for 40 minutes with 1 mg/mL DX-FITC or with 0.2 mg/mL OVA-FITC, then washed and analyzed by FACS. Histograms represent the uptake of FITC-conjugated antigens, expressed as percentage of FITC+ cells, in samples incubated with the Ag at 37°C and control cells incubated with Ag at 4°C. (B) Splenic DCs were incubated with 1 mg/mL HRP for 10 minutes at 37°C, then washed in cold PBS, cytospun on glass slides, and fixed; the enzymatic reaction was then developed. Slides were counterstained with hematoxylin. Brown spots (indicated by red arrows) reflect the presence of HRP in macropynosomal vesicles. Images were observed through an Achroplan 100 ×/1.25 oil-immersion objective lens. Scale bar represents 50 μm. (C) qRT-PCR analysis of mannose receptor (Mrc1) mRNA in ex vivo s-DCs. Total RNA from ICSBP–/– and WT s-DCs was used to generate cDNA that was analyzed by qRT-PCR. The cDNA concentrations were normalized to β-actin expression. Fold change of Mrc1 transcripts in WT s-DCs (▪) are expressed relative to those from ICSBP–/– mice (□) using the ΔΔCT method (“Materials and methods”). Results are represented as the mean ± SD of 3 separate experiments.
Lack of endocytic activity in CD8α+ DCs from ICSBP–/– mice
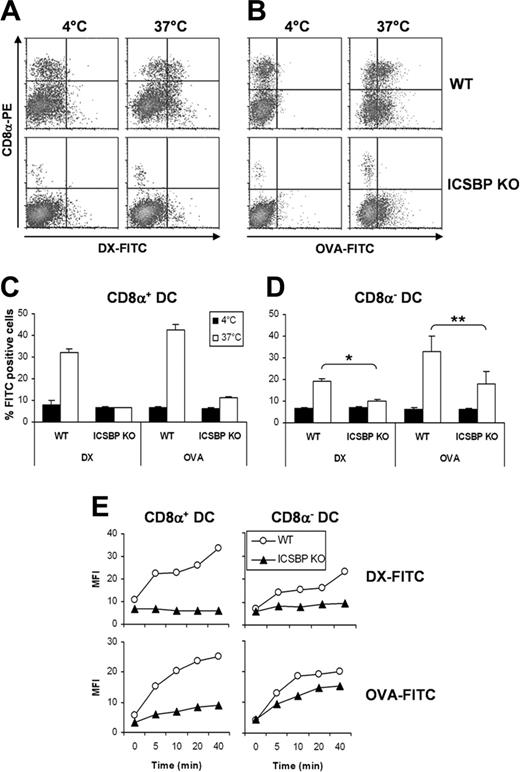
We previously reported that ICSBP–/– mice are characterized by an abnormally reduced frequency of the CD8α+ DC subset, which display a phenotype less mature than that of CD8α– DCs.21 It was therefore important to dissect the Ag uptake features of these 2 DC subsets. To this end, we analyzed the uptake of DX-FITC and OVA-FITC in CD8α+ and CD8α–\ DCs. As shown in Figure 2A and 2C, CD8α+ DCs from ICSBP–/– mice failed to exhibit any endocytic activity, whereas the same cells from WT mice displayed significant levels of DX uptake. Similarly, ICSBP–/– CD8α+ DCs exhibited almost undetectable endocytosis of OVA-FITC when compared with their WT counterparts (Figure 2B-C). CD8α– DCs from ICSBP–/– mice displayed very small ability to capture DX-FITC, whereas their WT counterparts were more efficient (Figure 2D). In addition, ICSBP–/– CD8α– DCs displayed a moderate reduction of OVA-FITC uptake with respect to WT cells (Figure 2D). We also examined the kinetics of uptake by CD8α+ and CD8α– DCs from WT and ICSBP–/– mice (Figure 2E). The mean fluorescence intensity (MFI) of WT CD8α+ DCs incorporating DX-FITC was increased 2-fold over control cells in as short a time as 5 minutes and increased over time to 3.5-fold higher at 40 minutes. Strikingly, no increase in the MFI was detected in ICSBP–/– CD8α+ DCs throughout the time of incubation, thus confirming the lack of DX-FITC uptake in these cells (Figure 2E). Furthermore, the MFI of WT CD8α+ DCs capturing OVA-FITC increased significantly over time, peaking at 4.5-fold higher compared with that of control cells. In contrast, the MFI of CD8α+ DCs from ICSBP–/– mice increased only to 2-fold higher compared with that of control cells. In WT CD8α– DCs the MFI increased up to 3.3-fold more than the incubation time with DX-FITC and up to 4.8-fold during incubation with OVA-FITC compared with that of control cells. Conversely, ICSBP–/– CD8α– DCs showed almost no increase in MFI during incubation with DX-FITC (1.5-fold more than control cells), and up to 3.4-fold MFI increase during incubation with OVA-FITC (Figure 2E).
CD8α+ and CD8α– DCs from ICSBP–/– mice exhibit defective ability to capture Ags. Splenic CD11c+ DCs were purified from ICSBP–/– and WT mice by magnetic cell sorting. DCs were incubated for 40 minutes either with 1 mg/mL DX-FITC (A,C-D) or with 0.2 mg/mL OVA-FITC (B-D), washed in cold PBS, and then stained with PE-labeled anti-CD8α mAb. (A-B) Density plots represent the uptake of FITC-conjugated OVA (A) or DX (B) and the expression of CD8α in CD11c+ s-DCs incubated with the Ag at 37°C or, as a control, at 4°C. (C-D) Histograms represent the uptake of Ag in CD8α+-gated (C) and CD8α–-gated (D) s-DCs populations. Data are expressed as the mean percentage of FITC+ cells ± SD of 3 separate experiments. *P < .01; **P < .05, ICSBP–/– versus WT. (E) CD11c+ s-DCs were incubated for various times with DX-FITC or OVA-FITC and then labeled with anti-CD8α mAb. Each dot represents the amount of FITC-conjugated Ag internalized by WT (○) and ICSBP–/– (▴) in CD8α+-gated (left panels) and CD8α–-gated (right panels) s-DCs. Zero time represents the background fluorescence of cells incubated with the Ag at 4°C. Data are representative of 2 separate experiments.
CD8α+ and CD8α– DCs from ICSBP–/– mice exhibit defective ability to capture Ags. Splenic CD11c+ DCs were purified from ICSBP–/– and WT mice by magnetic cell sorting. DCs were incubated for 40 minutes either with 1 mg/mL DX-FITC (A,C-D) or with 0.2 mg/mL OVA-FITC (B-D), washed in cold PBS, and then stained with PE-labeled anti-CD8α mAb. (A-B) Density plots represent the uptake of FITC-conjugated OVA (A) or DX (B) and the expression of CD8α in CD11c+ s-DCs incubated with the Ag at 37°C or, as a control, at 4°C. (C-D) Histograms represent the uptake of Ag in CD8α+-gated (C) and CD8α–-gated (D) s-DCs populations. Data are expressed as the mean percentage of FITC+ cells ± SD of 3 separate experiments. *P < .01; **P < .05, ICSBP–/– versus WT. (E) CD11c+ s-DCs were incubated for various times with DX-FITC or OVA-FITC and then labeled with anti-CD8α mAb. Each dot represents the amount of FITC-conjugated Ag internalized by WT (○) and ICSBP–/– (▴) in CD8α+-gated (left panels) and CD8α–-gated (right panels) s-DCs. Zero time represents the background fluorescence of cells incubated with the Ag at 4°C. Data are representative of 2 separate experiments.
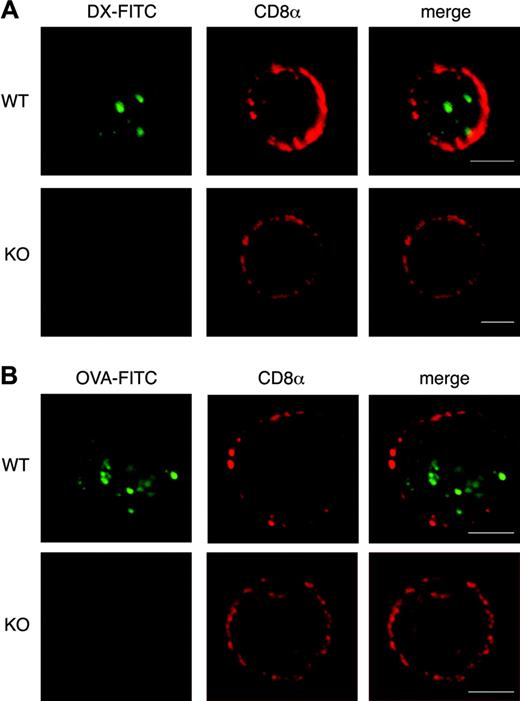
To better explore Ag endocytosis in CD8α+ s-DCs we used confocal laser-scanning microscopy. Strikingly, no evidence for uptake of FITC-labeled DX (Figure 3A) or OVA (Figure 3B) was found in any of the CD8α+ s-DCs from ICSBP–/– mice. In contrast, both DX-FITC (Figure 3A) and OVA-FITC (Figure 3B) were internalized by WT CD8α+ s-DCs.
Defective MHC-II presentation by s-DCs from ICSBP–/– mice
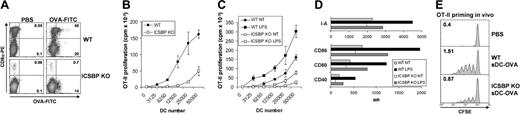
Priming of naive CD4+ T cells in draining lymphoid organs is largely the function of DCs that capture Ags in peripheral tissues.16,18 It was therefore important to evaluate the ability of s-DCs of ICSBP–/– mice to present Ags and to prime CD4+ T cells in vivo. To this end, we injected WT and ICSBP–/– mice intravenously with 1 mg OVA-FITC and analyzed OVA uptake in the CD8α+ and CD8α– DC subsets. As shown in Figure 4A, CD8α+ s-DCs from ICSBP–/– mice were unable to capture OVA-FITC in vivo. In contrast, OVA was efficiently taken up by the CD8α+ DC subset of WT mice. In keeping with the in vitro studies, CD8α– DCs from ICSBP–/– mice showed a moderately reduced endocytic activity when compared with their WT counterparts. Splenic DCs from ICSBP–/– and WT mice injected with OVA were also tested for their capacity to stimulate OT-II CD4+ T cells in vitro. As expected, the ability of ICSBP–/– s-DCs to stimulate OVA-specific CD4+ T-cell proliferation was greatly impaired when compared with the function of WT s-DCs (Figure 4B). To determine whether the defective Ag-presenting function of s-DCs from ICSBP–/– mice could be overcome by treatment with stimuli capable of activating DCs, s-DCs loaded with OVA in vivo were cultured overnight in presence or absence of LPS before testing their ability to stimulate the proliferation of OT-II CD4+ T cells. Notably, treatment of ICSBP–/– s-DC with LPS not only failed to increase their stimulatory capacity for CD4+ T cells, but seemed to actually diminish it when compared with the activity of untreated ICSBP–/– s-DCs (Figure 4C). In contrast, treatment of WT s-DCs with LPS markedly enhanced the proliferative response of OT-II CD4+ T lymphocytes over that observed using unstimulated WT s-DCs (Figure 4C). In line with these findings and with a previous report, treatment of ICSBP–/– s-DCs with LPS resulted in minor activation of MHC-II and costimulatory molecules with respect to LPS-exposed WT cells (Figure 4D).
Lack of endocytic activity in CD8α+ s-DCs from ICSBP–/– mice. WT and ICSBP–/– s-DCs were incubated for 40 minutes at 37°C with 2 mg/mL DX-FITC (A) or 0.3 mg/mL OVA-FITC (B), washed in cold PBS, and placed on a microscope slide. Cells were then stained with biotin-conjugated anti-CD8α mAb followed by labeling with streptavidin–Alexa 594. Slides were then fixed in cold acetone and analyzed by CLSM. Several cells for each labeling condition were analyzed and representative images are shown. Scale bars correspond to 5 μm. Images were observed through a 63 ×/1.40 PLApo oil-immersion objective lens.
Lack of endocytic activity in CD8α+ s-DCs from ICSBP–/– mice. WT and ICSBP–/– s-DCs were incubated for 40 minutes at 37°C with 2 mg/mL DX-FITC (A) or 0.3 mg/mL OVA-FITC (B), washed in cold PBS, and placed on a microscope slide. Cells were then stained with biotin-conjugated anti-CD8α mAb followed by labeling with streptavidin–Alexa 594. Slides were then fixed in cold acetone and analyzed by CLSM. Several cells for each labeling condition were analyzed and representative images are shown. Scale bars correspond to 5 μm. Images were observed through a 63 ×/1.40 PLApo oil-immersion objective lens.
Defective MHC-II presentation of s-DCs from ICSBP–/– mice in vivo. (A) ICSBP–/– and WT mice were injected intravenously with 1 mg OVA-FITC. Two hours later, s-DCs were isolated by magnetic cell sorting. CD11c+ cells were stained with PE-labeled anti-CD8α antibody and analyzed by FACS. Density plots represent the uptake of OVA-FITC and the expression of CD8α in CD11c+ s-DCs from mice injected with OVA-FITC or PBS. Numbers in top right and bottom right quadrants are the percentages of FITC+ cells in CD8α+- and CD8α–-gated DC subsets, respectively. Data are representative of 2 separate experiments. (B-C) ICSBP–/– and WT mice were intravenously injected with 1 mg OVA. Two hours later, CD11c+ s-DCs were isolated and used as stimulators of 105 OT-II CD4+ T cells in vitro. DC were either assayed ex vivo (B) or after overnight culture in the presence or absence of 0.5 μg/mL LPS (C). The cultures were pulsed with 3H-thymidine for 16 hours at the fourth day, and the incorporation into the cellular DNA was determined by liquid scintillation counting. Each point represents the mean counts per minute (cpm) ± SD in triplicate cultures of 1 out of 3 experiments. (D) Phenotype of s-DCs from ICSBP–/– and WT mice cultured overnight with or without LPS (0.5 μg/mL). (E) Splenic DCs from ICSBP–/– and WT mice injected with OVA were inoculated in the footpads of WT recipients that had been adaptively transferred with 5 × 106 CFSE-labeled OT-II CD4+ T cells the day before. Three days later, proliferation of CFSE+ cells in draining popliteal LN was evaluated by FACS. Results are representative of 2 similar experiments.
Defective MHC-II presentation of s-DCs from ICSBP–/– mice in vivo. (A) ICSBP–/– and WT mice were injected intravenously with 1 mg OVA-FITC. Two hours later, s-DCs were isolated by magnetic cell sorting. CD11c+ cells were stained with PE-labeled anti-CD8α antibody and analyzed by FACS. Density plots represent the uptake of OVA-FITC and the expression of CD8α in CD11c+ s-DCs from mice injected with OVA-FITC or PBS. Numbers in top right and bottom right quadrants are the percentages of FITC+ cells in CD8α+- and CD8α–-gated DC subsets, respectively. Data are representative of 2 separate experiments. (B-C) ICSBP–/– and WT mice were intravenously injected with 1 mg OVA. Two hours later, CD11c+ s-DCs were isolated and used as stimulators of 105 OT-II CD4+ T cells in vitro. DC were either assayed ex vivo (B) or after overnight culture in the presence or absence of 0.5 μg/mL LPS (C). The cultures were pulsed with 3H-thymidine for 16 hours at the fourth day, and the incorporation into the cellular DNA was determined by liquid scintillation counting. Each point represents the mean counts per minute (cpm) ± SD in triplicate cultures of 1 out of 3 experiments. (D) Phenotype of s-DCs from ICSBP–/– and WT mice cultured overnight with or without LPS (0.5 μg/mL). (E) Splenic DCs from ICSBP–/– and WT mice injected with OVA were inoculated in the footpads of WT recipients that had been adaptively transferred with 5 × 106 CFSE-labeled OT-II CD4+ T cells the day before. Three days later, proliferation of CFSE+ cells in draining popliteal LN was evaluated by FACS. Results are representative of 2 similar experiments.
Finally, we assessed how the reduced efficiency of Ag uptake and the altered T-cell stimulatory capacity of ICSBP–/– s-DCs affected Ag presentation to MHC-II–restricted T lymphocytes in vivo. To this end, s-DCs from ICSBP–/– and WT mice injected with OVA were inoculated into the footpads of WT recipients that had been adoptively transferred with CFSE-labeled OT-II CD4+ T cells. As shown in Figure 4E, s-DCs from ICSBP–/– mice induced only weak proliferation of OT-II cells, as revealed by a reduced number of division cycles (4 cycles) compared with those observed in mice immunized with WT OVA-DC (6 cycles). Of note, the absolute number of CFSE-positive cells recovered in LNs of mice primed with OVA-DCs from ICSBP–/– mice (0.87%) was significantly lower than the levels observed with recipients of OVA-DCs from WT counterparts (1.51%; Figure 4E). Thus, s-DCs in ICSBP–/– mice display marked defects in both capturing and presenting soluble Ags in a MHC-II–restricted context in vivo, and Ag presentation could not be enhanced by activation signals.
BM-DCs from ICSBP–/– mice efficiently capture Ag but are poor stimulators of naive CD4+ T cells
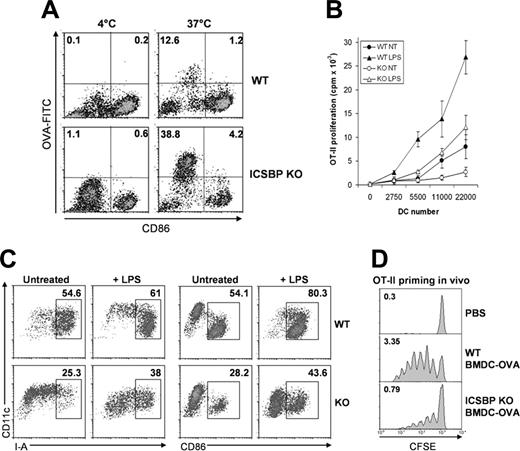
We have previously shown that ICSBP–/– BM-DCs display immature features resulting in defective chemotactic responses compared with BM-DCs from WT mice.25 We then asked whether ICSBP–/– BM-DCs, by virtue of their immature phenotype, exhibited a higher endocytic potential with respect to WT cells. For this purpose, we assessed the ability of BM-DCs from ICSBP–/– and WT mice to capture Ags. To define the maturational stage of the DCs, cells were stained for CD86 expression. As illustrated in Figure 5A, the frequency of ICSBP–/– BM-DCs taking up DX-FITC was 3-fold higher than for WT cells. Of interest, the population displaying endocytic activity in both WT and ICSBP–/– BM-DCs expressed CD86 at low levels or were negative (CD86–/low), indicating that the efficiency of DX-FITC uptake was associated with an immature state of DCs (Figure 5A). Previous studies have shown that the MR is expressed at high levels by immature DCs and that expression is down-regulated during the process of maturation.4,5 To test whether the efficiency of DX uptake correlated with the expression of its receptor, we analyzed the levels of Mrc1 transcripts in WT and ICSBP–/– BM-DCs at various stages of development. Interestingly, expression of Mrc1 in ICSBP–/– DCs increased 22-fold after 7 days in culture, whereas Mrc1 transcripts in WT cells increased only 2-fold during the same time frame (Figure 5B). To investigate receptor-independent pathways of endocytosis in ICSBP–/– BM-DCs, we tested the uptake of OVA. The frequency of ICSBP–/– BM-DCs taking up OVA-FITC was 3-fold higher than for BM cells of WT mice (Figure 6A). Again, the population exhibiting endocytic activity in both WT and ICSBP–/– BM-DCs displayed an immature phenotype (CD86–/low; Figure 6A). These findings suggest that the higher endocytic potential of BM-DCs from ICSBP–/– as compared to WT mice may be the result of a higher frequency of immature DCs.
Next, we studied the ability of ICSBP–/– BM-DCs to prime CD4+ T cells in vitro. Notably, OVA-loaded ICSBP–/– BM-DCs were much less effective than WT cells in stimulating the proliferation of OVA-specific CD4+ T lymphocytes (Figure 6B). To investigate whether Ag presentation by ICSBP–/– BM-DCs could be triggered by activation stimuli, OVA-loaded BM-DCs were treated with LPS and then tested for their ability to stimulate OT-II CD4+ T cell proliferation. Exposure to LPS enhanced the ability of both WT and ICSBP–/– BM-DCs to activate CD4+ T cells, although ICSBP–/– DCs were largely less stimulatory than WT DCs (Figure 6B). Notably, the magnitude of the proliferative response elicited by the BM-DC preparations correlated with their stage of maturation, as the frequency of ICSBP–/– BM-DCs expressing high levels of CD86 and MHC-II molecules was only about half that of WT DCs (Figure 6C). Following stimulation with LPS, the frequencies of mature cells increased to a similar extent for cells from both mice, leaving I-A+ and CD86+ cells in WT cultures present at frequencies that increased 1.6-fold and 1.8-fold, respectively, over stimulated cultures from ICSBP–/– mice (Figure 6C).
Increased Ag uptake of BM-DCs from ICSBP–/– mice. BM-DCs of ICSBP–/– and WT mice were cultured for 6 days in the presence of GM-CSF. (A) Cells were then incubated for 40 minutes with 1 mg/mL DX-FITC and then stained for CD11c and CD86 expression. Density plots represent the uptake of the DX and the relative expression of CD86 in a population gated on forward–side scatter properties and on CD11c positivity. Data are representative of 3 separate experiments. (B) qRT-PCR analysis of mannose receptor (Mrc1) transcript levels in BM-DCs at different times of culture. Total RNA was extracted from ICSBP–/– and WT BM-DCs at the indicated time points and used to generate cDNA that was analyzed by qRT-PCR. The cDNA concentrations were normalized to β-actin expression. The fold changes of Mrc1 transcripts of WT (•) and ICSBP–/– BM-DCs (○) are expressed relative to 0 days using the ΔΔCT method (“Materials and methods”). Results are representative of 3 separate experiments.
Increased Ag uptake of BM-DCs from ICSBP–/– mice. BM-DCs of ICSBP–/– and WT mice were cultured for 6 days in the presence of GM-CSF. (A) Cells were then incubated for 40 minutes with 1 mg/mL DX-FITC and then stained for CD11c and CD86 expression. Density plots represent the uptake of the DX and the relative expression of CD86 in a population gated on forward–side scatter properties and on CD11c positivity. Data are representative of 3 separate experiments. (B) qRT-PCR analysis of mannose receptor (Mrc1) transcript levels in BM-DCs at different times of culture. Total RNA was extracted from ICSBP–/– and WT BM-DCs at the indicated time points and used to generate cDNA that was analyzed by qRT-PCR. The cDNA concentrations were normalized to β-actin expression. The fold changes of Mrc1 transcripts of WT (•) and ICSBP–/– BM-DCs (○) are expressed relative to 0 days using the ΔΔCT method (“Materials and methods”). Results are representative of 3 separate experiments.
BM-DCs from ICSBP–/– mice are impaired in Ag presentation in vitro and in vivo. (A) BM-DCs of WT and ICSBP–/– mice were incubated for 40 minutes with 1 mg/mL OVA-FITC and then stained for CD11c and CD86 expression. Density plots represent the uptake of the OVA and the relative expression of CD86 in CD11c+-gated cells. Data are representative of 3 separate experiments. Three days later, the proliferation of CFSE+ cells in draining popliteal LN was evaluated by FACS. (B) WT and ICSBP–/– BM-DCs were magnetically sorted with anti-CD11c Microbeads and incubated with 1 mg/mL OVA for 40 minutes. Cells were washed, cultured overnight with 0.5 μg/mL LPS, and then assayed for their ability to stimulate the proliferation of OVA-specific OT-II CD4+ T cells. Thymidine incorporation was measured at the fourth day of coculture. Data represent the mean cpm of triplicate wells ± SD of 1 of 4 independent experiments. (C) BM-DCs were treated as in panel B and then analyzed by FACS for CD86 and I-A expression. (D) Sorted CD11c+ BM-DCs of WT and ICSBP–/– mice were loaded with OVA as in panel B. OVA-loaded DCs were injected in the footpads of WT recipients previously reconstituted with 5 × 106 CFSE-labeled OT-II CD4+ T cells. Three days later, proliferation of CFSE+ cells in draining popliteal LN was evaluated by FACS. Results are representative of 2 separate experiments.
BM-DCs from ICSBP–/– mice are impaired in Ag presentation in vitro and in vivo. (A) BM-DCs of WT and ICSBP–/– mice were incubated for 40 minutes with 1 mg/mL OVA-FITC and then stained for CD11c and CD86 expression. Density plots represent the uptake of the OVA and the relative expression of CD86 in CD11c+-gated cells. Data are representative of 3 separate experiments. Three days later, the proliferation of CFSE+ cells in draining popliteal LN was evaluated by FACS. (B) WT and ICSBP–/– BM-DCs were magnetically sorted with anti-CD11c Microbeads and incubated with 1 mg/mL OVA for 40 minutes. Cells were washed, cultured overnight with 0.5 μg/mL LPS, and then assayed for their ability to stimulate the proliferation of OVA-specific OT-II CD4+ T cells. Thymidine incorporation was measured at the fourth day of coculture. Data represent the mean cpm of triplicate wells ± SD of 1 of 4 independent experiments. (C) BM-DCs were treated as in panel B and then analyzed by FACS for CD86 and I-A expression. (D) Sorted CD11c+ BM-DCs of WT and ICSBP–/– mice were loaded with OVA as in panel B. OVA-loaded DCs were injected in the footpads of WT recipients previously reconstituted with 5 × 106 CFSE-labeled OT-II CD4+ T cells. Three days later, proliferation of CFSE+ cells in draining popliteal LN was evaluated by FACS. Results are representative of 2 separate experiments.
Last, we examined the ability of ICSBP–/– BM-DCs to present OVA to OT-II CD4+ T lymphocytes in vivo. BM-DCs were loaded with OVA and then injected in the hind footpads of WT recipients reconstituted with CFSE-labeled OT-II CD4+ T cells. We found that the ability of OVA-loaded ICSBP–/– BM-DCs to stimulate OT-II–cell proliferation was significantly reduced when compared with the activity of WT DCs (Figure 6D). In addition, recovery of CFSE-positive cells in mice primed with OVA-loaded BM-DCs from ICSBP–/– mice (0.79% of LN cells) was more than 4-fold less than the number of CFSE+ cells found in mice injected with WT DCs (3.35%; Figure 6D). Altogether, these data indicate that BM-DCs from ICSBP–/– mice are characterized by a high endocytic activity but a reduced ability to activate MHC-II–restricted T cells in vitro and in vivo, 2 hallmarks of immature DCs.
ICSBP–/– BM DCs fail to induce a CHS response when injected into WT host
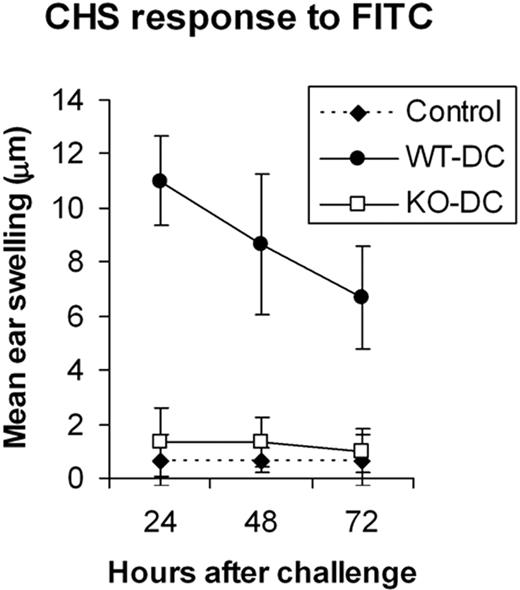
To further assess the ability of BM-DCs to prime a T-cell–mediated response in vivo, we examined their ability to sensitize mice for a CHS response to the hapten, FITC.36 BM-DCs from ICSBP–/– and WT mice were pulsed with FITC and injected in the footpad of WT hosts. Strikingly, only mice immunized with FITC-pulsed WT-DCs mounted a CHS response, as detected by specific ear swelling. No ear swelling was detected with recipients of FITC-pulsed DCs from ICSBP–/– mice (Figure 7).
Discussion
It is becoming increasingly clear that DCs comprise subsets of highly specialized cells primed to capture and to process Ags in distinct manners from other APCs. These distinctions are regulated by different activation stimuli in the developmental process termed “maturation.” Maturation links innate immunity with adaptive responses and plays a pivotal role in the discrimination of self-nonself differences that are orchestrated by DCs.2 It thus becomes extremely important to identify the factors and downstream signals that allow the maturation program to execute these distinctions. During the last few years, ICSBP has emerged as a transcription factor with a series of critical roles in regulating the differentiation and maturation of distinct subpopulations of DCs. Mice with a nonconditional knockout of ICSBP have essentially no plasmacytoid DCs and exhibit a selective reduction in the number and functionality of CD8α+ DCs, dermal DCs, and epidermal Langerhans cells.21-25 In addition, even DCs that develop in normal numbers in ICSBP–/– mice display a poorly differentiated phenotype, indicating that the developmental and maturation program of DCs may be controlled by ICSBP at multiple stages. In the present paper, we demonstrate that during the course of DC development, ICSBP is a crucial determinant for the ability of these cells to capture and to present exogenous Ags to MHC-II–restricted T lymphocytes.
Internalization of soluble Ags by DCs can occur by 3 major routes: micropinocytosis, involving fluid-phase uptake of small molecules (0.1 μm) via clathrin-coated pits; macropinocytosis, involving fluid-phase uptake of larger vesicles (0.5-3 μm) through cytoskeleton-dependent membrane ruffling; and receptor-dependent phagocytosis, mediated primarily by the MR. Both macropinocytosis and phagocytosis convey Ags to the endocytic route that leads to the presentation of derivative peptides by MHC-II molecules.4,37 In our studies, OVA-FITC, DX-FITC, and HRP were used as useful markers to monitor endocytosis in DCs of ICSBP–/– mice. OVA-FITC is known to be taken up exclusively by macropinocytosis, whereas DX-FITC and HRP use both the MR-dependent pathway and the macropinocytic route.2,4,5,33,34 We show that the ability of s-DCs of mutant mice to capture these antigens is greatly impaired. In particular, ICSBP–/– CD8α+ DCs were completely unable to capture DX and OVA, as revealed by both FACS and confocal microscopic analyses. Moreover, CD8α– s-DCs of ICSBP mice displayed impaired uptake of DX and, to a lesser extent, of OVA. These results were somehow unexpected, since s-DCs of ICSBP–/– mice, especially the CD8α+ subset, are characterized by a prominent immature phenotype,21,23 a feature that normally correlates with high endocytic potential. On the other hand, and consistent with their immature traits, ICSBP–/– BM-DCs were fully competent at capturing Ags. Precisely, uptake of DX or OVA was markedly increased in BM-DCs of ICSBP–/– mice compared with WT cells. Of note, the endocytic activity was exclusively observed in immature CD86–/low DCs, which represented the largest population in ICSBP–/– cultures. Thus, it appears that the enhanced level of endocytosis in ICSBP–/– BM-DCs is a result of a more immature phenotype of these cells. These findings suggest that ICSBP regulates Ag uptake only at late stages of DC development and is critical in maintaining efficient endocytic activity in s-DCs.
BM-DCs from ICSBP–/– mice fail to induce a CHS response in vivo. BM-DCs from WT and ICSBP–/– mice cultured for 7 days were magnetically sorted with anti-CD11c Microbeads and then labeled with FITC. FITC-loaded ICSBP–/– (□) and WT (•) DCs (5 × 105 cells) were then injected in the footpad of WT recipients. Six days after transfer, mice were challenged on 1 ear with 10 μL FITC. Ear thickness was measured on both ears 24 to 72 hours after challenge. Specific swelling was calculated by subtracting the thickness of the unchallenged ear from that of the FITC-challenged ear in each individual animal. Control swelling (♦) represents mice subjected to FITC challenge but not immunized. Results are expressed as the mean ± SD of at least 3 mice per group. One out of 3 separate experiments is shown.
BM-DCs from ICSBP–/– mice fail to induce a CHS response in vivo. BM-DCs from WT and ICSBP–/– mice cultured for 7 days were magnetically sorted with anti-CD11c Microbeads and then labeled with FITC. FITC-loaded ICSBP–/– (□) and WT (•) DCs (5 × 105 cells) were then injected in the footpad of WT recipients. Six days after transfer, mice were challenged on 1 ear with 10 μL FITC. Ear thickness was measured on both ears 24 to 72 hours after challenge. Specific swelling was calculated by subtracting the thickness of the unchallenged ear from that of the FITC-challenged ear in each individual animal. Control swelling (♦) represents mice subjected to FITC challenge but not immunized. Results are expressed as the mean ± SD of at least 3 mice per group. One out of 3 separate experiments is shown.
Although the mechanisms by which ICSBP controls the endocytic activity of s-DCs remain to be determined, we hypothesize that this function may be switched off at some point during maturation of these cells through the control of different processes. For instance, the strong impairment in the internalization of mannosylated Ags, such as DX and HRP, observed with ICSBP–/– s-DCs, may be correlated, at least in part, with reduced expression of MR. The idea that MR expression is regulated by ICSBP is worth considering since transcriptional regulation of MR gene is known to be under the control of PU.1, a transcription factor which interacts with ICSBP to bind target elements.19,38 In this regard, the high efficiency of DX uptake by ICSBP–/– BM-DCs correlated with elevated MR mRNA levels. On the other hand, since receptor-independent endocytosis of OVA was also impaired in s-DCs of ICSBP–/– mice, other mechanisms have to be taken into account. Thus, ICSBP may also be involved in structural mechanisms that allow macropinosomes to be adequately transported from the cell surface to the cytosol. This concept is supported by the strong reduction of HRP-containing macropinosomal vesicles in the cytosol of ICSBP–/– s-DCs, which indicates a defect in the internalization process used by these cells. In this regard, polymerization of cytoskeleton-bound actin is a key event regulating the selective mobilization of macropinosomes into DCs and affects both macropinocytosis and receptor-mediated endocytosis.39,40 The organization of actin cytoskeleton is regulated by the Rho family of guanosine triphosphatases (GTPases), whose activity is finely regulated during DC maturation and controls both the rates of endocytosis and the interaction of DCs with T cells during T-cell priming.41,42 Among these small GTPases, Rac1 and Cdc42 are the best-characterized members implicated in DC endocytosis.43-45 Interestingly, selective inhibition of Rac1 in DCs results in reduced endocytic activity and impaired development of murine CD8α+ DCs, 2 features shared with ICSBP–/– mice.46 Therefore, it may be hypothesized that ICSBP controls the endocytic potential of DCs through both the regulation of MR expression and possibly by affecting processes involved in the organization of acting cytoskeleton.
Ags that access the endocytic route of DCs are processed and loaded onto MHC-II molecules for presentation to naive CD4+ T cells.47 In our studies, we found that defective Ag uptake by s-DCs of ICSBP–/– mice reflects a functional impairment in presenting the Ag to MHC-II–restricted T cells. In fact, ICSBP–/– s-DCs were remarkably ineffective at presenting injected soluble OVA to OT-II CD4+ T cells both in vitro and in vivo. Although the impaired ability of ICSBP–/– s-DCs to stimulate CD4+ T cells strongly correlates with the incapacity of these cells to effectively capture Ags, we observed a complete lack of endocytic activity only in ICSBP–/– CD8α+ DCs, a subset that comprises a minor fraction of total s-DCs (3% in ICSBP–/– mice, 25%-30% in WT mice).21 Several studies indicate that in the MHC-II context, CD8α– s-DCs exhibit higher potential for the presentation of exogenous Ags, whereas CD8α+ s-DCs are specialized for presentation of cell-associated Ags.12,14,16,48 Thus, it can be envisaged that ICSBP–/– CD8α– s-DCs may convey additional functional alterations resulting in defective stimulation of CD4+ T cells. In this regard, previous work showed that CD8α– DCs of ICSBP–/– mice express reduced levels of MHC-II following activation in vitro or in vivo.21,23 Consistently, we found that exposure to LPS resulted in defective activation of ICSBP–/– s-DCs. On the other hand, ICSBP–/– s-DCs loaded in vitro with OVA peptide are able to stimulate OT-II CD4+ T cells as effectively as WT s-DCs49 (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). As peptide loading does not involve Ag internalization and processing, but direct recruitment to surface MHC molecules, these data suggest that when Ag is appropriately loaded onto MHC-II, ICSBP–/– s-DCs are competent at stimulating CD4+ T cells. In this view, the defects in Ag uptake in ICSBP–/– s-DCs appear to affect the fate of MHC-II Ag presentation critically. Deficiencies during the maturation process may ultimately contribute to the defective T-cell stimulatory ability of ICSBP critically s-DCs. Nonetheless, other pathways may be disrupted in ICSBP critically s-DCs, namely Ag processing into peptides, peptide targeting to MHC-II molecules in endocytic compartments, and transport of MHC-II peptide complexes to the cell surface. Of interest, a recent report showed that ICSBP directly regulates 3 cytoskeleton-related genes and 4 endosomal/lysozomal enzyme–related genes.50
Regardless of the mechanisms involved, it appears clear that ICSBP regulates endocytosis and Ag presentation independently and at distinct stages of DC development. This concept is supported by our finding that while ICSBP critically BM-DCs exhibit increased endocytic activity, these cells are significantly impaired at presenting OVA to CD4+ T lymphocytes both in culture and after in vivo injection into competent WT hosts. The impaired Ag-presenting function by ICSBP critically BM-DCs in vivo was revealed by the markedly reduced proliferation of OVA-specific CFSE-labeled OT-II cells as well as by the failure to induce a CHS response to FITC. From an immunologic perspective, CHS can be considered as a prototype of T-cell–mediated delayed-type hypersensitivity and is a reliable in vivo approach to follow the whole process of immune responses to some haptens where CD4+ T cells are thought to play a pivotal role.51,52 One mechanism that may account for defective MHC-II–restricted stimulation of T cells in vivo by Ag-loaded ICSBP–/– BM-DCs may stem from a defective ability of these cells to migrate from the injection site to the draining LN. This view is based on the observations that ICSBP–/– BM-DCs express low levels of CCR7 as well as impaired chemotactic response to the CCR7 ligands Mip-3β and 6CKine.25 However, the finding that ICSBP–/– BM-DCs were also impaired at stimulating CD4+ T cells in vitro clearly suggests that ICSBP–/– BM-DCs may have an intrinsic defect to undergo full maturation and to effect T-cell stimulation. This concept is supported by the finding that treatment with LPS did not activate ICSBP–/– BM-DCs as effectively as WT cells in terms of CD86 and MHC-II expression as well as of CD4+ T-cell stimulatory capacity, thus confirming the previously noted defective responses by BM-DCs of ICSBP–/– mice to maturation signals.24
In summary, our study demonstrates that in addition to its known roles in driving DC development, ICSBP critically affects key DC functions, including endocytosis of exogenous Ags and Ag-specific stimulation of CD4+ T cells. We suggest that ICSBP affects the capacity of lymphoid organ DCs to present blood-borne Ags to naive CD4+ T cells by controlling DC endocytosis and maturation. In parallel, ICSBP influences MHC-II presentation in BM-DCs by regulating the maturation process of these cells. Of interest, the concept that ICSBP is central in preserving the Ag uptake function of CD8α+ s-DCs may be particularly relevant since these cells are endowed with the unique ability to cross-present cell-associated antigens to CD8+ T cells, a crucial mechanism underlying immune responses to viral diseases and cancer.53 Given the potential of DCs to promote antitumor immunity due to their unique ability to prime T-cell responses, DC-based therapies hold promise for developing new effective immunoadjuvant therapies for cancer. Our studies clearly suggest that ICSBP may represent a crucial factor to be considered in efforts to enhance the immunopotency of therapeutic DCs.
Prepublished online as Blood First Edition Paper, March 28, 2006; DOI 10.1182/blood-2005-11-4490.
Supported by grants from the Italian Association for Cancer Research (Project AIRC n. F89), the Italian Ministry of Health (special project titled “Dendritic Cells and Type I IFN”), and Fondo per gli Investimenti della Ricerca di Base (FIRB)–Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) (Project RBNE01N9EE).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Annamaria Fattapposta and Angela Fresolone for excellent secretarial assistance. Special thanks also go to Maria Teresa D' Urso and Anna Maria Pacca for their excellent technical assistance with breeding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal