Platelet activation plays a central role in hemostasis and thrombosis. Many platelet agonists function through G-protein–coupled receptors. Epinephrine activates the α2A-adrenergic receptor (α2A) that couples to Gz in platelets. Although α2A was originally cloned from platelets, its role in thrombosis and hemostasis is still unclear. Through analysis of α2A-deficient mice, variable tail bleeding times were observed. In vitro, epinephrine potentiated activation/aggregation responses of wild-type but not α2A-deficient platelets as determined by flow cytometry and aggregometry, whereas perfusion studies showed no differences in platelet adhesion and thrombus formation on collagen. To test the in vivo relevance of α2A deficiency, mice were subjected to 3 different thrombosis models. As expected, α2A-deficient mice were largely protected from lethal pulmonary thromboembolism induced by the infusion of collagen/epinephrine. In a model of FeCl3-induced injury in mesenteric arterioles, α2A–/– mice displayed a 2-fold increase in embolus formation, suggesting thrombus instability. In a third model, the aorta was mechanically injured, and blood flow was measured with an ultrasonic flow probe. In wild-type mice, all vessels occluded irreversibly, whereas in 24% of α2A-deficient mice, the initially formed thrombi embolized and blood flow was reestablished. These results demonstrate that α2A plays a significant role in thrombus stabilization.
Introduction
At sites of vessel wall injury, components of the extracellular matrix (ECM) are exposed to the flowing blood, which triggers sudden platelet activation and platelet plug formation and is followed by coagulant activity and the formation of fibrin-containing thrombi that occlude the site of injury. These events are crucial to prevent posttraumatic blood loss, but they are also a major pathologic mechanism underlying arterial thrombosis.
Initial tethering and adhesion of platelets requires the action of surface receptors that function irrespectively of the activation state of the cell, namely GPIb-IX-V and the major collagen receptor GPVI, which then initiate signaling cascades and lead to platelet activation.1 However, once platelets have adhered to the ECM, extension of the thrombus requires rapid response of platelets to locally produced or released soluble agonists, including adenosine diphosphate (ADP), thrombin, thromboxane A2 (TXA2), and epinephrine. These agonists amplify and sustain the initial platelet responses and recruit circulating platelets from the flowing blood into a growing hemostatic plug. ADP and its receptors, P2Y1 and P2Y12, represent one well-described positive feedback system in this process. ADP is released from internal stores of activated cells and, by binding to its receptors, potentiates numerous platelet responses, including integrin αIIbβ3 activation, dense granule secretion, and platelet procoagulant activity. In vivo, P2Y1 and P2Y12 null mice display impaired hemostasis and thrombosis.2-4 The relevance of 2 other amplifiers of the platelet response, thrombin and TXA2, has also been demonstrated in vitro and in vivo.5,6
In contrast, little is known about the function of epinephrine within the process of platelet activation. Epinephrine and norepinephrine are endogenous catecholamines that belong to the family of biogenic amines. As neurotransmitters of the adrenergic nervous system, they are released from nerves or from adrenal chromaffin cells in response to physical or mental stress. It was reported that epinephrine can be taken up by human platelets and is concentrated and stored in platelet dense granules.7-9 Epinephrine is a weak activator of platelets and induces platelet responses through the α2A-adrenergic receptor, which is a low-copy receptor with approximately 300 molecules expressed per platelet.10-12 The activated α2A receptor contributes to platelet activation through Gz,13,14 which belongs to the Gi family.
In this study, we used α2A-deficient (–/–) mice15 to investigate the role of the receptor in platelet function in vitro and in vivo. Our results demonstrate that the α2A adrenergic receptor plays a significant role in hemostasis and in the formation and stabilization of pathologic thrombi in vivo.
Materials and methods
Animals
All experiments and care were approved by the local animal care and use committee. Mice lacking the α2A receptor (α2A–/–) were produced as described.15 α2A–/– mice were crossed back onto a C57BL6/J background for 12 generations. Wild-type C57BL6/J mice were used as controls.
Flow cytometry
Heparinized whole blood was diluted 1:20 with modified Tyrode-HEPES buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.0) containing 5 mM glucose, 0.35% bovine serum albumin (BSA), and 1 mM CaCl2. For glycoprotein expression and platelet count, blood samples were incubated with appropriate fluorophore-conjugated monoclonal antibodies for 15 minutes at room temperature and were directly analyzed on a FACScalibur (Becton Dickinson, Heidelberg, Germany). Activation studies were performed with blood samples washed twice with modified Tyrode-HEPES buffer, which then were activated with ADP or U46619 in the presence or absence of epinephrine for 15 minutes, stained with fluorophore-labeled antibodies16 for 15 minutes at room temperature, and directly analyzed.
Aggregometry
To determine platelet aggregation, light transmission was measured using platelet-rich plasma (PRP) (200 μL with 0.5 × 106 platelets/μL). Transmission was recorded in a Fibrintimer 4-channel aggregometer (APACT Laborgeräte und Analysensysteme, Hamburg, Germany) over 10 minutes and was expressed as arbitrary units with 100% transmission adjusted with plasma. Platelet aggregation was induced by the addition of U46619 (Alexis, Lausen, Switzerland) (1 or 0.1 μM) and ADP (5 μM) in the presence or absence of epinephrine (10 μM).
Adhesion under flow conditions
Rectangular coverslips (24 × 60 mm) were coated with 0.25 mg/mL fibrillar type I collagen (Nycomed, Munich, Germany) for 1 hour at 37°C and were blocked with 1% BSA. Perfusion of heparinized whole blood was performed as described.17 Briefly, transparent flow chambers with a slit depth of 50 μm, equipped with the collagen-coated coverslips, were rinsed with HEPES buffer and connected to a syringe filled with the anticoagulated blood. Perfusion was carried out at room temperature using a pulse-free pump at low (150 seconds–1) and high (1000 seconds–1) shear stress. During perfusion, microscopic phase-contrast images were recorded in real time. Thereafter, the chambers were rinsed by a 10-minute perfusion with HEPES buffer, pH 7.45, at the same shear stress, and phase-contrast pictures were recorded from at least 5 different microscopic fields (63× objectives). Image analysis was performed off-line using Metamorph software (Visitron, Munich, Germany). Thrombus formation results are expressed as the mean percentage of total area covered by thrombi.
Bleeding time experiments
Mice were anesthetized, and a 3-mm segment of the tail tip was cut off with a scalpel. Tail bleeding was monitored by gentle absorption of the bead of blood with a filter paper without contacting the wound site. When no blood was observed on the paper after a 15-second interval, bleeding was determined to have ceased.18 The experiment was stopped after 20 minutes.
Pulmonary thromboembolism model
Mice were anesthetized by intraperitoneal injection of Avertin (2,2,2-tribromoethanol and 2-methyl-2-butanol; Sigma), 0.15 mL/10 g body weight from 2.5% solution. Anesthetized mice received a mixture of collagen (0.8 mg/kg) and epinephrine (60 μg/kg) injected into the jugular vein. The incisions of surviving mice were stitched, and the mice were allowed to recover.
In vivo thrombosis model with FeCl3-induced injury
Mouse blood (1 vol) was collected into 0.5 vol HEPES buffer containing 20 U/mL heparin. The blood was centrifuged at 250g for 10 minutes, and platelet-rich plasma was gently transferred to a fresh tube. Platelets were labeled with 5-carboxyfluorescein diacetate succinimidyl ester (DCF) and adjusted to a final concentration of 200 × 106 platelets/250 μL.
Male and female mice (age range, 4-5 weeks) were anesthetized as described. Fluorescently labeled platelets were injected intravenously, and mesentery was gently exteriorized through a midline abdominal incision. Arterioles (35- to 60-μm diameter) were visualized with a Zeiss Axiovert 200 inverted microscope (× 10) equipped with a 100-W HBO fluorescent lamp source and a CCD camera (CV-M300) connected to an S-VHS video recorder (AG-7355; Panasonic, Matsushita Electric, Tokyo, Japan). Injury was performed by 10-second topical application of a 3-mm2 filter paper tip saturated with FeCl3 (20%). This caused the formation of free radicals and subsequent endothelium denudation that allowed circulated platelets to come in contact with subendothelial matrix. Arterioles were monitored for 40 minutes or until complete occlusion occurred (blood flow stoppage for more than 1 minute). Firm platelet adhesion was determined as the number of fluorescently labeled platelets that deposited on the vessel wall until 5 minutes after injury. A thrombus was defined as a platelet aggregate larger than 10 μm in diameter. Thrombus stability was characterized as the number of embolus events (thrombi larger than 10 μm in diameter) that detached during the observation period from the viewing field.
Aorta occlusion model
The abdominal cavity of anesthetized mice was longitudinally opened, and the abdominal aorta was prepared. An ultrasonic flow probe was placed around the aorta, and thrombosis was induced by one firm compression with a forceps. Blood flow was monitored until complete occlusion occurred; otherwise, experiments were stopped manually after 40 minutes.
Statistical analysis
Statistical analysis was performed with the Wilcoxon rank test.
Results
The role of α2A in platelet function was analyzed by comparing wild-type and α2A–/– mice. α2A adrenergic receptor–deficient mice did not show signs of spontaneous bleeding. Rather, they developed normally, were fertile, and appeared healthy.
Platelets from α2A–/– mice respond normally to thrombin-, CRP-, or collagen-induced activation
Wild-type and α2A-deficient mice had comparable platelet counts, as detected by flow cytometry (897 × 109 ± 226 × 109 platelets/μL vs 910 × 109 ± 150 × 109 platelets/μL). Deletion of α2A from the platelet surface did not lead to changes in any other membrane receptors tested, including GPIb and αIIbβ3 (Table 1).
Glycoprotein expression in α2A–/– mice
Glycoprotein . | Wild-type mice . | α2A-/- mice . |
|---|---|---|
| GPVI | 49.1 ± 2.3 | 48.6 ± 3.2 |
| Integrin α∥β3 | 396.1 ± 83.7 | 353.4 ± 46.6 |
| GPV | 264.6 ± 38.6 | 269.4 ± 26.4 |
| GPlbα | 641.8 ± 128.1 | 656.0 ± 71.4 |
| GPIX | 433.4 ± 60.4 | 405.6 ± 39.9 |
| Integrin α2 | 38.2 ± 2.3 | 36.6 ± 1.9 |
| Integrin β1 | 147.0 ± 38.8 | 138.1 ± 27.3 |
| CD9 | 1225.4 ± 106.8 | 1192.5 ± 85.7 |
Glycoprotein . | Wild-type mice . | α2A-/- mice . |
|---|---|---|
| GPVI | 49.1 ± 2.3 | 48.6 ± 3.2 |
| Integrin α∥β3 | 396.1 ± 83.7 | 353.4 ± 46.6 |
| GPV | 264.6 ± 38.6 | 269.4 ± 26.4 |
| GPlbα | 641.8 ± 128.1 | 656.0 ± 71.4 |
| GPIX | 433.4 ± 60.4 | 405.6 ± 39.9 |
| Integrin α2 | 38.2 ± 2.3 | 36.6 ± 1.9 |
| Integrin β1 | 147.0 ± 38.8 | 138.1 ± 27.3 |
| CD9 | 1225.4 ± 106.8 | 1192.5 ± 85.7 |
Platelet glycoprotein expression of wild-type and α2A adrenergic knockout mice as determined by flow cytometry. Results are expressed as mean ± SD fluorescence for groups of 6 mice.
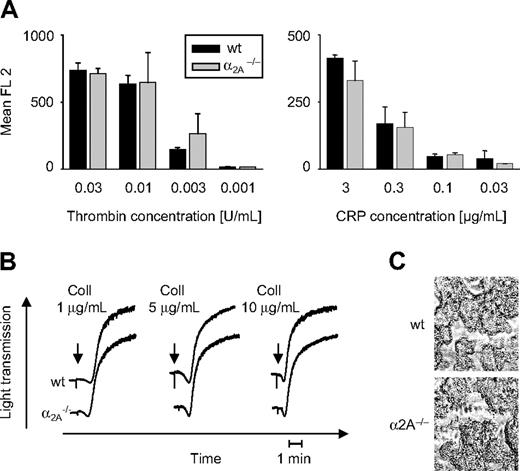
Dose-dependent activation of the αIIbβ3 receptor (Figure 1A) and P-selectin expression (not shown) induced by thrombin and collagen-related peptide (CRP) was not affected in platelets lacking α2A. Platelet aggregation in response to several collagen concentrations was not influenced by α2A deficiency (Figure 1B). Whole blood perfusion studies, performed under high (1000 seconds–1) shear flow conditions over a collagen-coated surface, did not show any difference in the surface coverage of formed thrombi from wild-type and α2A–/– blood samples (60% ± 15% vs 61% ± 14%) (Figure 1C). The same was true for perfusion studies performed under conditions of low shear (150 seconds–1) (not shown).
Defective epinephrine responses in α2A-deficient platelets
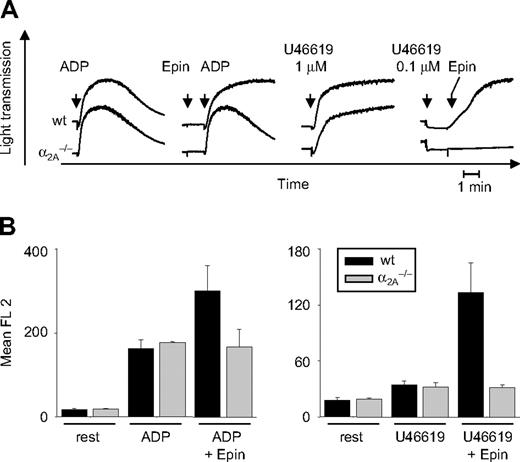
Aggregometry studies were performed with PRP obtained from wild-type and α2A–/– mice. ADP (5 μM) induced reversible aggregation of α2A–/– and wild-type platelets. In combination with epinephrine (10 μM), which alone did not induce aggregation, platelets from wild-type mice responded with full, irreversible aggregation. In contrast, epinephrine failed to enhance ADP-induced aggregation of α2A-deficient platelets (Figure 2A). High concentrations of the stable TxA2 analog U46619 (1 μM) induced full aggregation of wild-type and α2A–/– platelets. At low concentration (0.1 μM) of the agonist, wild-type and α2A–/– platelets responded with a pronounced shape change, as shown by the decrease in light transmission, but did not aggregate. However, the subsequent addition of epinephrine (10 μM) to these samples caused full aggregation of wild-type, but not α2A–/–, platelets (Figure 2A). The same effect was seen at higher concentrations of epinephrine (up to 100 μM), showing that this agonist is without effect on α2A–/– platelets under conditions of standard aggregometry.
Unaltered platelet responses to thrombin and collagen in α2A–/– mice. (A) Diluted whole blood was incubated with different concentrations of thrombin and CRP, as indicated. Activation of αIIbβ3 and P-selectin expression (not shown) was analyzed by flow cytometry. Results are presented as mean ± SD of 5 mice per group. (B) Heparinized PRP was stimulated with indicated concentrations of fibrillar collagen (“Coll”), and light transmission was recorded on a Fibrintimer 4-channel aggregometer. Results shown are representative of 6 individual experiments. (C) Whole blood was perfused over a “Horm-type” collagen-coated coverslip at high shear (1000 seconds–1). Representative phase-contrast images were taken at the end of the experiment. Images were captured with a Zeiss Axiovert 200 inverted microscope (Carl Zeiss, Jena, Germany) with a 63 ×/0.75 Ph2 objective, a 100-W HBO fluorescent lamp source, and a CCD camera (CV-M300; Visitron Systems, Puchheim, Germany) connected to an AG-7355 S-VHS video camera (Panasonic, Matsushita Electric, Osaka, Japan). Videotaped images were evaluated using a computer-assisted image analysis program, Meta View Version 5.0 (Visitron Systems).
Unaltered platelet responses to thrombin and collagen in α2A–/– mice. (A) Diluted whole blood was incubated with different concentrations of thrombin and CRP, as indicated. Activation of αIIbβ3 and P-selectin expression (not shown) was analyzed by flow cytometry. Results are presented as mean ± SD of 5 mice per group. (B) Heparinized PRP was stimulated with indicated concentrations of fibrillar collagen (“Coll”), and light transmission was recorded on a Fibrintimer 4-channel aggregometer. Results shown are representative of 6 individual experiments. (C) Whole blood was perfused over a “Horm-type” collagen-coated coverslip at high shear (1000 seconds–1). Representative phase-contrast images were taken at the end of the experiment. Images were captured with a Zeiss Axiovert 200 inverted microscope (Carl Zeiss, Jena, Germany) with a 63 ×/0.75 Ph2 objective, a 100-W HBO fluorescent lamp source, and a CCD camera (CV-M300; Visitron Systems, Puchheim, Germany) connected to an AG-7355 S-VHS video camera (Panasonic, Matsushita Electric, Osaka, Japan). Videotaped images were evaluated using a computer-assisted image analysis program, Meta View Version 5.0 (Visitron Systems).
The defective epinephrine response in α2A–/– platelets was confirmed in flow cytometry studies in which activation of the integrin αIIbβ3 was detected using the specific JON/A-PE19 antibody. Washed whole blood from wild-type and α2A–/– mice was incubated with ADP (5 μM) or U46619 (1 μM) in the presence or absence of epinephrine (10 μM). Activation of αIIbβ3 on wild-type platelets was enhanced when epinephrine was added, but there was no effect of epinephrine on α2A-deficient platelets (Figure 2B).
α2A receptor is involved in primary hemostasis
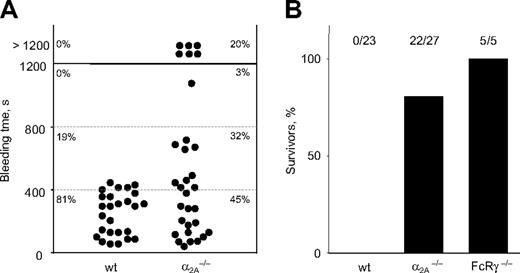
To define the role of α2A in hemostasis, tail bleeding time assays were performed. In wild-type mice, bleeding was arrested within 10 minutes after the tail tip segment was cut, whereas bleeding times in α2A–/– mice were highly variable. In 6 of 31 (20%) of the mutant mice, bleeding could not be stopped within the 20-minute observation period (Figure 3A).
In vitro characterization of α2A–/–-deficient platelets. (A) Heparinized PRP was stimulated with ADP (5 μM) and U46619 (1 μM or subthreshold concentration of 0.1 μM) in the presence or absence of 10 μM epinephrine (Epin), and light transmission was recorded on a Fibrintimer 4-channel aggregometer. Results shown are representative of 6 individual experiments. (B) Washed whole blood from wild-type or α2A–/– mice was incubated with 5 μM ADP and 1 μM U46619 (U46) in the presence or absence of 10 μM epinephrine (Epin) for 15 minutes at room temperature. Activation of αIIbβ3 and P-selectin expression was analyzed by flow cytometry. Results are presented as mean ± SD of 5 mice per group.
In vitro characterization of α2A–/–-deficient platelets. (A) Heparinized PRP was stimulated with ADP (5 μM) and U46619 (1 μM or subthreshold concentration of 0.1 μM) in the presence or absence of 10 μM epinephrine (Epin), and light transmission was recorded on a Fibrintimer 4-channel aggregometer. Results shown are representative of 6 individual experiments. (B) Washed whole blood from wild-type or α2A–/– mice was incubated with 5 μM ADP and 1 μM U46619 (U46) in the presence or absence of 10 μM epinephrine (Epin) for 15 minutes at room temperature. Activation of αIIbβ3 and P-selectin expression was analyzed by flow cytometry. Results are presented as mean ± SD of 5 mice per group.
To analyze platelet function in vivo, wild-type and α2A–/– mice were intravenously injected with collagen/epinephrine (0.8 mg/kg; 60 μg/kg), which causes lethal pulmonary thromboembolism.20 All wild-type mice died within 5 minutes of injection; more than 80% of mice lacking α2A survived and were able to completely recover. This suggests that the α2A receptor is the essential receptor for epinephrine-induced responses in vivo. Mice deficient in the Fc receptor (FcR) γ-chain, which also lack the major collagen receptor, GPVI, on the platelet surface, were used as controls. All injected FcRγ-chain–deficient mice survived the challenge, confirming previous results21 (Figure 3B).
Reduced thrombus stability in α2A–/–mice
To investigate the relevance of α2A in pathologic, occlusive thrombus formation in vivo, FeCl3 injury was induced on mesenteric arterioles, and thrombus formation was assessed by in vivo fluorescence microscopy. Mice were anesthetized, and injury was induced after intravenous injection of fluorescently labeled syngeneic platelets. Five minutes after injury, the adhesion of platelets to the denuded vessel wall was comparable between wild-type and α2A–/– mice (2508 ± 301/mm2 vs 3656 ± 618/mm2), suggesting that the α2A adrenergic receptor is not essential for platelet adhesion at high shear conditions in vivo.
Highly variable bleeding times and protection from collagen/epinephrine–induced thromboembolism in α2A–/– mice. (A) Mice were anesthetized, and a 3-mm segment of the tail tip was cut off with a scalpel. Tail bleeding times of wild-type (n = 26) and α2A–/– (n = 31) mice were monitored by gentle absorption of a drop of blood. When no blood was observed on the paper after a 15-second interval, bleeding was determined to have ceased. The experiment was stopped after 20 minutes. Each symbol represents one mouse, and horizontal lines indicate quartiles of bleeding time. (B) Thromboembolic death was observed after the injection of collagen (0.8 mg/kg) and epinephrine (60 μg/kg). All wild-type mice (23 of 23) died, whereas 81.5% (22 of 27) of α2A–/– mice and all mice injected with FcRγ-chain–/– (5 of 5) survived.
Highly variable bleeding times and protection from collagen/epinephrine–induced thromboembolism in α2A–/– mice. (A) Mice were anesthetized, and a 3-mm segment of the tail tip was cut off with a scalpel. Tail bleeding times of wild-type (n = 26) and α2A–/– (n = 31) mice were monitored by gentle absorption of a drop of blood. When no blood was observed on the paper after a 15-second interval, bleeding was determined to have ceased. The experiment was stopped after 20 minutes. Each symbol represents one mouse, and horizontal lines indicate quartiles of bleeding time. (B) Thromboembolic death was observed after the injection of collagen (0.8 mg/kg) and epinephrine (60 μg/kg). All wild-type mice (23 of 23) died, whereas 81.5% (22 of 27) of α2A–/– mice and all mice injected with FcRγ-chain–/– (5 of 5) survived.
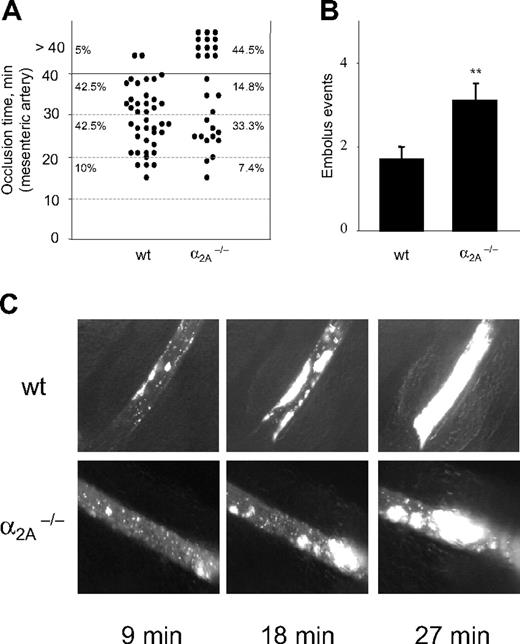
On average, 9 minutes after FeCl3 application, the first thrombi were observed in control and mutant mice (9.5 ± 0.7 minutes vs 9 ± 0.9 minutes). Interestingly, thrombi formed in α2A–/– mice were able to occlude the artery in only 55.5% of the injured vessels, whereas 95% of the injured vessels in wild-type mice occluded within 40 minutes (Figure 4A). This reduced occlusion rate was caused by thrombus instability and increased embolus formation compared with wild-type mice. The number of embolus events in α2A–/– mice was twice as high as in wild-type mice (3.1 ± 0.39 vs 1.7 ± 0.32) (Figure 4B), demonstrating for the first time that the α2A receptor plays a role in thrombus stabilization in vivo. Representative images of one experiment are shown in Figure 4C.
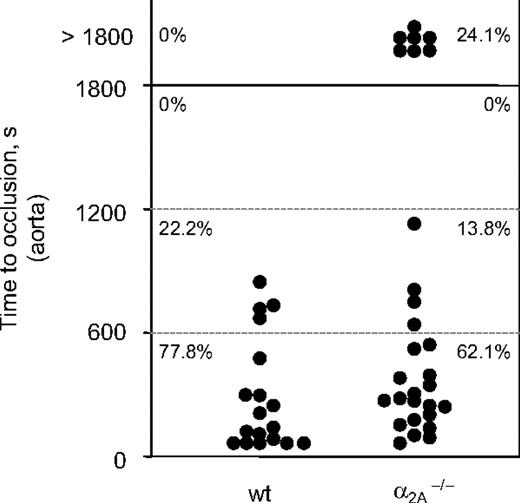
A second in vivo injury model was applied to confirm the finding that α2A is involved in the formation of stable thrombi. In this model, thrombosis was induced mechanically in the aorta by one firm compression with a forceps, and time to occlusion was determined.22 Blood flow was monitored with an ultrasonic flow probe until complete occlusion occurred. After a transient increase directly after injury, blood flow progressively decreased for several minutes in all animals. Subsequently, in all (17 of 17) tested wild-type mice, this decrease resulted in complete and irreversible occlusion of the vessel 0.5 to 13.1 minutes after injury (mean occlusion time, 4.0 ± 4.5 minutes). In contrast, stable thrombus formation was defective in 24.1% (7 of 29) α2A–/– mice because blood flow was reestablished between 10 and 453 seconds after initial occlusion (Figure 5). Second occlusion did not occur in any of these vessels. As a consequence, blood flow through the injured vessel was essentially normal at the end of the observation period (30 minutes). This result confirmed that the α2A adrenergic receptor plays a role in thrombus formation and stabilization in vivo.
Enhanced embolus formation in α2A–/– mice. Thrombosis was induced in mesenteric arteries by topical application of FeCl3. (A) In wild-type mice 95% of formed thrombi were able to occlude, whereas in α2A–/– mice only 55.5% of formed thrombi were able to occlude. Each symbol represents one monitored arteriole, and horizontal lines indicate quartiles of occlusion time. (B) Quantitative analysis of embolus formation in control (1.7 ± 0.32) and mutant (3.1 ± 0.39) mice are presented as the amount of thrombi that detached during the observation period from the viewing field. Results are presented as mean ± SEM. (**P = .01–.05) (C) Representative pictures of 1 experiment are shown for better illustration. Indicated time points represent minutes after FeCl3-induced injury. Images were captured as described in the caption for Figure 1C, except that a 10 ×/0.25 Ph1 objective was used.
Enhanced embolus formation in α2A–/– mice. Thrombosis was induced in mesenteric arteries by topical application of FeCl3. (A) In wild-type mice 95% of formed thrombi were able to occlude, whereas in α2A–/– mice only 55.5% of formed thrombi were able to occlude. Each symbol represents one monitored arteriole, and horizontal lines indicate quartiles of occlusion time. (B) Quantitative analysis of embolus formation in control (1.7 ± 0.32) and mutant (3.1 ± 0.39) mice are presented as the amount of thrombi that detached during the observation period from the viewing field. Results are presented as mean ± SEM. (**P = .01–.05) (C) Representative pictures of 1 experiment are shown for better illustration. Indicated time points represent minutes after FeCl3-induced injury. Images were captured as described in the caption for Figure 1C, except that a 10 ×/0.25 Ph1 objective was used.
Discussion
Thrombus stability largely depends on the release of second-wave mediators, including ADP and TxA2. They, together with epinephrine, share the common property of binding G-protein–coupled receptors. These receptors are known to act in concert to achieve full platelet activation by cyclic adenosine monophosphate (cAMP) suppression, increased Ca2+ concentration, phospholipase C (PLC) activation, and reorganization of the platelet cytoskeleton.23 The α2A receptor inhibits cAMP formation through stimulation of one of the Gi family members, Gz.24 Epinephrine alone is unable to promote platelet shape change, aggregation, or direct αIIbβ3 activation,25,26 but it potentiates different responses and thereby contributes to platelet activation in vitro.13,14 Although α2A has been known for many years, its role in thrombosis and hemostasis is poorly understood. Therefore, α2A–/– mice were used to analyze the process of in vitro and in vivo platelet adhesion and thrombus formation. Our results show that α2A is not essential for normal megakaryocyte maturation and platelet formation because platelet counts and expression of prominent glycoproteins were not affected in α2A–/– mice. However, α2A–/– mice displayed a mild bleeding tendency (Figure 3A), indicating that epinephrine-mediated platelet responses contribute to stable platelet plug formation. This finding is in accordance with previous reports from patients with reduced α2A levels on the platelet surface.27,28 In these patients, reduced amounts of α2A were accompanied by a bleeding tendency, and the platelets showed impaired responses to epinephrine in vitro. Comparable data were obtained with α2A–/– mice because their platelets remained unresponsive to epinephrine-enhanced αIIbβ3 activation and aggregation in the presence of low concentrations of ADP and U46619 (Figure 2).
Mice deficient in α2A are largely protected against collagen/epinephrine-induced pulmonary thromboembolism (Figure 3B), confirming that the contribution of epinephrine to platelet activation in vivo is mediated through the α2A receptor. These findings support results previously described by Yang et al29 showing that platelets obtained from Gzα–/– mice are unresponsive to epinephrine and that these animals are protected against collagen/epinephrine-induced lethal thromboembolism. Consistent with this, alterations in the number of α2A receptors on human platelets cause abnormalities in the platelet responses to epinephrine.11,30,31
Analysis of α2A–/– mice in different in vivo thrombosis models revealed that they display enhanced embolus formation and thrombus instability. In the model of FeCl3-induced injury on mesenteric arteries, 44.5% of the vessels in α2A–/– mice did not occlude because of frequent embolization (Figure 4). Similarly, in the aorta injury model, 24.1% of initially occlusive thrombi recanalized and remained open for the entire observation period (Figure 5). Our findings demonstrate for the first time that epinephrine-dependent signaling is also required for the formation of stable plugs.
These results revealed that α2A is the essential receptor for epinephrine on the platelet surface, which regulates epinephrine responses during platelet activation in vitro and in vivo. Generally, the contribution of epinephrine to thrombotic processes is thought to happen through its constrictive action to the vascular wall. It is important to note that we did not observe any differences in the vascular constriction upon FeCl3-induced injury. This strongly suggests that the observed defect in thrombus stabilization is based on reduced platelet activation caused by suboptimal activation of platelets from α2A–/– mice rather than by a defect in the vessel wall. Our findings lead us to speculate that epinephrine, through α2A, contributes to the progression of stable plug formation under pathologic conditions. It is known that the density of α2A on the platelet surface can vary in relation to physiologic, pathologic, and pharmacologic factors. Furthermore, it has been reported that age,32 ischemic heart failure,33 and plasmatic catecholamine levels34,35 can regulate α2A expression. In line with this, Wang and Cheng36 have found that strenuous exercise increases platelet α2A-adrenergic receptor density and can enhance platelet adhesiveness on fibrinogen-coated surfaces. This α2A/epinephrine–mediated platelet response during strenuous, acute exercise may enhance the risk for major ischemic cardiovascular events. Taken together, these results provide strong evidence that signaling initiated by epinephrine through the α2A receptor on the platelet surface contributes to in vivo thrombus stabilization under pathologic conditions.
Compromised thrombus stability in mice lacking the α2A receptor. Thrombosis was induced in the aorta by one firm compression with a forceps. Blood flow was monitored with a perivascular ultrasonic flow probe until complete occlusion. The experiment was stopped after 40 minutes. Each symbol represents one mouse, and horizontal lines indicate quartiles of occlusion time.
Compromised thrombus stability in mice lacking the α2A receptor. Thrombosis was induced in the aorta by one firm compression with a forceps. Blood flow was monitored with a perivascular ultrasonic flow probe until complete occlusion. The experiment was stopped after 40 minutes. Each symbol represents one mouse, and horizontal lines indicate quartiles of occlusion time.
Prepublished online as Blood First Edition Paper, February 28, 2006; DOI 10.1182/blood-2005-12-4835.
Supported by DFG grants Ni556/4-1 (B.N.) and SFB487 (L.H.). U.J.H.S. is a fellow of the German Foundation for Hemotherapeutic Research (Bonn, Germany).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Markus Bender for helpful suggestions and criticism throughout the study and Sabine Grüner for help with the bleeding time experiments.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal