Abstract
In utero hematopoietic-cell transplantation (IUHCT) can induce donor-specific tolerance to facilitate postnatal transplantation. Induction of tolerance requires a threshold level of mixed hematopoietic chimerism. CD26 is a peptidase whose inhibition increases homing and engraftment of hematopoietic cells in postnatal transplantation. We hypothesized that CD26 inhibition would increase donor-cell homing to the fetal liver (FL) and improve allogeneic engraftment following IUHCT. To evaluate this hypothesis, B6GFP bone marrow (BM) or enriched hematopoietic stem cells (HSCs) were transplanted into allogeneic fetal mice with or without CD26 inhibition. Recipients were analyzed for FL homing and peripheral-blood chimerism from 4 to 28 weeks of life. We found that CD26 inhibition of donor cells results in (1) increased homing of allogeneic BM and HSCs to the FL, (2) an increased number of injected animals with evidence of postnatal engraftment, (3) increased donor chimerism levels following IUHCT, and (4) a competitive engraftment advantage over noninhibited congenic donor cells. This study supports CD26 inhibition as a potential method to increase the level of FL homing and engraftment following IUHCT. The resulting increased donor chimerism suggests that CD26 inhibition may in the future be used as a method of increasing donor-specific tolerance following IUHCT.
Introduction
In utero hematopoietic-cell transplantation (IUHCT) has the potential to treat many congenital hematologic and genetic disorders.1,2 It is a nonmyeloablative approach that can, under some circumstances, achieve mixed allogeneic chimerism and associated donor-specific tolerance.3-6 This tolerance can facilitate postnatal cellular or organ transplantations with minimal or no myeloablative or immunosuppressive conditioning.3,4,6-15 Although engraftment of allogeneic or xenogeneic cells has been achieved, the levels of donor-cell chimerism in most circumstances following IUHCT alone have been below what would be considered therapeutic for most target diseases.3-7,16-21 Additionally, the frequency of engraftment following IUHCT of allogeneic or xenogeneic cells has also been relatively low.3,17-19,21 Thus, the ideal goal of a single in utero transplantation resulting in therapeutic levels of engraftment or a 100% frequency of donor-specific tolerance has been elusive. A significant barrier to allogeneic engraftment following IUHCT is limited space in the fetus due to host-cell competition from a robust and highly proliferative fetal hematopoeitic compartment.22 This has been supported by the presence of high-level engraftment after IUHCT in circumstances where a competitive or survival advantage exists for donor cells.23-28 Additionally, the inability of hematopoietic stem cells (HSCs) to home and engraft with absolute efficiency may limit donor-cell engraftment following allogeneic IUHCT.29 Thus, strategies that selectively increase donor HSC homing and engraftment in the fetal recipient may increase both the levels and frequency of engraftment following allogeneic IUHCT.
CD26/dipeptidylpeptidase IV is a membrane-bound ectopeptidase that is expressed on a subset of HSCs and early hematopoietic progenitor cells.30,31 It cleaves dipeptides from the N terminus of polypeptide chains that contain the N-terminal X-Pro or X-Ala motif.30,32 Among its many substrates, CD26 has been shown to have selectivity for stromal-cell–derived factor 1α (SDF-1α)/CXCL12.33 SDF-1α is a chemokine present at high levels in adult bone marrow (BM).34-36 The interaction of SDF-1α with CXCR4, the SDF-1α receptor expressed on hematopoietic cells, some of which also express high levels of CD26, is believed to play a key role in attracting circulating HSCs to the adult BM cavity.34,37,38 Recent in vitro studies have demonstrated that cleavage of SDF-1α by CD26 results in a loss of its chemotactic effect on primitive hematopoietic cells and that inhibition of this action results in increased SDF-1α–induced migration.30,39 In vivo, postnatal studies in a murine bone marrow transplantation (BMT) model found increased short-term BM homing of enriched HSCs and low-density BM donor cells after inhibition or absence of CD26 activity. Reduction of CD26 activity on low-density donor BM cells also resulted in increased long-term engraftment, competitive repopulation, secondary transplantation, and mouse survival following lethal irradiation.39 In addition to its role in the postnatal system, SDF-1α is known to be produced by multiple tissues during embryogenesis such as the fetal liver (FL) and fetal BM.34-36 SDF-1α and CXCR4 mutant mice have implicated the importance of the CXCR4–SDF-1α interaction in fetal hematopoiesis. In these mice, a significantly reduced number of HSCs and myeloid cells colonized the BM at gestational age E18.5. Similarly, fetal BM myelopoiesis and B-cell lymphopoiesis was severely impaired in SDF-1α and CXCR4 mutant mice. The hematopoietic defects in the FL of mutant mice were also present but not as severe. SDF-1α mutant mice demonstrated a 2-fold reduction in the number of HSCs in the FL at gestational age E16.5. Additionally, SDF-1α and CXCR4 mutant mice demonstrated defective FL B-cell lymphopoiesis but normal myelopoiesis at gestational age E16.5 to E18.5. Together, these studies implicate a potentially important role for SDF-1α and CXCR4 in the homing of HSCs, B-cell progenitors, and myeloid-cell progenitors to hematopoietic niches in the FL and BM during ontogeny.35-37,40,41
Based on the known production of SDF-1α by fetal hematopoietic tissues and its role in fetal hematopoiesis, as well as the recent studies demonstrating the relationship of CD26 activity to HSC homing and engraftment in postnatal BMT, we reasoned that a similar interaction may be important to homing and engraftment following allogeneic IUHCT. We hypothesized that inhibition of CD26 activity on donor cells would increase their ability to home to the FL, resulting in a subsequent increase in the frequency and levels of postnatal engraftment.
Materials and methods
Mice
BALB/c (H-2Kd, CD45.2, GFP–), B6Pep3b (H-2Kb, CD45.1, GFP–), and Swiss Webster (outbred strain, GFP–) mice were purchased from Jackson Laboratories (Bar Harbor, ME). C57BL/6TgN(act-EGFP)OsbY01 (H-2Kb, CD45.2, GFP+) mice were kindly provided by Dr Okabe (Osaka University, Genome Information Research Center) and have been maintained in our breeding colonies for 6 years (referred to as B6GFP in this report). Animals were housed in the Laboratory Animal Facility of the Abramson Research Center at The Children's Hospital of Philadelphia. The experimental protocols were approved by the Institutional Animal Care and Use Committee at The Children's Hospital of Philadelphia and followed guidelines set forth in the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Donor BM harvest
Adult BM was harvested from 6- to 8-week-old donors by flushing the tibias and femurs with Ca++/Mg++-free phosphate-buffered saline (PBS; Gibco, Rockville, MD). A single-cell suspension was created by gently passing the flushed BM through a 26-gauge needle several times. The BM was filtered through a 70 μm nylon mesh filter and then layered over Ficoll (Histopaque 1077, Sigma, St Louis, MO). After centrifugation at 600g at room temperature for 25 minutes, the light-density mononuclear cell (LDMC) layer was carefully removed and washed with cold PBS twice. Cells were counted prior to transplantation and more than 95% viability confirmed by trypan blue exclusion. On average, 20 × 106 LDMCs with more than 95% viability were obtained from a single donor mouse.
Enriched donor HSC preparation
For homing experiments involving the injection of enriched B6GFP HSCs, donor BM was harvested as described under “Donor BM harvest” with the exception that PBS with 1% fetal calf serum was used. After Ficoll gradient centrifugation, the LDMCs underwent c-kit enrichment using c-kit microbeads and the quadroMACs separation unit (Miltenyi Bitotec, Auburn, CA) as per the manufacturer's instructions. The c-kit–enriched cells were subsequently labeled with anti–c-kit APC, anti–Sca-1 phycoerythrin (PE), and anti-Lineage (anti-Lin) biotin (CD5, CD45R, CD11b, Gr-1, Ter-119) antibodies with secondary staining with streptavidin PerCP-Cy5.5. Labeled cells were sorted on a BD FACSVantage (BD Biosciences, San Jose, CA) for GFP+c-kit+Sca-1+Lin– cells and confirmed to be more than 90% pure after sorting. The lineage panel was purchased from Miltenyi Biotec, and all additional antibodies were purchased from BD Biosciences.
CD26 inhibition
The 3–amino acid peptide Diprotin A (Peptides International, Louisville, KY) was used to inhibit CD26 activity on donor cells. LDMCs or enriched HSCs were incubated in PBS with 5 mM Diprotin A at room temperature for 15 minutes. After incubation, the cells were washed once in PBS. The cells were recounted, and a greater than 95%-cell viability was confirmed by trypan blue exclusion prior to transplantation.
In utero transplantation
Fetuses of time-dated pregnant BALB/c (n = 144) or Swiss Webster (n = 81) mice were injected at gestational day 14. BALB/c mice were chosen as a recipient strain to evaluate IUHCT across a defined full MHC barrier, and Swiss Webster mice were used to evaluate IUHCT in outbred recipients. Under isoflurane anesthesia, a midline laparotomy was performed, and the uterine horns were exposed. Under a dissecting microscope, the vitelline vein was identified, and each fetus was injected with either 20 × 106 whole B6GFP BM cells with or without CD26 inhibition or 1 × 105 enriched B6GFP HSCs with or without CD26 inhibition in a total volume of 20 μL and 10 μL, respectively, using a 100 μm beveled glass micropipette. A successful injection was confirmed by visualization of clearance of the blood in the vein by the injectate and the absence of extravasation at the site of injection. The uterus was returned to the maternal peritoneal cavity and the abdomen closed with 2 layers of absorbable 5-0 vicryl suture. Of note, the vitelline vein was chosen as the site of injection because of its accessibility following maternal laporotomy, the ability to safely inject a larger volume of cells (20 μL) compared with the intraperitoneal route (5 μL), and the ability to visually confirm a successful injection. The vitelline vein enters the portal circulation, resulting in first-pass distribution of the cells to the portal circulation and to the systemic circulation via shunting through the ductus venosus. Using this route of injection, we have found a trend toward increased mortality with the injection of volumes of more than 20 μL and cell concentrations of more than 1 × 106 cells per microliter. Thus, 20 × 106 whole B6GFP BM cells were injected to maximize cell dose. A total of 1 × 105 enriched HSCs, a cell dose that we found detectable by flow cytometry and stereomicroscopy in preliminary studies, were injected in a volume of 10 μL to maximize the number of fetuses injected and fetal survival.
In utero competition assay
Whole BM was harvested from B6GFP mice and congenic B6Pep3b mice as described above. The LDMCs from the B6GFP donor were subjected to CD26 inhibition as described under “Donor BM harvest”, and the LDMCs from the B6Pep3b donor were unmanipulated. A total of 10 × 106 cells from each donor were coinjected into a gestational day-14 BALB/c fetus. The injected fetuses were analyzed for GFP+ and CD45.1+ donor cells in the peripheral blood at 1 month, 2 months, and 4 months of age as described under “FL and peripheral-blood sampling for flow cytometric analysis.”
Monoclonal antibodies used for flow cytometric analysis
The PE-conjugated antibodies included antibodies against CD45, CD45.1, CD3, B220, CD11b, and Gr-1. CD45 conjugated to PerCP was also used for the analysis of the in utero competition assay. Nonspecific Fcγ receptor binding was blocked by the monoclonal antibody (mAb) against mouse Fcγ receptor 2.4G2. Conjugated mAbs with irrelevant specificities served as negative controls. Propidium iodide staining was used to exclude dead cells in dual-color flow cytometry. All antibodies were purchased from PharMingen (San Diego, CA), and flow cytometry was performed on a FACSCalibur (Becton Dickinson).
FL and peripheral-blood sampling for flow cytometric analysis
The presence of B6GFP donor cells in the FL was assessed at 4, 24, 48, and 96 hours after transplantation for analysis of homing. FLs were individually harvested from injected fetuses and placed in petri dishes filled with cold heparinized PBS. Prior to disruption, the superior and inferior surface of the livers were examined under a fluorescent stereoscopic microscope (MZ16FA; Leica, Heerburg, Switzerland) for qualitative assessment of engraftment. The FLs were then passed repeatedly through a 1-mL syringe to form a single-cell suspension that was subsequently Ficoll separated for enrichment of LDMCs. The percentage of GFP+ cells in the FL was then assessed by flow cytometry on a FACSCalibur. Peripheral-blood B6GFP donor-cell frequency was assessed in BALB/c and Swiss Webster fetal recipients of whole BM at 4 weeks of age. Chimerism levels were followed on a biweekly schedule until 8 weeks of age, after which they were assessed monthly until 6 months of age. Chimerism was assessed by flow cytometry as the percentage of CD45+ cells that were GFP+ using an anti-CD45 PE antibody. At 4 months of age, chimeric mice that had received CD26-inhibited B6GFP BM in utero were also assessed for peripheral-blood multilineage engraftment by dual-color flow cytometry using anti-CD3, B220, CD11b, and Gr-1 PE-conjugated antibodies. Finally, the peripheral blood from BALB/c fetal recipients of CD26-inhibited B6GFP BM coinjected with noninhibited B6Pep3b BM (the in utero competition assay) was analyzed at 4, 6, 8, 12, and 16 weeks of age by 3-color flow cytometry to assess the contribution of the 2 donor-cell populations to total donor chimerism. In this analysis the percentage of CD45 cells that were GFP+ and CD45.1+ was assessed using anti-CD45.1 PE and anti-CD45 PerCP antibodies. For each peripheral-blood analysis, approximately 200 μL peripheral blood was collected in heparinized capillary tubes via retro-orbital vein puncture and diluted to 10 mL with heparinized PBS. The sample was layered over a Ficoll gradient. The LDMCs were collected after centrifugation at 600g for 25 minutes and subsequently washed with PBS prior to flow cytometric analysis. A minimum of 10 000 events was analyzed for each determination.
Assessment of GVHD
Mice were weighed at 4, 6, 8, 12, and 16 weeks of age and were monitored for clinical signs of graft-versus-host disease (GVHD), including runting, fur loss, and serositis at these time points. Groups analyzed included BALB/c mice that underwent IUHCT but were not chimeric, chimeric recipients of CD26-inhibited BM, and chimeric recipients of non–CD26-inhibited BM.
Statistical methods
Data are graphically represented as the mean of the respective group ± 1 standard error of the mean. Statistical comparisons between groups were performed with the Student t test for 2 samples assuming unequal variances. A 2-tailed P ≤ .05 was considered significant.
Results
CD26 inhibition increases fetal liver homing of enriched HSCs and whole BM
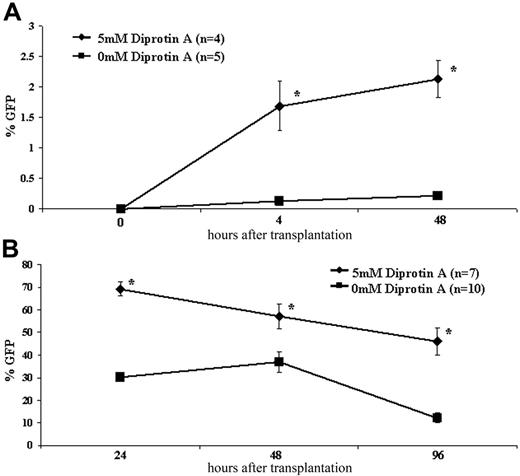
CD26 inhibition significantly increased homing to the FL of both B6GFP whole BM and enriched HSCs at all time points analyzed (Figure 1; Table 1). The increased homing efficiency of whole BM and enriched HSCs following CD26 inhibition ranged from 1.6-fold to 3.8-fold and from 10-fold to 12.5-fold, respectively. The effect on enriched HSCs was most striking (Figures 1, 2; Table 1). Without CD26 inhibition, a minimal number of allogeneic HSCs home to the FL with well under 1% of FL MNCs at 4 and 48 hours being of donor origin (0.135% ± 0.09% and 0.212% ± 0.1%, respectively) compared with levels above 1% (1.69% ± 0.8% and 2.13% ± 0.6% at 4 and 48 hours, respectively) with CD26 inhibition. These differences are also reflected in the absolute number of transplanted cells that home to and engraft in the FL. Assuming an absolute CD45+ FL cell number of 0.26 × 106 and 2.61 × 106 at 4 and 48 hours after enriched HSC transplantation, respectively, and that 80.75% and 89.56% of FL cells are CD45+ at these time points, respectively (data not shown), one can extrapolate the difference in absolute number of donor cells that homed to the FL between the inhibited and noninhibited groups.42 Accordingly, at 4 hours, a time point reflective of only homing, 5392 ± 1304 CD26-inhibited enriched HSCs (or 5.4% ± 1.3% of injected cells) compared with 432 ± 137 noninhibited enriched HSCs (or 0.43% ± 0.13% of injected cells) homed to the FL (P = .031 comparing inhibited versus noninhibited). At 48 hours, a time point representative of both homing and proliferation of donor cells and representing both HSCs and progenitors, 61 770 ± 8897 CD26-inhibited donor cells compared with 6148 ± 1246 noninhibited donor cells were present in the FL (P = .008 comparing inhibited versus noninhibited). These data suggest that CD26 blockade can restore a homing defect present in highly enriched HSCs.
The absolute number of enriched HSCs that homed to and engrafted in the FL at 4 and 48 hours was extrapolated and compared for CD26-inhibited and noninhibited donors
. | 4 hours . | 48 hours . |
|---|---|---|
| CD26-inhibited enriched HSCs | 5392 ± 1304 | 61 770 ± 8897 |
| Non-CD26-inhibited enriched HSCs | 432 ± 137 | 6148 ± 1246 |
. | 4 hours . | 48 hours . |
|---|---|---|
| CD26-inhibited enriched HSCs | 5392 ± 1304 | 61 770 ± 8897 |
| Non-CD26-inhibited enriched HSCs | 432 ± 137 | 6148 ± 1246 |
P < .05 for both time points.
Early kinetics following IUHCT. E14 BALB/c fetal mice were injected intravenously with 1 × 105 B6GFP-enriched HSCs (A) or 20 × 106 B6GFP BM cells (B) with or without CD26 inhibition with 5 mM Diprotin A. Fetal livers of recipient mice were harvested at short time points after injection and assessed for GFP chimerism. *P < .05.
Early kinetics following IUHCT. E14 BALB/c fetal mice were injected intravenously with 1 × 105 B6GFP-enriched HSCs (A) or 20 × 106 B6GFP BM cells (B) with or without CD26 inhibition with 5 mM Diprotin A. Fetal livers of recipient mice were harvested at short time points after injection and assessed for GFP chimerism. *P < .05.
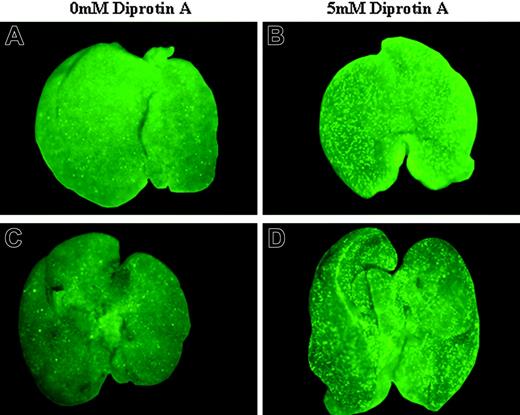
Stereomicroscope analysis of FL homing of enriched HSCs following IUHCT. E14 BALB/c fetal mice were injected intravenously with 1 × 105 B6GFP-enriched HSCs without (A,C) or with (B,D) CD26 inhibition with 5 mM Diprotin A. Fetal livers of recipient mice were harvested at 4 hours (A-B) and 48 hours (C-D) after injection and assessed for the presence of GFP donor cells by fluorescence stereomicropscope. All images were obtained with a Leica MZ16FA stereoscopic fluorescent microscope (Leica Microsystems, Bannockburn, IL) at an exposure time of 800 milliseconds and 25× microscope (1×/0.25 Leica Planapochromatic objective). Images were photographed with a Leica DFC350FX camera, acquired by Openlab 4.0 (Improvision, Lexington, MA) image acquisition software, and combined into a single image using Photoshop 7.0 (Adobe Systems, San Jose, CA) without image enhancement.
Stereomicroscope analysis of FL homing of enriched HSCs following IUHCT. E14 BALB/c fetal mice were injected intravenously with 1 × 105 B6GFP-enriched HSCs without (A,C) or with (B,D) CD26 inhibition with 5 mM Diprotin A. Fetal livers of recipient mice were harvested at 4 hours (A-B) and 48 hours (C-D) after injection and assessed for the presence of GFP donor cells by fluorescence stereomicropscope. All images were obtained with a Leica MZ16FA stereoscopic fluorescent microscope (Leica Microsystems, Bannockburn, IL) at an exposure time of 800 milliseconds and 25× microscope (1×/0.25 Leica Planapochromatic objective). Images were photographed with a Leica DFC350FX camera, acquired by Openlab 4.0 (Improvision, Lexington, MA) image acquisition software, and combined into a single image using Photoshop 7.0 (Adobe Systems, San Jose, CA) without image enhancement.
CD26 inhibition increases the frequency of postnatal engraftment following IUHCT
The peripheral blood from BALB/c mice that had received an IUHCT with either CD26-inhibited or noninhibited whole BM was analyzed at 1 month of age for B6GFP donor-cell chimerism. Eleven of 22 (50%) analyzed mice that had received CD26-inhibited BM demonstrated allogeneic engraftment compared with 9 of 40 (22.5%) analyzed mice that received noninhibited BM. All of the 11 chimeric mice that received CD26-inhibited BM had donor-cell engraftment levels of more than 1% at 1 month of age (range, 4.97% to 51.57% donor-cell chimerism with a standard error of the mean of 4.23%) and maintained chimerism until the time of killing at 6 months of age. Conversely, 2 of the 9 chimeric mice that received noninhibited BM had initial chimerism levels of more than 1% at 1 month of age (0.18% and 0.79% donor-cell chimerism), and 3 of the 9 chimeric mice lost their chimerism by the time of killing (initial donor chimerism levels at 1 month of age: 0.18%, 0.79%, and 2.27%) (Table 2). This suggests that CD26 inhibition increases the frequency of allogeneic engraftment following IUHCT and does so at the level of both the committed progenitor cell and the HSCs.
Efficiency of engraftment following IUHCT with CD26-inhibited and noninhibited allogeneic BM
. | 5 mM Diprotin A: CD26 inhibition . | 0 mM Diprotin A: no inhibition . |
|---|---|---|
| Chimerism at any level, no. of chimeric mice/no. of mice analyzed (%) | 11/22 (50) | 9/40 (22.5) |
| Macrochimerism, no. of macrochimeric mice/no. of chimeric mice (%) | 11/11 (100) | 7/9 (77.7) |
. | 5 mM Diprotin A: CD26 inhibition . | 0 mM Diprotin A: no inhibition . |
|---|---|---|
| Chimerism at any level, no. of chimeric mice/no. of mice analyzed (%) | 11/22 (50) | 9/40 (22.5) |
| Macrochimerism, no. of macrochimeric mice/no. of chimeric mice (%) | 11/11 (100) | 7/9 (77.7) |
The number of BALB/c mice that were chimeric at 1 month of age after receiving either CD26-inhibited or noninhibited B6 GFP BM in utero was assessed.
*More than 1% donor chimerism.
CD26 inhibition increases the level of allogeneic engraftment following IUHCT
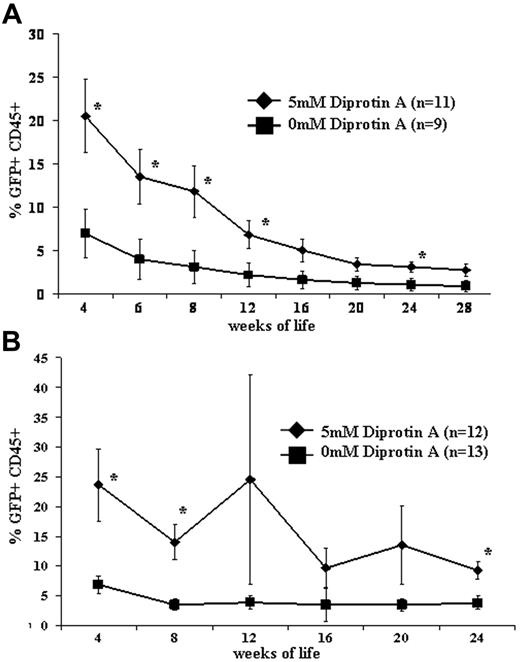
Peripheral-blood allogeneic donor-cell chimerism levels were assessed at 1 month of age and followed until the time of killing at 6 months of age in recipients of CD26-inhibited or noninhibited in utero whole BM transplantation. Two recipient strains were assessed: BALB/c recipients were used to assess engraftment across full major histocompatibility complex (MHC) barriers, and Swiss Webster recipients were used to assess engraftment in an outbred strain. As demonstrated in Figure 3, levels of engraftment were significantly higher following the in utero transplantation of CD26-inhibited BM in both strain combinations. Although the trend continued until the time of killing, the engraftment advantage conferred by CD26 blockade was most significant within the first 2 to 3 months of life.
Allogeneic engraftment following CD26-inhibited IUHCT is stable and multilineage
Donor engraftment decreased between 1 month of age and 4 months of age, after which time it remained stable in recipients of both CD26-inhibited and noninhibited BM. Persistence of stable engraftment to 6 months of age supports engraftment at the level of the HSCs. This is further supported by the presence of multilineage engraftment at 4 months of age in recipients of CD26-inhibited IUHCT (Figure 4). Analysis of the percent GFP+ cells that are lineage positive demonstrated no significant difference between recipients of CD26-inhibited BM in utero and GFP control mice. This argues against CD26 inhibition, favoring the engraftment of a lineage-specific progenitor cell.
Postnatal engraftment following IUHCT. E14 BALB/c (A) or Swiss Webster (B) fetal mice were injected intravenously with 20 × 106 B6GFP BM cells with or without CD26 inhibition with 5 mM Diprotin A. *P < .05.
Postnatal engraftment following IUHCT. E14 BALB/c (A) or Swiss Webster (B) fetal mice were injected intravenously with 20 × 106 B6GFP BM cells with or without CD26 inhibition with 5 mM Diprotin A. *P < .05.
CD26 inhibition provides a competitive advantage to donor cells following IUHCT
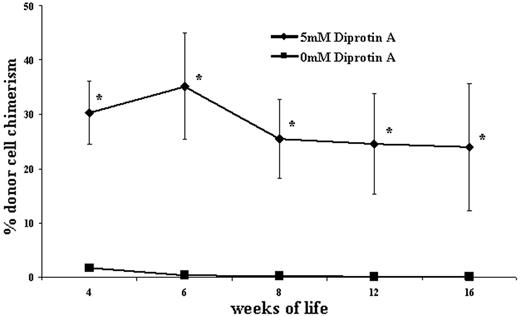
An in utero competition analysis was performed to mechanistically evaluate the effect of CD26 inhibition on donor-cell engraftment following IUHCT. A total of 10 × 106 CD26-inhibited B6GFP BM cells were coinjected with 10 × 106 noninhibited congenic B6Pep3b BM cells into 14-day–gestation BALB/c fetuses. Postnatally, peripheral-blood analysis demonstrated 18-fold and 200-fold increases in engraftment of CD26-inhibited cells compared with noninhibited cells at 1 and 4 months, respectively (Figure 5). This argues for a role of CD26 inhibition in increasing the ability of donor HSCs or early progenitor cells to compete with host HSCs or early progenitor cells for a limited number of hematopoietic niches.
Multilineage engraftment following IUHCT with CD26-inhibited allogeneic BM. E14 BALB/c fetal mice were injected intravenously with 20 × 106 B6GFP BM cells that had been incubated with 5 mM Diprotin A. Peripheral blood from chimeric mice was analyzed at 4 months of age for the presence of GFP cells that expressed CD3 (T cells), B220 (B cells), Gr-1 (granulocytes), and CD11b (macrophages) to assess for donor-cell multilineage engraftment. There was no significant difference in multilineage chimerism levels between control mice and mice injected in utero with CD26-inhibited BM.
Multilineage engraftment following IUHCT with CD26-inhibited allogeneic BM. E14 BALB/c fetal mice were injected intravenously with 20 × 106 B6GFP BM cells that had been incubated with 5 mM Diprotin A. Peripheral blood from chimeric mice was analyzed at 4 months of age for the presence of GFP cells that expressed CD3 (T cells), B220 (B cells), Gr-1 (granulocytes), and CD11b (macrophages) to assess for donor-cell multilineage engraftment. There was no significant difference in multilineage chimerism levels between control mice and mice injected in utero with CD26-inhibited BM.
CD26 inhibition confers a competitive engraftment advantage on donors. An in utero competition analysis was performed to mechanistically evaluate the effect of CD26 inhibition on allogeneic donor BM cells used for IUHCT. A total of 10 × 106 CD26-inhibited B6GFP BM cells were coinjected with 10 × 106 noninhibited congenic B6Pep3b cells into 14-day–gestation BALB/c fetuses. The contribution to peripheral-blood engraftment of the 2 donor-cell populations was assessed postnatally and is expressed as the percentage of CD45+ cells that are from the inhibited and noninhibited donor; n = 5. *P < .05 when comparing chimerism levels between the 2 donor-cell populations.
CD26 inhibition confers a competitive engraftment advantage on donors. An in utero competition analysis was performed to mechanistically evaluate the effect of CD26 inhibition on allogeneic donor BM cells used for IUHCT. A total of 10 × 106 CD26-inhibited B6GFP BM cells were coinjected with 10 × 106 noninhibited congenic B6Pep3b cells into 14-day–gestation BALB/c fetuses. The contribution to peripheral-blood engraftment of the 2 donor-cell populations was assessed postnatally and is expressed as the percentage of CD45+ cells that are from the inhibited and noninhibited donor; n = 5. *P < .05 when comparing chimerism levels between the 2 donor-cell populations.
CD26 inhibition does not result in significant deleterious effects on IUHCT
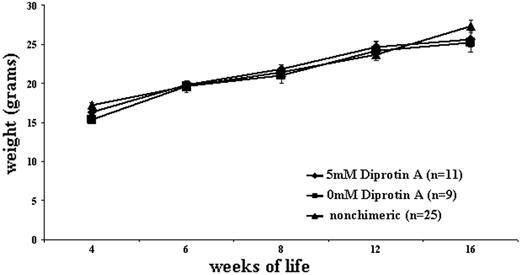
Fetal survival rate as well as GVHD in surviving chimeric animals were assessed to determine if CD26 inhibition had any negative effects on recipients of IUHCT. No decrease in survival to weaning at 1 month of age was seen following the in utero injection of CD26-inhibited BM compared with noninhibited BM. Of a total 59 BALB/c fetal recipients of CD26-inhibited B6GFP BM, 22 survived to weaning for a fetal survival rate of 37.3%. This compares with 40 surviving out of 112 BALB/c fetal recipients of noninhibited B6GFP BM, for a fetal survival rate of 35.7%. Overall survival in this model is a reflection of procedure-related loss and perinatal loss related to maternal neglect or cannibalism. The survival in this study is comparable to our previous experience with the strains used. All surviving chimeric animals that received either CD26-inhibited BM or noninhibited BM in utero demonstrated appropriate weight gain and displayed no evidence of runting, fur loss, or serositis (Figure 6).
Discussion
IUHCT has the potential to treat many congenital disorders, either by providing clinically significant levels of donor-cell engraftment or by the induction of donor-specific tolerance to facilitate postnatal cellular and organ transplantion. However, for IUHCT to reach its full clinical potential, more consistent and higher levels of donor-cell engraftment are required. One of the major barriers to engraftment after IUHCT is host-cell competition for a limited number of hematopoietic niches.1,22 Strategies to overcome this barrier theoretically include any approach that would improve donor-cell competitive capacity relative to the host. Myeloablative regimens used in postnatal BMT to provide a competitive advantage for donor cells are associated with prohibitive toxicity for application in the fetus. A more appealing approach would involve manipulation of the donor cells to selectively improve their competitive capacity in the fetal environment.
Early events following IUHCT that are critical for long-term engraftment include homing of donor HSCs to fetal hematopoietic organs, transendothelial migration and engraftment of HSCs in hematopoietic niches, and their subsequent proliferation and migration from one hematopoietic organ to another as hematopoiesis progresses with ontogeny. Specifically at 14 days of gestation in the mouse, successful IUHCT requires that donor HSCs home to and engraft in the FL, which is the only active hematopoietic compartment at that developmental time point. The cells must subsequently successfully compete with host hematopoietic cells, a task that includes proliferation and successful migration and engraftment in the BM and spleen.40 Strategies that provide a competitive advantage for donor HSCs at any point along this path may help overcome the barrier of host-cell competition and improve engraftment. A critical initial component that is subject to manipulation is donor-cell homing. In fact, in the sheep model, manipulation of the very late activation antigen-4–vascular-cell adhesion molecule (VLA-4–VCAM) adhesion interaction resulted in increased homing of human donor cells to the FL after IUHCT.43 The current study took advantage of the SDF-1α–CXCR4 interaction that has proven important for the homing and engraftment of HSCs to BM in postnatal transplantation models. Specifically, inhibition of CD26, a peptidase that cleaves SDF-1α and disrupts the ability of CXCR4 to attract HSCs to hematopoietic niches postnatally, resulted in an increased FL homing capacity of both enriched allogeneic HSCs and whole BM. CD26 inhibition of allogeneic whole BM also resulted in increased levels of engraftment at 1 month of age that remained significantly higher for the first 3 months of life following IUHCT. Multilineage chimerism remained stable from 4 to 6 months of age, supporting the engraftment of allogeneic HSCs following IUHCT.
Our data support the concept that CD26 inhibition preferentially improves homing and engraftment of HSCs and early progenitors. Specifically, without CD26 inhibition, the homing of enriched allogeneic HSCs to the FL appears defective relative to whole BM. In previous homing studies in our model as well as in this study, approximately 4.9% of injected whole BM cells will home to the FL at 4 hours after injection.42 However, only 0.43% of injected highly enriched HSCs homed to the FL at 4 hours. With CD26 inhibition, however, homing of HSCs to the FL was restored to at least that of whole BM at 5.4% of injected cells, a 12.5-fold increase in homing capacity. Additionally, while one third of chimeric recipients (3 of 9) lost chimerism following in utero transplantation of noninhibited whole BM, all mice initially chimeric after in utero transplantation of CD26-inhibited BM remained chimeric until the time of killing. These data are consistent with the engraftment of long-term repopulating cells in all of the mice rendered chimeric with CD26-inhibited BM but perhaps not in some of the mice that lost engraftment in the noninhibited group. However, the drop in chimerism levels between 1 month and 4 months of age in both groups suggests that CD26 inhibition augments progenitor-cell engraftment as well as the engraftment of HSCs.
Appropriate weight gain in in utero recipients of CD26-inhibited and noninhibited allogeneic BM. Chimeric BALB/c mice that had received CD26-inhibited or noninhibited BM in utero were serially weighed to assess for any deleterious effects, such as GVHD, of IUHCT. BALB/c mice that had received an in utero injection of B6GFP BM but were not chimeric postnatally were used for the control group.
Appropriate weight gain in in utero recipients of CD26-inhibited and noninhibited allogeneic BM. Chimeric BALB/c mice that had received CD26-inhibited or noninhibited BM in utero were serially weighed to assess for any deleterious effects, such as GVHD, of IUHCT. BALB/c mice that had received an in utero injection of B6GFP BM but were not chimeric postnatally were used for the control group.
Potentially the most important finding of the current study is the increased frequency of macrochimerism (more than 1%) following IUHCT with CD26-inhibited BM. The goal of high, therapeutically relevant levels of chimerism following a single in utero transplantation has been difficult to achieve. The induction of tolerance with low levels of chimerism to facilitate postnatal cellular or organ transplantations using minimally ablative conditioning regimens is a more realistic immediate goal for clinical application of IUHCT. Multiple studies have indicated that the ability of IUHCT to induce donor-specific tolerance directly correlates with the level of mixed hematopoietic chimerism.3,14 These studies suggest that consistent tolerance induction can be achieved with allogeneic chimerism levels of more than 1% following IUHCT. The ability of CD26 inhibition to more consistently result in macrochimerism suggests that it may also improve the frequency of donor-specific tolerance following IUHCT and therefore the efficiency of combined prenatal and postnatal strategies based on tolerance induction.
An interesting observation of this study was the relatively high level of donor-cell chimerism derived from the CD26-inhibited donor cells in the in utero competition analysis. In this experiment, 10 × 106 CD26-inhibited BM cells were coinjected with 10 million noninhibited BM cells. Levels of chimerism resulting from the inhibited BM ranged from 30% to 24% from 1 to 4 months of age. This compares with chimerism levels ranging from 20% to 5% from 1 to 4 months of age in fetal recipients of 20 × 106 CD26-inhibited BM cells. The higher levels of chimerism derived from one half the number of CD26-inhibited donor cells in the in utero competition analysis are an unexplained observation of this study. We speculate that CD26 inhibition may effect HSC engraftment by more than one mechanism and that these may be opposite in effect. In our study, CD26 inhibition clearly enhanced HSC homing to the FL, presumably via its effect on SDF1-α. However, the observation that cotransplantation with non–CD26-inhibited cells markedly increased long-term engraftment, even with transplantation of a smaller number of donor HSCs, suggests that CD26 inhibition may also have a negative effect on the ability of another cell population in the donor inoculum to facilitate HSC engraftment following in utero transplantation. Cotransplantation of noninhibited BM cells would theoretically maintain the facilitating potential of the donor-cell population while capitalizing on the enhanced homing of HSCs provided by CD26 inhibition. A likely candidate population for CD26-mediated effects is T lymphocytes. Subsets of T cells within the donor BM inoculum are known to provide important engraftment-facilitating properties.10,18,44,45 CD26 is expressed at low density on T cells with markedly enhanced expression after T-cell activation. CD26 plays an important role in T-cell activation and has been shown to be a receptor capable of generating T-cell costimulatory signals.46,47 Inhibitors of CD26 have been reported to inhibit antigen- and mitogen-induced T-cell proliferation in vitro, and some have even been reported to suppress an immune reaction in vivo.48-51 A closer look at the function of CD26 enzyme activity in T-cell activation suggests that it is required for the costimulatory activity of CD26 and that it may contribute to but is not absolutely required for IL-2 production after T-cell stimulation with anti-CD26 and anti-CD3 antibodies.47-49 Thus, the inhibition of CD26 activity in the donor BM inoculum prior to IUHCT may negatively affect the ability of T cells within the inoculum to facilitate the engraftment of HSCs by immune-mediated mechanisms. Another more direct mechanism that has been documented for facilitation of human CD34+ cell engraftment by CD8+ cells is via a nonimmune-mediated mechanism of modulation of homing and cell migration.52 This phenomenon requires cell-cell interaction and acts through the SDF-1α signaling pathway, but the specific molecular interactions between CD8+ cells and CD34+ cells responsible for the effect are unknown. If activation of CD26 on CD8+ cells is required for this interaction, CD26 blockade may inhibit a facilitating effect explaining our observation.
The potential applicability of these results clinically remains to be defined. The ability of CD26 inhibition to improve enriched HSC homing and engraftment is important from a mechanistic standpoint and may be valuable in improving engraftment after IUHCT using populations of cells processed to avoid GVHD. However, successful transplantation of highly enriched human HSCs across allogeneic barriers has been problematic, and the improved engraftment of whole BM may be more clinically important. An important question is the applicability of our murine studies to human IUHCT. The expression of CD26 on human CD34+ cells in cord blood is only 8% to 9%, much lower than its expression on murine progenitors.30 However, as much as 30% of mobilized peripheral-blood CD34+ cells express CD26, suggesting that this molecule may be up-regulated on progenitors in response to physiologic stimuli.31 In addition, there is evidence that the subpopulation of cells expressing CD26 may regulate homing and migration in the CD34+ population as a whole.30 Finally, CD34+ cell expression of CD26 may not be reflective of HSC expression of CD26. While the CD26+ population contained fewer clonogenic progenitors, enriched stem-cell populations also are poorly clonogenic.30 The degree of expression of CD26 on human HSCs remains unknown. In view of the dramatic effects of CD26 blockade observed in this murine fetal system, we remain optimistic that the strategy may be applicable to human transplantation. In any case, however, the results of this study are proof in principle that the manipulation of donor-cell homing may be an effective approach to improve donor-cell engraftment and the consistency of tolerance induction. In the future, successful IUHCT will most likely require a combination of multiple strategies to facilitate engraftment and tolerance induction. CD26 inhibition may be an important adjunct to other strategies that are directed toward overcoming the barriers to fetal engraftment.
Authorship
Contribution: W.H.P. designed the research, performed research, analyzed data, and wrote the paper; M.E. performed research and contributed vital analytic and technical skills; O.O.A. performed research and analyzed data; A.M. contributed vital technical skills and performed research; P.W.Z. designed research and performed vital analytic skills; and A.W.F. designed research, contributed vital analytic skills, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, September 5, 2006; DOI 10.1182/blood-2006-04-018986.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants RO1 HL64715 and U54 HL070596-01 from the National Institutes of Health (A.W.F.); T32 HD046402 (W.H.P.; A.W.F., Program Director); and the Ruth and Tristram C. Colket, Jr, Chair of Pediatric Surgery (A.W.F.).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal