Abstract
Tryptophan (Trp) catabolism mediated by indoleamine 2,3-dioxygenase (IDO) plays a central role in the regulation of T-cell–mediated immune responses. In this study, we also demonstrate that natural killer (NK)–cell function can be influenced by IDO. Indeed, l-kynurenine, a Trp-derived catabolite resulting from IDO activity, was found to prevent the cytokine-mediated up-regulation of the expression and function of specific triggering receptors responsible for the induction of NK-cell–mediated killing. The effect of l-kynurenine appears to be restricted to NKp46 and NKG2D, while it does not affect other surface receptors such as NKp30 or CD16. As a consequence, l-kynurenine–treated NK cells display impaired ability to kill target cells recognized via NKp46 and NKG2D. Instead, they maintain the ability to kill targets, such as dendritic cells (DCs), that are mainly recognized via the NKp30 receptor. The effect of l-kynurenine, which is effective at both the transcriptional and the protein level, can be reverted, since NK cells were found to recover their functional competence after washing.
Introduction
Indoleamine 2,3-dioxygenase (IDO) is an enzyme endowed with powerful immunomodulatory effects, resulting from its enzymatic activity that leads to catabolism of the amino acid tryptophan (Trp).1-3 IDO-generated, Trp-derived catabolites, in particular l-kynurenine, have been reported to block Ag-driven specific T-cell proliferation and even to induce T-cell death.4,5 In addition, Trp deprivation that would result from the IDO enzymatic activity may also play a role in such an immune regulation.2,6,7
IDO is generally absent or inactive in cells of the immune system, but it can be induced or activated in macrophages and in given dendritic-cell (DC) subsets by specific cytokines, in particular IFN-γ.2,8-11 On the other hand, IDO has been found in various tumors of different histotypes, and increments in IDO activity correlate with tumor progression.12,13 Therefore, the activity of IDO may play a role in the regulation of the immune response exerted by the antigen-presenting cells and also provide transformed cells with a potent tool to escape from the immune system assault.
While the mechanisms of IDO-dependent inhibition of T-cell function have been elucidated, less is known on the possible role of IDO in regulating natural killer (NK)–cell activity. NK cells are known to display a potent antitumor activity14-19 and also to interact, in a reciprocal crosstalk, with different DC subsets.20-29 Therefore, NK cells may represent a target for the IDO-mediated suppression exerted by either regulatory leukocytes or tumors. In this context, it has recently been shown that IDO-generated l-kynurenine, in addition to T-cell proliferation, is also able to inhibit the IL-2–induced proliferation of NK cells.4 However, while the regulation of the T-cell response can be efficiently exerted through the control of Ag-specific clonal expansion, the regulation of NK-cell responses, which do not need a clonotypic expansion, may rather require a direct modulation of the NK-cell killing capability. In this context, it is well established that activated NK cells display improved efficiency in clearing susceptible target cells compared with freshly isolated, resting NK cells.14,30,31 This appears to be consequent to the enhancement of their cytolytic machinery as well as to the up-regulation of certain surface receptors and coreceptors32-34 that, upon engagement by specific ligands on target cells, trigger the NK-cell cytotoxicity. These receptors include molecules, such as NKp30, NKp46, and NKG2D,15,35,36 that are expressed on both resting and activated NK cells, and other molecules, such as NKp4433 and CD69,32 that are expressed only upon activation. Therefore, in order to assess the nature and the significance of IDO influence on the NK-cell activity, l-kynurenine or other IDO-generated Trp catabolites should be analyzed for their ability to affect the NK cytolytic function as well as the expression and function of the NK-triggering receptors.
In this study, we demonstrate that l-kynurenine interferes with the activity of NK cells by modulating their cytotoxic capacity. This effect is specific, since it selectively interferes with the cytokine-induced up-regulation of NKp46- and NKG2D-activating receptors, while it only minimally affects NKp30, CD16, and other triggering receptors or coreceptors. Our results provide new insights into the complex regulatory network that controls NK-cell functions and indicate IDO as a regulatory agent able to shape, by means of l-kynurenine, the repertoire of the NK-cell responses.
Materials and methods
Monoclonal antibodies (mAbs) and reagents
The following mAbs, produced in our lab, were used in this study: JT3A (IgG2a, anti-CD3), c127 (IgG1, anti-CD16), c218 (IgG1, anti-CD56), AZ20 and F252 (IgG1 and IgM, respectively, anti-CD337 [NKp30]), BAB281 and KL247 (IgG1 and IgM, respectively, anti-CD335 [NKp46]), Z231 (IgG1, anti-CD336 [NKp44]), BAT221 (IgG1, anti-CD314 [NKG2D]), c227 (IgG1, anti-CD69), EB6b (IgG1, anti-CD158a [KIR2DL1] and CD158h [KIR2DS1]), GL183 (IgG1, anti-CD158b1 [KIR2DL2], CD158b2 [KIR2DL3], and CD158j [KIR2DS2]), ECM41 (IgM, anti-CD158b2 [KIR2DL3]), FES172 (IgG2a, anti-CD158i [KIR2DS4]), Z27 (IgG1, anti-CD158e1 [KIR3DL1]), Z199 (IgG2b, anti-CD159a [NKG2A]), PP35 (IgG1, anti-CD244 [2B4]), MA127 (IgG1, anti-NTB-A), GN18 (IgG3, anti-CD226 [DNAM-1]), ON251 (IgG1, anti-CD59), QA79 (IgG1, anti-CDw328 [p75/AIRM1]), DF305 (IgM, anti–LFA-3), M7E22 (IgG1, anti-CD54 [ICAM-1]), A6-136 (IgM, anti–HLA class I), FM184 (IgM, anti-CD1a), and FM95 (IgG1 anti-CD86).
Anti-CD14 (IgG2a) mAb was purchased by Immunotech (Marseille, France).
T96H6 (IgG1, anti-CD11a) mAb was provided by Dr A. Poggi (Genova, Italy); D1.12 (IgG2a, anti–HLA-DR) mAb was provided by Dr R. S. Accolla (Varese, Italy).
l-kynurenine is commercially available (Sigma Aldrich, St Louis, MO).
Isolation of NK cells and proliferation assays
To obtain purified NK cells, the RosetteSep NK cell Enrichment kit (StemCell Technologies, Vancouver, BC) was used. The kit includes a cocktail of tetrameric antibody complexes that target unwanted cells and cross-link them to red blood cells, forming rosettes. According to the manufacturer's instructions, whole blood was incubated with the RosetteSep antibodies cocktail and then centrifuged on Ficoll-Hypaque gradients in order to separate rosetted cells from NK cells. After centrifugation, the recovered NK cells were assessed for purity by cytofluorimetric analysis. Only those populations displaying more than 95% of CD56+CD3–HLA-DR–CD14– NK cells were selected. To assess proliferation, NK cells labeled with the green fluorescent dye CFSE were cultured in the presence of either IL-2 or IL-12 for 2 days and then analyzed by fluorescence-activated cell sorting (FACS).
Analysis of the effect of l-kynurenine on NK cells
Freshly drawn NK cells were seeded in 96-well, round-bottomed plates at 2 × 105 cells/well and cultured in RPMI medium (Sigma Aldrich) supplemented with 10% FCS. When indicated, cytokines and/or l-kynurenine were added at the onset of culture. IL-2 (Proleukin; Chiron, Emeryville, CA) was added at the final concentration of 300 U/mL, while IL-12 (Peprotech, London, United Kingdom) was added at the final concentration of 2.5 or 5 ng/mL. After the time period indicated in “Results”, the cells were collected, washed, counted, assessed for viability (by trypan blue staining), and analyzed in cytofluorimetric analysis or functional assays.
Real-time RT-PCR
Total RNA was extracted from freshly derived NK cells and from IL-2–activated NK cells either untreated or treated with 0.6 mM l-kynurenine using RNeasy Mini Kit (Qiagen, Germantown, MD). Oligo(dT)-primed cDNA was prepared by standard techniques. Relative quantitation of gene expression after IL-2 activation and l-kynurenine treatment was performed in real-time reverse-transcription–polymerase chain reaction (RT-PCR) using Taqman probes. To internally standardize the levels of gene expression, we used the β-actin housekeeping gene. Amplifications were performed with the Icycler IQ System (Biorad, Hercules, CA) in a 25-μL final volume, using primers at 300-nM and probe at 200-nM concentration for 40 cycles at 30 seconds at 60°C and 30 seconds at 95°C. Primers and probes for NKp46, NKG2D, and NKp30 have already been described28 ; primers for β-actin are as follows: β-actin frw, 5′-CATGGTGCATCTCTGCCTTACA and β-actin rev, 5′-GGATAGCACAGCCTGGATAGCA. β-Actin probe (5′-TTTGAGACCTTCAACACCCCAGCCAT) has been labeled with 6-carboxyfluorescein (6FAM) and N, N, N′,N′-tetramethyl-6-carboxyrhodamine (XTp).
Relative expression of each transcript was obtained by calculating the ΔCT as the difference between the PCR threshold cycle number of the analyzed transcripts (NKp46, NKG2D, or NKp30), and the housekeeping transcript β-actin was used as reference.
The difference in expression levels between freshly derived and cultured NK cells was calculated by comparing the ΔCT of samples from freshly derived NK cells (used as control) with that of samples from IL-2–activated or IL-2–activated and l-kynurenine–treated cells
Monocyte-derived dendritic cells (Mo-DCs)
Mo-DCs were generated as previously described.24 Briefly, peripheralblood mononuclear cells (PBMCs) were derived from healthy donors and plastic adherent cells were cultured in the presence of IL-4 (Peprotech) and GM-CSF (Peprotech) at the final concentration of 20 ng/mL and 50 ng/mL, respectively. After 6 days of culture, cells were characterized by the CD14–, CD1a+, CD86– phenotype corresponding to immature Mo-DCs (iDCs).
Flow cytofluorimetric analysis and cytolytic activity
For cytofluorimetric analysis (FACSCalibur; Becton Dickinson, Mountain View, CA), cells were stained with the appropriate unlabeled mAb. Staining was followed by PE- or FITC-conjugated isotype-specific goat antimouse second reagent (Southern Biotechnology Associated, Birmingham, AL).
NK cells were tested for cytolytic activity in a 4-hour 51Cr-release assay33 against either the P815 FcγR+ murine cell line, human cell lines (including 721.221 B-lymphoblastoid cell line, M14 melanoma cell line, and K562 erythroleukemia cell line), or allogeneic immature Mo-DCs. The concentration of the various mAbs added in the assays was 10 μg/mL in the masking experiments and 1 μg/mL in the redirected killing experiments. The E/T ratios are indicated in the figure captions.
Cytokine secretion assays
NK cells were stimulated as follows: 5 × 104 NK cells/well were cultured overnight in 96-well microtiter plates precoated with goat antimouse (GAM; ICN, Santa Ana, CA) in the presence of the following mAb supernatants: c218 (IgG1, anti-CD56), AZ20 (IgG1, anti-NKp30), BAB281 (IgG1, anti-NKp46), and c127 (IgG1, anti-CD16). The culture supernatants were then collected and analyzed for the presence of TNF-α and IFN-γ by using specific enzyme-linked immunosorbent assay (ELISA) kits from BioSource International (Camarillo, CA).
Results
l-kynurenine affects the surface phenotype of activated NK cells
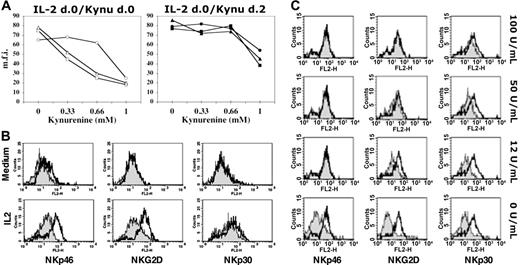
NK cells, freshly isolated from healthy donors, were cultured in complete medium supplemented with rhIL-2 either in the absence or in the presence of increasing doses of l-kynurenine. The range of l-kynurenine concentrations was chosen on the basis of a previous study reporting the thresholds of toxicity (1 mM) and efficacy (approximately 0.2 mM) of Trp catabolites on cultured PBMCs.4 After 48 hours of culture, treated and untreated NK cells were analyzed by flow cytometry for the surface expression of the main receptors involved in induction of the NK-cell response. These included NKp46, NKp30, and NKG2D. As shown in Figure 1A (left panel), at doses ranging from 0.3 to 0.6 mM, l-kynurenine clearly prevented the cytokine-induced up-regulation of NKp46 and NKG2D surface expression, while it only minimally affected the expression of the NKp30 receptor. At such concentrations, l-kynurenine did not significantly affect cell viability (the percent cell death ranged between 10% in untreated cells to 20% in treated cells). When the l-kynurenine concentration was increased up to 1 mM, NKp30 was also strongly down-regulated; in this case, however, in line with the study cited above,4 a toxic effect was detected, since cell viability dropped below 30%.
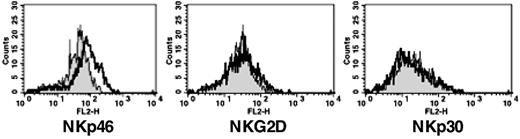
l-kynurenine induces modulation of certain NK-cell–triggering surface receptors. (A) (Left) Freshly isolated NK cells were cultured in the presence of IL-2 either alone or in combination with increasing concentrations of l-kynurenine (indicated on the x-axis). After 48 hours of culture, cells were evaluated by cytofluorimetric analysis for the surface expression of the main NK-cell–triggering receptors. (Right) Freshly isolated NK cells were cultured in the presence of IL-2. At day 2, l-kynurenine was added at the indicated concentrations. After a further 48 hours, cells were analyzed as in the left panel. For each determination, the mean fluorescence intensity (mfi) was calculated and reported: NKp46 (triangles), NKG2D (squares), or NKp30 (circles). (B) Freshly drawn NK cells were cultured for 2 days in medium alone or in the presence of IL-2. At the onset of the cultures, NK cells were given either l-kynurenine (at the final concentration of 0.5 mM) or dilution buffer alone. Treated (gray profiles) and untreated (white profiles) NK cells were analyzed by flow cytometry for the expression of the indicated triggering receptors. (C) Freshly drawn NK cells were cultured in the presence of IL-2 at the indicated concentrations. Either at the onset (0 U/mL) or after 2 days of culture (12, 50, 100 U/mL), NK cells were analyzed for the expression of the indicated triggering receptors (gray profiles). For comparison, in each panel the profile (white) indicating the maximum expression level for each receptor obtained by culturing cells for the same time period in the presence of 300 U/mL IL-2 is also reported. Results shown in panels A-C are representative of those obtained in 10 independent experiments.
l-kynurenine induces modulation of certain NK-cell–triggering surface receptors. (A) (Left) Freshly isolated NK cells were cultured in the presence of IL-2 either alone or in combination with increasing concentrations of l-kynurenine (indicated on the x-axis). After 48 hours of culture, cells were evaluated by cytofluorimetric analysis for the surface expression of the main NK-cell–triggering receptors. (Right) Freshly isolated NK cells were cultured in the presence of IL-2. At day 2, l-kynurenine was added at the indicated concentrations. After a further 48 hours, cells were analyzed as in the left panel. For each determination, the mean fluorescence intensity (mfi) was calculated and reported: NKp46 (triangles), NKG2D (squares), or NKp30 (circles). (B) Freshly drawn NK cells were cultured for 2 days in medium alone or in the presence of IL-2. At the onset of the cultures, NK cells were given either l-kynurenine (at the final concentration of 0.5 mM) or dilution buffer alone. Treated (gray profiles) and untreated (white profiles) NK cells were analyzed by flow cytometry for the expression of the indicated triggering receptors. (C) Freshly drawn NK cells were cultured in the presence of IL-2 at the indicated concentrations. Either at the onset (0 U/mL) or after 2 days of culture (12, 50, 100 U/mL), NK cells were analyzed for the expression of the indicated triggering receptors (gray profiles). For comparison, in each panel the profile (white) indicating the maximum expression level for each receptor obtained by culturing cells for the same time period in the presence of 300 U/mL IL-2 is also reported. Results shown in panels A-C are representative of those obtained in 10 independent experiments.
At variance with that study, we did not prestimulate NK cells with mitogens (ie, PHA); therefore, cultured NK cells displayed poor proliferation (not shown), and the effect of l-kynurenine could be related mainly to the surface phenotype (see also Table 1).
Influence of L-kynurenine treatment on KIR2DL1/S1+, KIR2DL3+, and KIR3DL1+ NK-cell subsets at different culture time points
. | KIRs analyzed* . | . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | KIR2DL1/S1 . | . | KIR2DL3 . | . | KIR3DL1 . | . | Total cell counts, cell/mL‡ . | . | |||||
| Kynurenine treatment,*lasting from d 0 to d 2 . | - . | + . | - . | + . | - . | + . | - . | + . | |||||
| Day 0 | 37† | — | 21 | — | 15 | — | 1.0 × 106 | — | |||||
| Day 2 | 35 | 34 | 23 | 24 | 16 | 17 | 0.9 × 106 | 0.8 × 106 | |||||
| Day 5 | 33 | 36 | 20 | 22 | 18 | 16 | 1.2 × 106 | 1 × 106 | |||||
. | KIRs analyzed* . | . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | KIR2DL1/S1 . | . | KIR2DL3 . | . | KIR3DL1 . | . | Total cell counts, cell/mL‡ . | . | |||||
| Kynurenine treatment,*lasting from d 0 to d 2 . | - . | + . | - . | + . | - . | + . | - . | + . | |||||
| Day 0 | 37† | — | 21 | — | 15 | — | 1.0 × 106 | — | |||||
| Day 2 | 35 | 34 | 23 | 24 | 16 | 17 | 0.9 × 106 | 0.8 × 106 | |||||
| Day 5 | 33 | 36 | 20 | 22 | 18 | 16 | 1.2 × 106 | 1 × 106 | |||||
—indicates not applicable.
Cells were cultured as indicated in Figure 4 and analyzed by immunofluorescence staining and cytofluorimetric analysis (data are referred to as 1 representative donor of 5 analyzed).
Numbers are referred to as the percentage of cells positive for the indicated KIRs within the whole NK-cell population analyzed.
In these columns, numbers indicate the cell counts (cells/mL) at various time intervals and in the different culture conditions.
Notably, l-kynurenine was effective only when it was added simultaneously to IL-2, while it did not substantially modify the phenotype of NK cells that have been cultured previously in IL-2 (Figure 1A right panel). Similarly, resting NK cells (ie, cells cultured in medium without IL-2) were virtually unaffected by l-kynurenine treatment (Figure 1B). This would suggest that l-kynurenine might interfere with the process that leads to the up-regulation of certain triggering receptors at the NK-cell surface. As shown in Figure 1B, following culture in IL-2, NK cells up-regulated the expression of NKp46, NKG2D, and NKp30. However, when cells were simultaneously treated with IL-2 and l-kynurenine, such increments appeared to be limited to NKp30, whereas the expression of NKp46 and NKG2D remained at levels comparable with those detected in resting NK cells. In some experiments, l-kynurenine also limited the full expression of the activation marker CD69 (not shown). The effect on NKp44 could not be evaluated since this receptor is generally induced on NK cells at later stages of activation.33
The effect of l-kynurenine on the phenotype of NK cells cultured in IL-2 appears to be specific since, apart from NKp46, NKG2D, and CD69, it did not modify the surface density of many other triggering receptors or coreceptors,36 including CD16, 2B4, NTB-A, DNAM-1, and CD59, nor affect that of inhibitory receptors36 such as killer Ig-like receptors (KIRs), NKG2A, and p75/AIRM-1 or adhesion molecules such as LFA-1, LFA-3, and ICAM-1 (not shown).
l-kynurenine may contrast the effects induced by IL-2 on NK cells. Further analyses, however, indicate that l-kynurenine should act directly on selected receptors rather than by interfering with the ability of NK cells to respond to IL-2. For example, l-kynurenine did not substantially modify the expression of the IL-2 receptors (CD25, CD122, CD132) (not shown). In addition, titration of IL-2 added in NK-cell cultures (without l-kynurenine) indicates that up-regulation of NKp30 receptor requires IL-2 at doses even higher than those required to induce increments in NKp46 or NKG2D expression (Figure 1C). Therefore, if l-kynurenine inhibited the overall response of the cells to IL-2, up-regulation of NKp30, rather than that of NKp46 or NKG2D, would be first blocked by treatment.
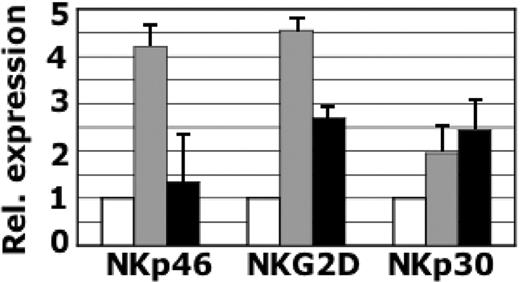
Effect of l-kynurenine on NKp46, NKG2D, and NKp30 transcript expression in NK cells. Real-time RT-PCR was performed on freshly derived NK cells (□) and on NK cells cultured for 48 hours in IL-2 either untreated (▦) or treated with 0.6 mM l-kynurenine (▪). Bar histograms indicate the expression levels of the different transcripts. The endogenous control β-actin was used to internally standardize the levels of gene expression. Data are expressed as fold difference between freshly derived cells (whose levels of gene expression have been assumed as 1) and cells cultured in the presence of either IL-2 or IL-2 + l-kynurenine. The histograms are representative of results obtained with NK cells derived from 2 different donors. Each experiment was run in triplicate; standard deviation (SD) is indicated.
Effect of l-kynurenine on NKp46, NKG2D, and NKp30 transcript expression in NK cells. Real-time RT-PCR was performed on freshly derived NK cells (□) and on NK cells cultured for 48 hours in IL-2 either untreated (▦) or treated with 0.6 mM l-kynurenine (▪). Bar histograms indicate the expression levels of the different transcripts. The endogenous control β-actin was used to internally standardize the levels of gene expression. Data are expressed as fold difference between freshly derived cells (whose levels of gene expression have been assumed as 1) and cells cultured in the presence of either IL-2 or IL-2 + l-kynurenine. The histograms are representative of results obtained with NK cells derived from 2 different donors. Each experiment was run in triplicate; standard deviation (SD) is indicated.
To verify whether l-kynurenine treatment affected not only the surface expression but also the transcription of NKp46 and NKG2D, real-time RT-PCR was performed on either untreated or treated NK cells. In these experiments, NK cells freshly derived from 2 different donors were analyzed immediately and after 48-hour culture in the presence of IL-2 either alone or in combination with l-kynurenine. Expression levels of NKp46, NKG2D, and NKp30 transcripts were evaluated using β-actin as internal control. Figure 2 shows that following IL-2 culture, expression levels of NKp46, NKG2D, and NKp30 were up-regulated. The presence of l-kynurenine in the culture, however, resulted in a significant decrease of NKp46 and NKG2D transcript expression, while NKp30 transcription levels were not decreased. This was in line with the unaltered NKp30 surface expression (Figures 1, 2). These data suggest that the decrease of NKp46 and NKG2D surface expression that follows l-kynurenine treatment may be due, at least in part, to the regulation of NKp46 and NKG2D transcription levels.
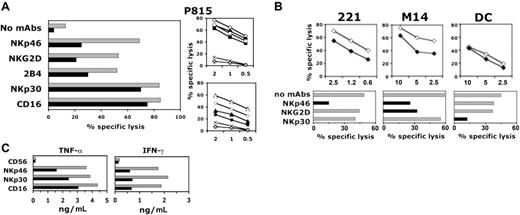
l-kynurenine affects NK-cell function. Freshly isolated NK cells were cultured for 2 days in the presence of IL-2 either alone or in combination with l-kynurenine at the final concentration of 0.3 mM and then assessed either for their cytolytic activity or for their ability to produce cytokines. (A) NK-cell cytotoxicity was assessed in a redirected killing assay against the FcγR+ P815 target cell line either in the absence of mAbs or in the presence of the mAbs to the indicated receptors. (Left) Gray bars indicate untreated NK cells; black bars, l-kynurenine–treated NK cells. (E/T ratio: 2:1.) (Right) Different E/T ratios are shown (indicated in the x-axis). Symbols indicate the specificity of the mAbs added in the assay. Diamonds indicate anti-CD16 mAb; squares, anti-NKp30 mAb; triangles, anti-NKp46 mAb; circles, anti-NKG2D mAb; X symbols, spontaneous lysis (ie, no mAbs added); white symbols, untreated NK cells; and black symbols, l-kynurenine–treated NK cells. (NK cells analyzed in the right panels are not the same as those shown in the left panel.) (B, upper panels) Treated (black diamonds) and untreated (white diamonds) NK cells were evaluated for their ability to kill the indicated target cell types. The E/T ratios are indicated in the horizontal axis. (Bottom panels) To determine which triggering receptors are involved in the NK-mediated recognition and killing of the various target cell types, the same (untreated) effector cells were assessed for their ability to kill the indicated targets either in the absence of mAbs or in the presence of mAbs that specifically mask the indicated receptors. (C) Treated (black bars) or untreated (gray bars) NK cells were stimulated overnight by plastic-bound goat antimouse antiserum plus one or another of the mAbs to the indicated receptors (anti-CD56 specific mAb was used as negative control). NKG2D was not analyzed since mAb-mediated triggering of this receptor does not result in cytokine release.37 Supernatants were harvested and assessed for cytokine content by specific ELISA.
l-kynurenine affects NK-cell function. Freshly isolated NK cells were cultured for 2 days in the presence of IL-2 either alone or in combination with l-kynurenine at the final concentration of 0.3 mM and then assessed either for their cytolytic activity or for their ability to produce cytokines. (A) NK-cell cytotoxicity was assessed in a redirected killing assay against the FcγR+ P815 target cell line either in the absence of mAbs or in the presence of the mAbs to the indicated receptors. (Left) Gray bars indicate untreated NK cells; black bars, l-kynurenine–treated NK cells. (E/T ratio: 2:1.) (Right) Different E/T ratios are shown (indicated in the x-axis). Symbols indicate the specificity of the mAbs added in the assay. Diamonds indicate anti-CD16 mAb; squares, anti-NKp30 mAb; triangles, anti-NKp46 mAb; circles, anti-NKG2D mAb; X symbols, spontaneous lysis (ie, no mAbs added); white symbols, untreated NK cells; and black symbols, l-kynurenine–treated NK cells. (NK cells analyzed in the right panels are not the same as those shown in the left panel.) (B, upper panels) Treated (black diamonds) and untreated (white diamonds) NK cells were evaluated for their ability to kill the indicated target cell types. The E/T ratios are indicated in the horizontal axis. (Bottom panels) To determine which triggering receptors are involved in the NK-mediated recognition and killing of the various target cell types, the same (untreated) effector cells were assessed for their ability to kill the indicated targets either in the absence of mAbs or in the presence of mAbs that specifically mask the indicated receptors. (C) Treated (black bars) or untreated (gray bars) NK cells were stimulated overnight by plastic-bound goat antimouse antiserum plus one or another of the mAbs to the indicated receptors (anti-CD56 specific mAb was used as negative control). NKG2D was not analyzed since mAb-mediated triggering of this receptor does not result in cytokine release.37 Supernatants were harvested and assessed for cytokine content by specific ELISA.
Effect of l-kynurenine on the NK-cell function
To assess whether the l-kynurenine–induced modulation of NKp46 and NKG2D receptors could result in an alteration of the NK-cell activity, treated and untreated NK cells were compared in a series of cytolytic assays.
As a first step, NK cells were analyzed in a redirected killing assay against the FcγR+ P815 target cell line to evaluate the effect of l-kynurenine on the activity of the different triggering receptors and coreceptors. As shown in Figure 3A, l-kynurenine treatment strongly reduced the ability of anti-NKp46 and anti-NKG2D mAbs to induce NK-cell–mediated killing, while it minimally affected the responsiveness to mAbs specific for other triggering receptors such as NKp30 or CD16. Of interest, the function of 2B4, which is known to act primarily as coreceptor of NKp46,15 was also modulated by l-kynurenine treatment (Figure 3A).
We next analyzed whether the selective loss of function of NKp46 and NKG2D receptors that follows l-kynurenine treatment could affect the ability of NK cells to kill NK-susceptible targets. To this end, different cell lines, each expressing a unique pattern of ligands for the different NK-cell–triggering receptors, were used as target cells in a cytolytic assay and analyzed for susceptibility to killing by either treated or untreated NK cells. Although some of the cellular ligands for NK-triggering receptors are still unknown, their presence at the target cell surface can be predicted in experiments of mAb-mediated masking of one or another triggering receptor (Figure 3B lower panels). Based on such experiments and consistent with previous data,15 the 721.221 lymphoblastoid cell line appears to be prevalently recognized and killed via the NKp46 receptor since antibodies to this receptor strongly reduced cytotoxicity. In the same series of experiments, the M14 melanoma cell line appears to be recognized and killed via NKp46 and NKG2D, while Mo-DCs were recognized and killed via NKp3022 (Figure 3B lower panels). As shown in Figure 3B (upper panels), l-kynurenine–treated NK cells showed a reduced ability to kill 721.221 or M14 cells (which are recognized and killed via NKp46 or via NKp46 and NKG2D, respectively), while maintaining their capability to kill immature Mo-DCs (which are recognized and killed upon engagement of NKp30).
In these functional experiments, optimal specific inhibitory effect was observed by using 0.3 mM l-kynurenine, while doses of 0.5 mM or more resulted in an overall, nonspecific inhibition of the NK-cell cytotoxicity.
Further experiments indicated that l-kynurenine treatment also reduced the ability of activating receptors to promote cytokine production (ie, IFN-γ and TNF-α) by NK cells. In this case, however, the inhibitory effect was less specific, since the function of NKp30- and CD16-activating receptors was, at least in part, also affected by l-kynurenine treatment (Figure 3C), although their expression remained unchanged. This finding suggests that l-kynurenine may affect NK-cell function not only by interfering with the expression and the function of NKp46- and NKG2D-triggering receptors, but also by decreasing the production of NK cytokines by a mechanism independent of receptor down-modulation.
The effect of l-kynurenine on NK cells is reversible
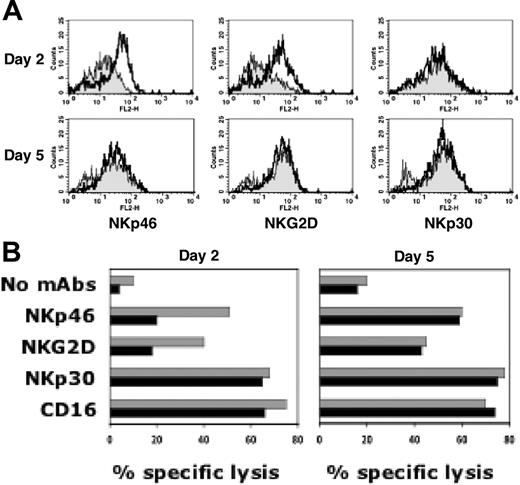
We next analyzed whether the ability to up-regulate NKp46 and NKG2D receptors in response to IL-2 is stably lost by NK cells upon l-kynurenine treatment or, on the contrary, whether it can be recovered once l-kynurenine has been removed. In order to answer this question NK cells were first cultured for 2 days in the presence of either IL-2 alone or IL-2 in combination with l-kynurenine and then washed and cultured for an additional 3 days in the presence of IL-2 alone. NK cells were then assessed for their surface phenotype by cytofluorimetric analysis and for their function by redirected killing assay. As expected, after 2 days in the presence of l-kynurenine, NK cells showed a reduced expression (Figure 4A) and function (Figure 4B) of both NKp46 and NKG2D receptors compared with untreated NK cells. However, after removal of l-kynurenine, NK cells returned within 3 days to the original surface phenotype (day 5, Figure 4A) and function (day 5, Figure 4B). Although not shown, a similar functional recovery could also be detected in experiments in which NK cells were analyzed for cytotoxicity against targets, such as K562 and M14 cell lines, that are recognized and killed via the NKp46 and NKG2D receptors.
Under the same experimental conditions, NK cells were further analyzed for the expression of different members of the KIR family. In healthy subjects, such receptors are clonally distributed within the circulating NK-cell population and allow the identification of discrete NK-cell subsets. Our analysis revealed that none of the subsets analyzed was modified in size by the l-kynurenine treatment. Indeed, as shown in Table 1, neither the percentage of KIR2DL1/S1+ cells nor that of KIR2DL3+ or KIR3DL1+ cells was affected in any of the culture time points. Thus, the possible effect of l-kynurenine on cell proliferation seems to be exerted homogeneously on the whole NK-cell population, and, apparently, does not determine any selection of phenotypically defined NK-cell subsets. On the other hand, cell count indicated that at such time intervals l-kynurenine induces only slight decrement in cell numbers (Table 1).
The effect of l-kynurenine on NK cells is transient. Freshly isolated NK cells were cultured in the presence of IL-2 either alone or in combination with l-kynurenine. At day 2, cells from both culture conditions were harvested, washed, and cultured for a further 3 days in medium containing IL-2 alone. At days 2 and 5, the cells were analyzed for the expression and function of the NKp46-, NKG2D-, and NKp30-triggering receptors. (A) NK cells were assessed by immunofluorescence and flow cytometry analysis for the surface expression of the indicated receptors. Gray profiles indicate cells that have been cultured in the presence of l-kynurenine (0.5 mM) for the first 2 days; white profiles, cells that have never been treated with l-kynurenine. (B) NK cells were analyzed for cytolytic activity against the P815 target cell line either in the absence of mAbs or in the presence of the mAbs to the indicated receptors. ▦ indicate untreated NK cells; ▪, NK cells that have been cultured in the presence of l-kynurenine (0.3 mM) for the first 2 days. (E/T ratio: 2:1.)
The effect of l-kynurenine on NK cells is transient. Freshly isolated NK cells were cultured in the presence of IL-2 either alone or in combination with l-kynurenine. At day 2, cells from both culture conditions were harvested, washed, and cultured for a further 3 days in medium containing IL-2 alone. At days 2 and 5, the cells were analyzed for the expression and function of the NKp46-, NKG2D-, and NKp30-triggering receptors. (A) NK cells were assessed by immunofluorescence and flow cytometry analysis for the surface expression of the indicated receptors. Gray profiles indicate cells that have been cultured in the presence of l-kynurenine (0.5 mM) for the first 2 days; white profiles, cells that have never been treated with l-kynurenine. (B) NK cells were analyzed for cytolytic activity against the P815 target cell line either in the absence of mAbs or in the presence of the mAbs to the indicated receptors. ▦ indicate untreated NK cells; ▪, NK cells that have been cultured in the presence of l-kynurenine (0.3 mM) for the first 2 days. (E/T ratio: 2:1.)
In conclusion, these data indicate that the effect of l-kynurenine is transient since, once it has expired, NK cells can regain full competence. This is further supported by the fact that the NK-cell “repertoire” appears to be maintained throughout the experiment.
Effect of l-kynurenine on NK cells stimulated by IL-12
Since during the first phase of innate immune responses one of the major sources of NK-cell activation is represented by Mo-DC–derived IL-12,25,38-40 we next assessed whether the effects of this cytokine on NK cells could also be affected by l-kynurenine. In our culture conditions, upon exposure to IL-12, NK cells seem to up-regulate the expression at the cell surface of NKp46, while NKG2D is only slightly modified (not shown). In addition, IL-12 did not induce substantial cell proliferation.39 As shown in Figure 5, the surface phenotype of NK cells cultured in the presence of IL-12 was influenced by l-kynurenine. In this case, however, only the expression of the NKp46 molecule appeared to be modulated by the treatment, while NKG2D was substantially unmodified. These results correlated with functional data, assessed by redirected killing assay. Indeed, in NK cells cultured in the presence of IL-12, l-kynurenine treatment selectively reduced the ability of NKp46 receptor to induce a cytolytic response (not shown).
Discussion
In the present study, we demonstrate that the induction of Trp catabolism may represent a novel potential mechanism capable of regulating NK-cell responses. Our experiments show that l-kynurenine, an IDO-generated Trp catabolite, is able to affect the process of NK-cell activation mediated by different cytokines. In particular, when NK cells are exposed to exogenous IL-2, l-kynurenine acts by interfering with the IL-2–driven up-regulation of NKp46- and NKG2D-triggering receptors. This modulation affects both surface expression and function of these receptors and consequently modifies the ability of NK cells to kill certain target cell types. Indeed, following l-kynurenine treatment, NK cells were less efficient in killing those targets that are recognized and killed via NKp46 and/or NKG2D receptor(s), but still maintained the ability to kill targets, such as immature Mo-DCs, that are recognized and killed via NKp30. Although the molecular mechanism responsible for the l-kynurenine–induced modulation of NKp46 and NKG2D is still to be defined, our experiments indicate that this occurs (at least in part) at the transcriptional level, since mRNAs for both receptors are clearly decremented.
l-kynurenine affects surface phenotype of NK cells cultured in the presence of IL-12. NK cells cultured for 2 days either in the presence of IL-12 alone (2.5 ng/mL; white profiles) or in combination with l-kynurenine (0.5 mM; gray profiles) were analyzed by immunofluorescence and flow cytometry analysis for the expression of the indicated receptors. Identical results were also obtained when NK cells were cultured in the presence of 5 ng/mL IL-12.
l-kynurenine affects surface phenotype of NK cells cultured in the presence of IL-12. NK cells cultured for 2 days either in the presence of IL-12 alone (2.5 ng/mL; white profiles) or in combination with l-kynurenine (0.5 mM; gray profiles) were analyzed by immunofluorescence and flow cytometry analysis for the expression of the indicated receptors. Identical results were also obtained when NK cells were cultured in the presence of 5 ng/mL IL-12.
In the last years, many different and often opposite functions have been assigned to the enzymatic activity of IDO. Due to its ability to degrade Trp, an essential amino acid for both eukaryotic and prokaryotic organisms, IDO has been proposed to synergize with the immune system in controlling pathogen proliferation as well as tumor growth.1,41-43 On the other hand, IDO enzymatic activity has also been reported to induce tolerance or immunosuppression by inhibiting T-cell proliferation.2,4-7,44 The IDO-mediated T-cell tolerization appears to be advantageous in protecting a semiallogeneic fetus during pregnancy,45 as well in avoiding insurgence of autoimmune phenomena.46 Conversely, IDO-mediated immune suppression may also be exploited by viruses or tumors to escape the immune surveillance.12,47 In addition, the IDO-generated Trp catabolites have recently been demonstrated to be active in blocking T- and NK-cell proliferation.4,5 All of these apparently conflicting data could be reconciled by considering IDO as a factor characterized by pleiotropic regulatory functions rather than as a mere suppressor of cell proliferation. This concept finds a confirmation in a recent paper by Bozza et al48 who demonstrated how IDO intervenes in regulating different stages of the response against fungal infections. Also, our data are in line with this concept. Indeed, we demonstrate that, at appropriate doses, l-kynurenine does not induce an overall suppression of the NK-cell function; rather, it tunes its spectrum of action by acting directly on specific NK-cell–triggering receptors. The idea that l-kynurenine acts as a regulatory agent able to confer plasticity to the NK-cell response is further supported by the observation that its effect appears to be transient (Figure 4) and confined to the period of time required for the initiation of the NK-cell activation. Indeed, the NK-cell phenotype could not be modified by l-kynurenine either before (Figure 1) or after (not shown) IL-2 treatment. This was also suggested by a recent report showing that resting NK cells were not affected in their cytolytic capability by kynurenine exposure.49 This study also showed that, in the presence of an inhibitor of IDO, resting NK cells might even decrease their ability to kill NK-susceptible targets. In these studies, however, NK cells were treated for a very limited time period since either kynurenine or IDO inhibitor was added during the cytolytic assays only.
In our experiments, l-kynurenine was used at doses that are in line with those indicated in previous reports.4,5 Such l-kynurenine concentrations could be reasonably achieved in tissues at least in certain pathologic conditions. It has to be noted that adequately primed macrophages produce, via IDO, 20 μmol/106 per hour l-kynurenine.2 Tumor cells can also be endowed with IDO activity, and many human solid tumors display a strong positivity.12 It can be reasoned that a massive tissue infiltration by IDO-expressing cells (for instance macrophages) in certain inflammatory diseases, or by mutated cells in tumors, could produce enough l-kynurenine in the tissue microenvironment to affect NK-cell function.
A further experiment on this subject could be the assessment of the phenotype and function of NK cells that have been cultured together with IDO+ cells. By this approach, however, the effect of kynurenine may not be easily dissected from that of other IDO-generated Trp catabolites4,5 or from the intrinsic capability to affect NK-cell function possibly shown by different types of IDO+ cells. The answer to these questions is not the object of our study, however these assessments would be mandatory in future studies aimed at elucidating the meaning of IDO expression in different cell types. Along this line, it is noteworthy that the analysis of how IDO enzymatic activity is regulated in different cell types is still the object of intense studies.9-11,44,50-53
The regulation of the IDO activity and, consequently, of the Trp catabolite production may be one of the keys to determine whether this enzyme is acting as a suppressor or as a regulator. Our experiments indicate that in the case of a limited exposure time (2 days) l-kynurenine induces reversible effects on NK cells (Figure 4), while, as demonstrated in a recent report,5 for longer exposure times (more than 3 days) it induces cell death (not shown). Therefore, cells that can appropriately control IDO activity and l-kynurenine production could exert a regulatory effect on NK cells, while cells (if they do exist) that constitutively express IDO in an active form would induce NK-cell suppression. In this context, it could be of interest to assess whether, in contrast to what occurs in monocytes or DCs, l-kynurenine could be produced constitutively by IDO+ tumor cells. A recent study by Uyttenhove et al,12 reporting how certain tumor cell lines show constitutive l-kynurenine production, supports this notion.
An additional regulation on IDO+ cells may also be exerted by NK cells. As strong producers of IFN-γ, NK cells may induce IDO activity on DCs or macrophages. On the other hand, NK cells are also able to kill immature DCs,22,24 thus limiting one of the sources of IDO+ cells. Of interest, l-kynurenine is able to inhibit NK-cell cytokine production, while it does not affect their ability to kill DCs (Figure 3). Therefore, l-kynurenine could take part in a regulatory loop allowing NK cells to control excessive IDO-mediated suppression.
The inhibitory effect mediated by l-kynurenine on NK cells is reminiscent of previous data indicating that the immunomodulatory cytokine TGF-β1 could alter the pattern of triggering surface receptors expressed on NK cells.34 Such modulation, however, is different from the one herein described, since the former involved NKp30 (and not NKp46) and NKG2D. Therefore, at variance with l-kynurenine, TGF-β1 also affects NK cells in their ability to interact with DCs, since the NKp30 receptor is prevalently involved in such interaction.22,27 On the basis of these observations, it may be speculated that the large panel of triggering receptors and coreceptors that equips NK cells may supply defined and differentiated functions. Each of these functions would be enhanced or temporarily blocked by the combined action of different regulatory factors (cytokines, kynurenine, and/or others) able to modulate one or another NK-triggering receptor. This plasticity of the NK-cell repertoire should allow a subtle tuning of the NK-cell functions by the cells of immune system but also could be exploited by pathogens and tumors to escape the NK-cell–mediated attack.
Authorship
Contribution: M.D.C. and S.C. were responsible for most experiments and contributed to study design; G.F. had the initial idea and advised on the preparation of the report; M.B. did some of the experiments and prepared the figures; C.C. and R.C. did RT-PCR experiments and analyzed data; L.M. contributed to writing the article and searching funds; A.M. analyzed data, and contributed to writing the article and searching funds; M.V. contributed to study design, analyzed data, and wrote the article.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 10, 2006; DOI 10.1182/blood-2006-03-006700.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro (AIRC); Istituto Superiore di Sanità (ISS); Ministero della Salute–RF 2002/149; Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST); Ministero dell'Istruzione dell'Università e della Ricerca (MIUR); FIRB-MIUR progetto-codRBNE017B4; European Union FP6; LSHB-CT-2004-503319-Allostem (the European Commission is not liable for any use that may be made of the information contained); Fondazione Compagnia di San Paolo; and Fondazione Cariverona, Bando 2004, indirizzo biomedico. M.D.C. is the recipient of a fellowship awarded by Federazione Italiana Ricerca sul Cancro (FIRC).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal