While extrahepatic factor VIII (FVIII) synthesis suffices for hemostasis, the extrahepatic production sites are not well defined. We therefore investigated the ability of the human lungs to produce FVIII. Lungs from heart-beating donors who were declined for transplantation were perfused and ventilated in an isolated reperfusion model for 2 hours. A progressive accumulation of FVIII and von Willebrand factor (VWF) was recorded in the perfusion medium in 3 of 4 experiments. By contrast, factor V, fibrinogen, and immunoglobulin G (IgG) levels remained constant during the perfusion period, indicating that the accumulation of FVIII and VWF was not due to diffusion from the intercellular medium into the vascular system. Purified human lung microvascular endothelial cells produced FVIII during at least 2 passages in vitro. Altogether, these data identify the lung endothelial cells as a FVIII production site in humans.
Introduction
Factor VIII (FVIII) is synthesized in the liver, although additional production sites exist but are not yet identified. Transplantation of a normal liver to a recipient with hemophilia A corrects plasma FVIII levels,1,2 whereas transplantation of the liver of a hemophilic dog to a normal animal does not change the phenotype of the receiver into that of a hemophilic animal.3
Extrahepatic FVIII synthesis sites are important for the acute and chronic regulation of FVIII production, as illustrated by the normal or increased FVIII levels during severe liver diseases, which result in low plasma levels of the other coagulation factors.4 However, the study of the regulation of extrahepatic FVIII production has been hampered by the lack of unequivocal identification of extrahepatic tissues able to produce FVIII.1,3,5-9
Here we report that perfused human lungs and pulmonary microvascular endothelial cells produce FVIII.
Study design
Ex vivo lung perfusion study
Organs from brain-dead donors were recovered in accordance with the Belgian Transplantation Law. The Institutional Review Board approved a study protocol on ex vivo reperfusion of human lungs following extensive preservation studies on porcine lungs.10
Donor lungs were flushed with 4°C low-potassium dextran glucose (Perfadex; Vitrolife AB, Gothenburg, Sweden) and inflated with 50% oxygen. The double lung blocks, stored at 4°C in low-potassium dextran for less than 12 hours, were mounted in the perfusion/ventilation system as described.10 The system was filled with 2 L of ABO-compatible leukocyte-depleted erythrocyte concentrate diluted to a hematocrit of 15% with Steen Solution (Vitrolife AB). This extracellular medium was finalized with CaCl2, heparin, nitroglycerin, and sodium bicarbonate.
Lungs were reperfused for 120 minutes at 15 mmHg with deoxygenated perfusate at 37°C and ventilated with 100 mL air/min/kg donor body weight. Pulmonary physiologic variables remained stable during the total perfusion time. When indicated, the medium was supplemented with 100 nM phorbol 12-myristate 13-acetate (PMA; Sigma, Bornem, Belgium) or 1 pg/mL desmopressin acetate (Ferring, Malmö, Sweden). As control, complete medium was perfused for 2 hours with no lung inserted in the system.
FVIII activity (FVIII:C) in 5-fold–diluted perfusion medium was measured using a chromogenic assay (Dade Behring, Marburg, Germany). Factor V (FV) and fibrinogen were measured using the FV and Fibrinogen enzyme-linked immunosorbent assay (ELISA) sets, respectively (Kordia, Leiden, The Netherlands). FVIII:antigen (Ag), von Willebrand factor (VWF), and IgG were measured by ELISA.11,12 A pool of human plasma was used as standard. To compare the variation of one variable to another in a perfusion experiment, the concentrations measured at each time point were divided by the concentration at 15 minutes. A 2-sided Wilcoxon paired test was used to compare the ratios.
Microvascular lung endothelial culture
Human adult pulmonary microvascular endothelial cells (HPMECs) were obtained from normal portions of lung specimens surgically resected from patients who underwent lobectomies for early-stage lung cancer. The study was approved by the Ethics Committee of the University of Mainz and informed consent as defined by the Helsinki declaration was obtained from each patient. About 99% pure HPMEC preparations were isolated from lung specimens by 2 separations with CD31 beads (Dynal, Hamburg, Germany) as previously described.13 Alveolar macrophages were purified by Ficoll density gradient both after mechanically disrupting the lung parenchyma and after enzymatic treatment of the minced lung tissue with dispase. Fibroblasts, smooth muscle cells, and epithelial cells contaminating the HPMEC preparations were enriched by immunodepletion of endothelial cells with CD31 beads.13 The cells were cultivated in endothelial cell growth medium for microvascular cells (ECGM-MV; PromoCell, Heidelberg, Germany) on gelatin-coated tissue-culture flasks.
FVIII:C of culture supernatants and plain medium was measured with a FVIII chromogenic assay (Dade Behring). In control experiments, cell-culture supernatants were incubated with a human monoclonal antibody to FVIII (B02C11; 5 μg/mL14 ) before measuring residual FVIII:C. A 2-sided Wilcoxon paired test was used to compare the activities recorded in the presence and absence of BO2C11.
Results and discussion
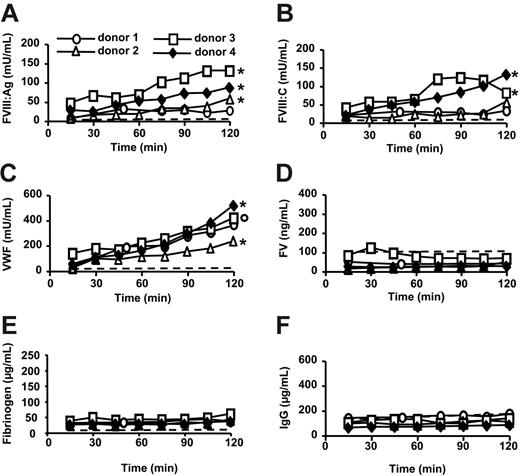
A significant FVIII:Ag accumulation was recorded during the first 45 minutes of perfusion when comparing with FV, fibrinogen, or IgG concentration (P ≤ .02; Figure 1A,D-F). The amounts of FVIII:Ag and FVIII:C released during the first 45 minutes were 19 ± 14 mU/mL and 20 ± 7 mU/mL (mean ± SD; n = 4), respectively, which corresponds to a total production of about 50 U FVIII/h. A significant accumulation of FVIII was recorded up to 120 minutes in donor 4 (P ≤ .03 compared with FV, fibrinogen, or IgG concentration; Figure 1A-B,D-F).
FVIII secretion is dependent on the presence of VWF.15,16 VWF concentrations significantly increased during the first 45 minutes of perfusion when comparing with FV, fibrinogen, or IgG concentrations (P < .01; Figure 1C-F). During this time period, VWF accumulation reached 113 ± 37 mU/mL (mean ± SD; n = 4). The VWF/FVIII:Ag ratio was 6.3 ± 2.7 (mean ± SD; n = 4), whereas in plasma that ratio is 1, which is in agreement with the fact that most FVIII is produced by the liver.1-3
FVIII, VWF, IgG, FV, and fibrinogen levels in ex vivo perfusion medium of human lungs. Double lung blocks retrieved from brain-dead donors were ventilated through an endotracheal tube and perfused for 2 hours (donor 4). Alternatively, the perfusion medium was supplemented with desmopressin acetate (donor 1 and 2) or PMA (donor 3) 60 minutes after the start of the perfusion. In a control experiment, erythrocytes in complete medium were perfused for 2 hours with no lung inserted in the system (dashed line). FVIII:Ag (A), FVIII:C (B), VWF (C), FV (D), fibrinogen (E) and IgG (F) were measured in the perfusion medium. To compare the variations of FVIII:Ag, FVIII:C, or VWF concentrations to those of FV, fibrinogen, or IgG, the values of each variable in a whole perfusion experiment were divided by the corresponding concentration measured at 15 minutes. A 2-sided Wilcoxon paired test was then used to compare FVIII:Ag, FVIII:C, or VWF ratios with FV, fibrinogen, or IgG ratios obtained at the corresponding time points in the same perfusion experiment; °P ≤ .06; *P ≤ .03 for each of the comparisons with FV, fibrinogen, or IgG.
FVIII, VWF, IgG, FV, and fibrinogen levels in ex vivo perfusion medium of human lungs. Double lung blocks retrieved from brain-dead donors were ventilated through an endotracheal tube and perfused for 2 hours (donor 4). Alternatively, the perfusion medium was supplemented with desmopressin acetate (donor 1 and 2) or PMA (donor 3) 60 minutes after the start of the perfusion. In a control experiment, erythrocytes in complete medium were perfused for 2 hours with no lung inserted in the system (dashed line). FVIII:Ag (A), FVIII:C (B), VWF (C), FV (D), fibrinogen (E) and IgG (F) were measured in the perfusion medium. To compare the variations of FVIII:Ag, FVIII:C, or VWF concentrations to those of FV, fibrinogen, or IgG, the values of each variable in a whole perfusion experiment were divided by the corresponding concentration measured at 15 minutes. A 2-sided Wilcoxon paired test was then used to compare FVIII:Ag, FVIII:C, or VWF ratios with FV, fibrinogen, or IgG ratios obtained at the corresponding time points in the same perfusion experiment; °P ≤ .06; *P ≤ .03 for each of the comparisons with FV, fibrinogen, or IgG.
Neither desmopressin acetate nor PMA substantially increased the rate of FVIII or VWF secretion (Figure 1A-C), possibly due to alteration of Weibel-Palade body physiology17-19 by cold preservation.20,21 In addition, lungs from donor 1 showed signs of severe edema at the onset of perfusion.
It is unlikely that the accumulation of FVIII and VWF was due to release of plasma from vessels reopened during ex vivo perfusion or to diffusion of FVIII and VWF from the intercellular fluid to the perfusion medium. Indeed, extensive flushing of the lungs had been performed immediately after organ collection and the levels of FV, fibrinogen, and IgG in the perfusion medium did not increase during ex vivo perfusion experiments (Figure 1D-E). Neither FVIII nor VWF was released from erythrocytes in perfusion medium (Figure 1A-C).
Altogether, the data indicated that the human lung is a site of release of FVIII, whether synthesized or stored locally. As genetically manipulated human umbilical vein endothelial cells can produce FVIII,22 we determined whether isolated HPMECs secreted FVIII. After passages 1 and 2 in vitro, a significant FVIII production was observed in 6 of 7 HPMEC cultures (Table 1). FVIII:C activity was inhibited by an antibody to FVIII (Table 1). Fibroblasts, smooth muscle cells, or epithelial cells in HPMEC preparations are unlikely to contribute to FVIII production because FVIII was undetectable in the supernatant of enriched contaminating cells obtained by immunodepletion of CD31+ cells. In addition, cultures of purified lung macrophages did not contain any measurable FVIII.
FVIII and VWF production by purified human lung microvascular endothelial cells
Donor . | Passage (d)* . | FVIII, mU/mL . | Inhibition, %† . | VWF, mU/mL . |
|---|---|---|---|---|
| 5 | 1 (4) | 2.0 | > 95 | 0.4 |
| 6 | 1 (4) | < 1.5 | ND | < 0.1 |
| 7 | 2 (6) | 6.0 | > 95 | 16.0 |
| 5 | 2 (4) | 1.5 | ND | 9.4 |
| 5 | 2 (6) | 1.5 | ND | 18.1 |
| 6 | 2 (4) | 2.5 | > 95 | 15.3 |
| 6 | 2 (6) | 7.0 | > 95 | 23.7 |
Donor . | Passage (d)* . | FVIII, mU/mL . | Inhibition, %† . | VWF, mU/mL . |
|---|---|---|---|---|
| 5 | 1 (4) | 2.0 | > 95 | 0.4 |
| 6 | 1 (4) | < 1.5 | ND | < 0.1 |
| 7 | 2 (6) | 6.0 | > 95 | 16.0 |
| 5 | 2 (4) | 1.5 | ND | 9.4 |
| 5 | 2 (6) | 1.5 | ND | 18.1 |
| 6 | 2 (4) | 2.5 | > 95 | 15.3 |
| 6 | 2 (6) | 7.0 | > 95 | 23.7 |
ND indicates not done.
Passage number, with days after passage given in parentheses.
Cell-culture supernatant was mixed with the human monoclonal antibody BO2C11 and the residual FVIII activity measured after 15 minutes of incubation at room temperature. The results are expressed as the percentage inhibition of FVIII activity compared with samples incubated without antibody. The sensitivities of the FVIII and of the VWF assays were 1.5 mU/mL and 0.1 mU/mL, respectively.
The maximal rate of FVIII synthesis recorded in in vitro experiments was 1.3 mU FVIII/48 h/cm2 of confluent HPMECs. Taking into account that the surface area of capillary endothelium in the lungs of a healthy individual is evaluated at 120 m2,23 such a production rate would represent a production of 32 U FVIII/h. By comparison, FVIII turnover in an adult of 70 kg has been evaluated at about 140 U/h.24,25
Altogether, these data identify the human lung as a significant source of FVIII. This first identification of extrahepatic cells spontaneously producing FVIII opens the way to the study of the acute and chronic regulation of FVIII production.
Prepublished online as Blood First Edition Paper, March 28, 2006; DOI 10.1182/blood-2005-11-4571.
Supported by grant E/B41G/1G302/1A402 from the German Bundesministerium für Verteidigung (BMVg) and by grants from the Flemish Research Foundation (G.0231.05 and G.0093.02) and from the Catholic University Leuven (OT/03/55). D.V.R. is a senior research fellow of the Flemish Research Foundation (FWO G3C04.99).
A.N., D.V.R., and F.R. designed research, performed lung ex vivo perfusion, analyzed data, and wrote the paper; M.I.H. and C.J.K. designed research, performed cell culture, analyzed data, and wrote the paper; R.L. designed research and measured protein concentration; and M.J., K.P., and J.-M.S.-R. designed research, analyzed data, and wrote the paper.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Caroline Van de Wauwer, Nele Geudens, Nicole Jannis, and Magda Mathys for their technical and administrative support, Michaela Bock for her assistance with the cell culture, and Jos Vermylen for discussions and comments on the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal