Abstract
In eukaryotic cells the phospholipid phosphatidylserine (PS) is restricted to the inner plasma-membrane leaflet. This lipid asymmetry, which is maintained by the concerted action of phospholipid transport proteins, is mainly lost during apoptosis. Here, we demonstrate that primary human CD8+ cytotoxic T lymphocytes (CTLs) expose PS on T-cell receptor (TCR)–mediated antigen (Ag) recognition. In contrast to PS externalization on apoptotic cells, activation-induced PS exposure is less pronounced and reversible. Fluorescence microscopic analysis revealed that PS is distributed nonhomogenously over the plasma membrane and concentrated in membrane lipid raft domains at the immunologic synapse. By studying the activity of PS transport proteins using a fluorescence-labeled PS analogue, we found that activation of CTLs inhibited the flippase-mediated inward-directed PS transport without affecting the outward transport. Shielding of exposed PS by annexin V protein during Ag recognition diminished cytokine secretion, activation, and cell-to-cell clustering of Ag-specific CTLs. In summary, our data demonstrate for the first time that externalized PS on Ag-stimulated CTLs is linked to T-cell activation and probably involved in cell-to-cell contact formation at the immunologic synapse.
Introduction
Most eukaryotic cells exhibit an asymmetric distribution of phospholipids (PLs) in their plasma membrane with phosphatidylcholine and sphingomyelin concentrated in the outer leaflet and phosphatidylethanolamine and phosphatidylserine (PS) predominantly located in the cytosolic leaflet.1 PS is the only PL thereof that is completely restricted to the inner membrane layer.
This PL asymmetry is maintained by an energy-dependent flippase, known as the aminophospholipid translocase (APTL), that uses ATP hydrolysis to catalyze a fast, inward-directed transport of aminophospholipids PS and phosphatidylethanolamine across the plasma membrane.2 A second class of ATP-dependent lipid transporters, the floppases, mediates outward-directed PL transfer. In contrast to these energy-dependent lipid transporters the activation of a Ca2+-dependent PL scramblase, a putative membrane protein facilitating a rapid equilibration of PLs between the 2 plasma-membrane leaflets, causes the disruption of lipid asymmetry. The appearance of PS at the cell surface as a consequence of the loss of lipid asymmetry is described predominantly during early apoptosis and is reported to be a phylogenetically conserved process.3-6 For apoptotic lymphocytes PS exposure has been ascribed to an inhibition of the APTL activity in parallel to an activation of the PL scramblase. Both processes rapidly lead to a randomization of the trans-bilayer lipid distribution.7-9
Annexin V (annV) is a member of a large family of Ca2+- and PL-binding proteins.10 In the presence of physiologic concentrations of Ca2+ annV has a high affinity for PS. Fluorescence-labeled annV is widely used to detect early apoptotic cells by flow cytometry.11,12 However, an externalization of PS is not restricted to apoptotic cells but has also been described for several cell types including platelets,13 neutrophils,14 B cells,15 erythrocytes,16 mast cells,17 and T-cell hybridomas18 on cellular activation. Stimulation of the particular cell type leads to an elevated cytoplasmic Ca2+ level followed by an induction of the PL scramblase and an accelerated PL movement. Williamson et al18 investigated the PL scramblase activation in murine T-cell hybridoma cells and found that apoptosis and activation, fundamentally different processes, both result in the activation of the same PL scramblase.
Functional implications of activation-induced PS exposure have been described in detail for procoagulant platelets, on which external PS strongly propagates the coagulation cascade.19,20 Moreover, for mast cells and neutrophils it is hypothesized that PS exposure influences granular exocytosis.14,17 An involvement of PS has been reported for the formation of myotubes during muscle development21 and for the adhesion of erythrocytes to endothelium.22,23 For other cell types including T cells, the biologic function of activation-induced PS exposure is largely unclear. Recently, Elliott et al24 described a potential role of PS distribution in the modulation of transport proteins, shedding of CD62L, and T-cell migration in nonapoptotic CD4+ murine T lymphocytes.
In this report, we investigated the role of activation-induced PS exposure on human antigen (Ag)–specific CD8+ T cells recognizing an HLA-A2–binding 10-mer Melan-A peptide. Melan-A is a melanocyte differentiation Ag and frequently overexpressed on melanoma cells.25
We found that PS exposure in Ag-experienced effector CD8+ T cells is linked to T-cell activation and may play a more profound role at the immunologic synapse.
Materials and methods
Media, cytokines, and peptides
T cells were cultured in supplemented RPMI 1640 medium (Gibco, Karlsruhe, Germany) plus 10% human AB serum (PAN Biotech, Aidenbach, Germany). The following recombinant human cytokines were used: 50 U/mL or 200 U/mL interleukin-2 (IL-2; Chiron, Muenchen, Germany) and 2000 U/mL interferon-γ (IFN-γ; PromoCell, Heidelberg, Germany). Preparation of T-cell growth factor (TCGF) was described previously.26 The HLA-A2–binding peptides Melan-A26-35 (ELAGIGILTV) and gp100280-288 (YLEPGPVTA) were used (Clinalfa, Laeufelfingen, Switzerland).
MHC peptide tetramers and mAbs
Phycoerythrin (PE)–conjugated HLA-A*0201 tetramer folded around Melan-A26-35 was from Beckman Coulter (Fullerton, CA). The following monoclonal antibodies (mAbs) were used: CD4-APC, CD8-APC, CD69-FITC, CD25-PE, CD45RO-PE, CD95-PE, HLA-DR-FITC (all from BD PharMingen, Heidelberg, Germany), T-cell receptor (TCR) αβ-PE, and CD71-FITC (Immunotech, Krefeld, Germany). For blocking studies a defined supernatant of anti–MHC class I mAb (W6/32) was used. For apoptosis induction, cytotoxic T lymphocytes (CTLs) were treated with anti-fas mAb (CH-11, Biomol, Hamburg, Germany).
Generation of Melan-A–specific CD8+ T cells
Peripheral-blood mononuclear cells (PBMCs) were obtained by leukapheresis of healthy donors, followed by density gradient centrifugation (Biocoll, Biochrom, Berlin, Germany). The study was approved by the University of Regensburg Institutional Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki. Melan-A–specific CTL lines were generated as described previously27 and used for all experiments if not indicated otherwise. Briefly, CD8+ T cells were enriched magnetically from PBMCs (Miltenyi Biotec, Bergisch-Gladbach, Germany). Mature monocyte-derived dendritic cells (DCs)27 were pulsed with 30 μg/mL Melan-A26-35 peptide and 10 μg/mL human β2-microglobulin for 2 hours at 37°C in serum-free medium. CD8+ T cells (6 × 106) were restimulated weekly with 2 × 106 autologous peptide-loaded DCs in 96-well U-bottom plates. Complete medium supplemented with 3% TCGF was replenished twice a week. CTLs were used for experiments 4 to 7 days after last restimulation. The Melan-A–specific CTL clone S2Cl14 was expanded by stimulation with irradiated allogeneic PBMCs and PHA-L (Sigma, Steinheim, Germany) in Iscove medium (Biochrom) supplemented with 50 U/mL IL-2.
Cell lines
The transporter associated with antigen processing (TAP)–deficient T2 cell line28 was cultured in complete medium plus 10% FCS. T2 cells were kept for 6 hours at room temperature (RT) in X-Vivo 15 medium (Cambrex, Verviers, Belgium) and were pulsed overnight with 5 μg/mL Melan-A peptide (if not indicated otherwise) and 10 μg/mL β2-microglobulin in serum-free medium. After Ag loading, T2 cells were washed 4 times with complete medium to remove redundant free peptide. HLA-A2+ melanoma cell lines MeI493 (Melan-A+) and Na8 (Melan-A–)29 were cultured in complete medium plus 10% FCS.
Flow cytometry and cell sorting
Prior to flow cytometric analysis, cells were stimulated either with cytokines (IL-2, IFN-γ), anti-CD3/CD28–coated DynaBeads (CTL/bead ratio of 1:1; Dynal Biotech, Oslo, Norway), ionomycin, melanoma cells, or Melan-A–pulsed T2 cells. Depending on the respective experiment, cells were stained with Melan-A tetramers (30 minutes at 37°C), then with mAbs, and finally with 5 μL annV-FITC in 150 μL annV-binding buffer (both from BD PharMingen) for 15 minutes at RT in the dark. Propidium iodide (PI; 0.1 μg/150 μL; Calbiochem, Laeufelfingen, Switzerland) was added immediately prior to fluorescence-activated cell scanning (FACS) analysis. Cytokines were quantified using the Th1/Th2 cytokine bead array (BD Biosciences, San Diego, CA) following the manufacturer's instructions. Flow cytometry was performed on a FACSCalibur (BD Biosciences). Data were analyzed with CellQuest (BD Biosciences) or WinMDI software (J. Trotter, Scripps Institute, San Diego, CA). For cell sorting (FACSAria, BD Biosciences) CTLs were stained with anti-CD8 mAb and annV-FITC and resuspended in annV-binding buffer. Gated vital CD8+ T cells were sorted for annVint and annVhigh binding. Labeling of CTLs with membrane dye PKH-26 has been described previously.30
TUNEL assay
The terminal transferase-mediated DNA nick end labeling (TUNEL) assay (BD PharMingen) was performed according to the manufacturer's instructions. Briefly, cells were fixed for 45 minutes with 1% paraformaldehyde (PFA; Sigma), washed twice, and stored overnight in 70% ethanol at –20°C. Fixed cells were incubated for 60 minutes at 37°C with the terminal deoxynucleotidyl-transferase enzyme and FITC-dUTP solution to label 3′ end DNA fragments. Samples were then subjected to flow cytometry.
Fluorescent PL transport assay
Palmitoyl-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-sn-glycero-3-phospho-l-serine (NBD-PS) was from Avanti Polar Lipids (Alabaster, AL). For determination of PS outward transport, 5 × 106 CTLs were labeled with 3 μM NBD-PS for 15 minutes at 37°C. During this period, analogues redistributed to the cytoplasmic membrane leaflet mediated by an inward-directed flippase activity present in the plasma membrane. Hydrolysis of analogues was blocked by addition of 1 mM Pefabloc (Serva, Heidelberg, Germany) to all labeling and washing buffers. After incubation for 15 minutes, NBD-PS remaining on the cell surface was extracted by washing cells 3 times with Hanks buffer containing 10% HSA(Octapharma, Dessau, Germany). Subsequently, CTLs were resuspended in Ca2+-containing medium (1% HSA, 1 mM CaCl2; Sigma), stimulated with 5 μM ionomycin or Melan-A–loaded T2 cells (stained with anti-CD4–APC to allow out-gating of T2 cells) at a CTL/T2 ratio of 3:1. After various time points, aliquots were analyzed flow cytometrically after addition of 10 mM freshly dissolved sodium dithionite to quench NBD fluorescence in the medium. Immediately prior to flow cytometry, 0.1 μg/150 μL PI was added for 1 minute.
For determination of PS inward transport, cells were preincubated in 5 μM ionomycin for 15 minutes or with Melan-A–loaded T2 cells (CTL/T2 ratio of 3:1) or left untreated for 45 minutes at 37°C in serum-free medium containing 1 mM CaCl2 and subsequently labeled with NBD-PS (5 minutes on ice). An anti-CD8–APC staining was performed to allow gating of T cells. Noninserted analogues were removed by washing with ice-cold Hanks buffer. Inward redistribution of analogues was initiated by incubation at 37°C. Before flow cytometric analysis, HSA (final 2%) and freshly dissolved sodium dithionite (final 10 mM) were added to extract and reduce NBD lipids from the cell surface. The relative amount of NBD-PS present in the HSA/dithionite-treated sample (% nonaccessible) was calculated as a percentage of the total amount present in a control sample not treated with HSA/dithionite. Immediately prior to flow cytometry, 0.1 μg/150 μLPI was added for 1 minute.
Fluorescence microscopy
CTLs were stimulated with anti-CD3/CD28–coated DynaBeads (Dynal) for 4 hours at a ratio of 1:1. After magnetic depletion of beads, CTLs were stained for lipid rafts with 1 μg/mL cholera toxin B (CTx-B) followed by an incubation with a 1:200 dilution of an anti–CTx-B–AlexaFluor594–conjugated rabbit serum (both from Molecular Probes, Eugene, OR). Exposed PS was detected by staining with 1.3 × 106 cells with 20 μL annV-FITC in annV-binding buffer (both from BD PharMingen) for 30 minutes at RT. Cells were fixed with 2% PFA in annV-binding buffer, applied onto slides coated with poly-l-lysine (Sigma), and mounted (DakoCytomation, Hamburg, Germany). As a control, CTLs were treated with an apoptosis-inducing anti-fas mAb for 24 hours. For visualization of immunologic synapses, Melan-A–specific CTLs were labeled with an anti–MHC-I mAb followed by a goat anti–mouse 9-amino-6-chloro-2-methoxyacridine (AMCA)–conjugated secondary antibody to allow discrimination between T cells and APCs prior to lipid raft staining. CTLs were then cocultured with Melan-A–pulsed T2 cells for 2 hours. Finally, cells were harvested, stained with annV-FITC, transferred onto poly-l-lysin–coated glass slides, and mounted. Images were acquired with an Axioscope2plus fluorescence microscope equipped with an AxioCam-HR CCD camera using a Plan-Neofluar 100 ×/1.3 oil objective (all from Zeiss, Goettingen, Germany).
Blocking of exposed PS
CTLs were cocultured with peptide-pulsed T2 cells at a ratio of 3:1 in complete medium containing 1.5 mM CaCl2 and 6.55 mM HEPES (Sigma) in 96-well U-bottom plates. Unconjugated annV (BD PharMingen) was added into the culture at a final concentration of 50 μg/mL to block exposed PS during the assay. Control cocultures were treated with PBS. Transmission light images of cluster formation were taken with an Axiovert25 microscope (Zeiss) after 24 hours.
Results
Human Ag-specific CD8+ T cells expose PS after Ag recognition
Melan-A–specific CTL lines recognizing the Melan-A26-35 peptide were generated by weekly stimulation with autologous Melan-A–pulsed mature DCs. The frequency of Melan-A–specific T cells as determined by Melan-A–MHC tetramer staining was greater than 90% among total CD8+ T cells (data not shown).
We investigated the phenomenon of PS exposure on Melan-A–specific CTLs after 4 hours of stimulation with Melan-A–loaded T2 cells demonstrating a marked exposure of PS as determined by annV-FITC staining, a highly specific PS-binding protein (Figure 1A). PS exposure was Ag-specific (> 80% annV+ T cells) but control peptide (gp100) pulsed and unpulsed T2 cells also induced a background PS exposure on Melan-A–specific CTLs. Furthermore, Melan-A–specific CTLs did not show a significant PS exposure on stimulation with 200 U/mL IL-2 or 2000 U/mL IFN-γ stimulation (Figure 1B). However, stimulation of CTLs with anti-CD3/CD28–coated beads led to the induction of annV+ CTLs (Figure 1B). This finding indicates that only TCR/CD3 complex ligation is able to induce PS exposure on Ag-specific CTLs. As expected, ionomycin, an ionophore that increases the intracellular Ca2+ concentration, mimicking the result of TCR ligation, also induced PS exposure on Melan-A–specific CTLs (Figure 1C). Furthermore, an HLA-A2+, Melan-A+ melanoma cell line was used to analyze if endogenously processed and presented Melan-A Ag is also able to induce PS exposure in Ag-specific CTLs. Indeed, after 4 hours of coculture with HLA-A2+ Melan-A+ melanoma cells (MeI493), CTLs exhibited an annV-FITC+ phenotype, whereas no PS exposure could be detected after coincubation with HLA-A2+ Melan-A– tumor cells (Na8; Figure 1D). Pretreatment of MeI493 with a neutralizing anti–MHC-I mAb completely inhibited PS exposure on Melan-A–specific CTLs (Figure 1D).
Human CD8+ Melan-A–specific T cells expose elevated amounts of PS after Ag recognition. Flow cytometric analysis of annV-FITC binding on the cell surface of Melan-A–specific CTLs. (A) CTLs were labeled with membrane dye PKH-26 and stimulated for 4 hours either with unloaded, Melan-A–loaded, or gp100-loaded T2 cells at a T2/CTL ratio of 1:2.5 and stained with annV-FITC. Unstimulated CTLs (0 and 4 hours) served as a control. Shown is one representative flow cytometric analysis of 5. (B) Melan-A–specific CTLs were activated with different types of stimuli for 4 hours to induce PS exposure: T2 cells loaded with 5μg/mL Melan-A peptide, 200 U/mL IL-2, 2000 U/mL IFN-γ, anti-CD3/CD28–coated beads, or complete medium as a control. Cells were stained with annV-FITC and dead/apoptotic cells were excluded via PI staining. One representative experiment of 5 is shown. Numbers in panels A and B represent percentages of annV+/PKH26+ T cells. (C) Melan-A–specific CTLs were stimulated with 2.5 μM ionomycin (▴) or left unstimulated (○) at 37°C in medium containing 1.5 mM CaCl2 and annV-FITC. Flow cytometric analysis was performed at indicated time points. Mean values ± SEM from 4 independent experiments are shown. (D) CTLs were labeled with PKH26 and coincubated for 4 hours with HLA-A2+ Melan-A–expressing (MeI493) or Melan-A– (Na8) melanoma cell lines at different tumor cell/CTL ratios. Preincubation of MeI493 with an anti–MHC-I–blocking mAb (clone W6/32) was performed to demonstrate MHC class I-restricted Ag recognition. Results represent percent annV-FITC+ cells of total PKH-26+ CTLs. Data show one representative experiment of 2.
Human CD8+ Melan-A–specific T cells expose elevated amounts of PS after Ag recognition. Flow cytometric analysis of annV-FITC binding on the cell surface of Melan-A–specific CTLs. (A) CTLs were labeled with membrane dye PKH-26 and stimulated for 4 hours either with unloaded, Melan-A–loaded, or gp100-loaded T2 cells at a T2/CTL ratio of 1:2.5 and stained with annV-FITC. Unstimulated CTLs (0 and 4 hours) served as a control. Shown is one representative flow cytometric analysis of 5. (B) Melan-A–specific CTLs were activated with different types of stimuli for 4 hours to induce PS exposure: T2 cells loaded with 5μg/mL Melan-A peptide, 200 U/mL IL-2, 2000 U/mL IFN-γ, anti-CD3/CD28–coated beads, or complete medium as a control. Cells were stained with annV-FITC and dead/apoptotic cells were excluded via PI staining. One representative experiment of 5 is shown. Numbers in panels A and B represent percentages of annV+/PKH26+ T cells. (C) Melan-A–specific CTLs were stimulated with 2.5 μM ionomycin (▴) or left unstimulated (○) at 37°C in medium containing 1.5 mM CaCl2 and annV-FITC. Flow cytometric analysis was performed at indicated time points. Mean values ± SEM from 4 independent experiments are shown. (D) CTLs were labeled with PKH26 and coincubated for 4 hours with HLA-A2+ Melan-A–expressing (MeI493) or Melan-A– (Na8) melanoma cell lines at different tumor cell/CTL ratios. Preincubation of MeI493 with an anti–MHC-I–blocking mAb (clone W6/32) was performed to demonstrate MHC class I-restricted Ag recognition. Results represent percent annV-FITC+ cells of total PKH-26+ CTLs. Data show one representative experiment of 2.
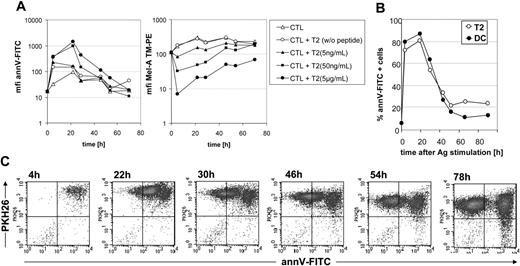
Next, we studied the kinetics and reversibility of PS exposure in parallel to TCR internalization, which is accepted as an indicator for MHC-peptide recognition.31 T2 cells were loaded with increasing concentrations of the Melan-A peptide and used as stimulator cells for Melan-A–specific CTLs. We found that high amounts of the peptide (5 μg/mL) provoked a strong PS exposure on CTLs (determined by mean annV-FITC binding) for more than 30 hours, whereas lower amounts of peptide (5 ng/mL) only induced a moderate PS exposure on CTLs (Figure 2A). This phenomenon is also reflected by an increase in the percentage of annV-FITC+ cells (data not shown). In parallel, we determined TCR internalization via Melan-A–MHC tetramer binding (Figure 2A) and found that both PS exposure and TCR internalization occur in parallel. To exclude that PS exposure is caused by an artificial overstimulation by peptide-loaded T2 cells, we analyzed the PS exposure on Melan-A–specific CTLs after stimulation with autologous peptide-pulsed DCs. Therefore, mature DCs were loaded with 30 μg/mL Melan-A peptide. In comparison to T2 cells (5 μg/mL), a higher amount of peptide is necessary for loading of DCs to replace endogenously processed peptides bound on MHC complexes. Data demonstrate that Melan-A–pulsed DCs induce PS exposure on CTLs to a similar extent and with similar kinetics as peptide-pulsed T2 cells (Figure 2B). Together these data demonstrate that CTLs pass through an annV+ state as a result of Ag recognition.
AnnVint CTLs do not undergo apoptosis
To follow more precisely the fate of annV+ CTLs after Ag-specific stimulation, we labeled Melan-A–specific CTLs with the membrane dye PKH-26 and isolated all annV+ PKH-26+ CTLs 4 hours after stimulation with Melan-A–loaded T2 cells by cell sorting. We could demonstrate that the annV+ T-cell population is heterogenous; whereas the annVhigh T-cell population retained the annVhigh phenotype, the annVint T-cell population revealed a constant decrease of annV binding and became annVneg at 54 to 72 hours after stimulation (Figure 2C).
We hypothesized that the annVhigh CTL population consists mainly of apoptotic cells, whereas the annVint CTL population is composed of vital T cells that transiently expose PS on activation. To prove this hypothesis, we characterized annVint CTLs in more detail. Phenotypic analysis of annVint cells in Melan-A–specific CTLs was performed at 4 hours after Ag-specific stimulation. Cells were gated on annVint CTLs and analyzed for expression of CD69, CD71, CD95, HLA-DR, and TCRαβ (Figure 3A). We found that annVint CTLs exhibit a phenotype that is typical for early T-cell activation: an up-regulation of the early activation marker CD69 and a down-regulation of the TCRαβ chain due to TCR internalization. Moreover, annVint CTLs expressed other markers of an activated, mature phenotype: CD71, CD95, HLA-DR. Note that most of these Ags are constitutively expressed on unstimulated annVneg Melan-A–specific CTLs due to weekly in vitro restimulation in the course of T-cell expansion (Figure 3A dashed line).
Kinetics of PS exposure on activated human CTLs. (A) T2 cells were loaded with different concentrations of Melan-A peptide and used as stimulator cells for Melan-A–specific CTLs. At indicated time points CTLs were harvested, stained with PE-conjugated Melan-A–MHC tetramers, CD8-APC, annV-FITC, and PI.. PI–CD8+ CTLs were gated and analyzed for annV-FITC binding by flow cytometry. Mean fluorescence intensity (MFI) of one representative experiment of 3 is shown. (B) CTLs were stimulated with peptide-loaded (5 μg/mL) T2 cells or peptide-loaded (30 μg/mL) mature DCs. One representative kinetics of 3 is shown. (C) Melan-A–specific CTLs were labeled with the membrane dye PKH-26, stimulated for 3 hours with Melan-A–loaded T2 cells and isolated by cell sorting. Sorted CTLs were then cultured in TCGF-containing medium and PS exposure was determined by staining with annV-FITC at different time points.
Kinetics of PS exposure on activated human CTLs. (A) T2 cells were loaded with different concentrations of Melan-A peptide and used as stimulator cells for Melan-A–specific CTLs. At indicated time points CTLs were harvested, stained with PE-conjugated Melan-A–MHC tetramers, CD8-APC, annV-FITC, and PI.. PI–CD8+ CTLs were gated and analyzed for annV-FITC binding by flow cytometry. Mean fluorescence intensity (MFI) of one representative experiment of 3 is shown. (B) CTLs were stimulated with peptide-loaded (5 μg/mL) T2 cells or peptide-loaded (30 μg/mL) mature DCs. One representative kinetics of 3 is shown. (C) Melan-A–specific CTLs were labeled with the membrane dye PKH-26, stimulated for 3 hours with Melan-A–loaded T2 cells and isolated by cell sorting. Sorted CTLs were then cultured in TCGF-containing medium and PS exposure was determined by staining with annV-FITC at different time points.
To further prove the hypothesis that annVint CTLs are nonapoptotic T cells, we determined the percentage of late apoptotic/necrotic cells in both, annVint and annVhigh T cells via PI staining. To avoid contamination with Melan-A nonresponsive T cells, we used the Melan-A–specific CTL clone (S2Cl14) for these experiments. The CTL clone was labeled with the membrane dye PKH-26, stimulated with Melan-A–loaded T2 cells, and analyzed for up to 96 hours after stimulation. PI+ dead cells could only be detected in the annVhigh T-cell population, whereas annVint cells were completely negative (Figure 3B). We cannot exclude that a certain percentage of stimulated CTLs die from activation-induced cell death and are found at later time points in the annVhigh subpopulation, but cells with a stable annVint phenotype apparently are vital.
In addition, we sorted annVhigh and annVint CTLs stimulated overnight with Melan-A–pulsed T2 cells and performed a TUNEL assay to detect DNA rupture in both cell populations. In line with PI staining we could demonstrate that FITC-dUTP+ cells in a late stage of apoptosis are found only in the annVhigh CTL subpopulation (Figure 3C). AnnVneg CTLs from an unstimulated sample served as a negative control. These T cells exhibited a typical slightly decreased background FITC-dUTP fluorescence intensity (Figure 3C). To confirm these data we determined the presence of active caspase-3 in annVhigh and annVint CTLs and found, in line with previous findings, that active caspase-3 could only be detected in annVhigh but not in annVint T cells (data not shown).
Exposed PS on Ag-specific T cells colocalizes with lipid rafts
Next, we analyzed the spatial distribution of exposed PS on Ag-specific T cells after stimulation and performed fluorescence microscopy to clarify if exposed PS is distributed homogenously over the cell membrane or is located in distinct membrane regions. Melan-A–specific CTLs were stimulated for 4 hours with anti-CD3/CD28–coated beads followed by annV-FITC staining. In all samples we found a certain percentage of annVhigh CTLs probably deriving from activation-induced cell death. However, annVint CTLs with a patchy PS distribution could only be observed in anti-CD3/CD28–stimulated T cells (Figure 4A middle). This population was completely absent in the samples treated with anti-fas mAb containing predominantly annVhigh apoptotic/dead CTLs with a strong PS exposure (Figure 4A right). As a negative control, unstimulated CTLs were stained with annV-FITC (Figure 4A left). We next addressed the question whether PS exposure is colocalized with membrane lipid raft regions, special microdomains of the cell membrane rich in cholesterol and signal-transducing molecules. We visualized the cell-surface distribution of these lipid rafts via CTx-B, a protein that binds to ganglioside GM-1 and is thus enriched in lipid rafts. Hence, CTLs were stimulated for 4 hours with anti-CD3/CD28–coated beads and consecutively stained with CTx-B, anti–CTx-B–Alexa594 rabbit serum, and finally with annV-FITC. Overlays of green and red fluorescence revealed a colocalization of PS exposure and lipid raft regions (Figure 4B yellow color). These data suggest that PS exposure occurs predominantly in membrane patches that are involved in signal transduction, indicating a profound relevance of exposed PS for T-cell function. To further visualize the PS exposure at the immunologic synapse Melan-A–specific CTLs were labeled with an anti–MHC-I mAb to allow discrimination between T cells and T2 cells (blue fluorescence in Figure 4C). After an additional lipid raft staining with Alexa594-conjugated CTx-B (red fluorescence), CTLs were cocultured for 2 hours with Melan-A–pulsed T2 cells. Subsequent annV-FITC staining (green fluorescence) revealed that PS externalization predominantly occurs at the contact side between CTLs and T2 cells. PS as well as raft domains are concentrated at the immunologic synapse (Figure 4C yellow color). Of note, Ag-loaded T2 cells are also annV+ most probably due to the cytotoxic attack by Ag-specific CTLs.30,32
Ag-primed annVint CTLs are activated but nonapoptotic T cells. (A) Phenotypic analysis of annVint cells by flow cytometry. Melan-A–specific CTLs were stimulated for 4 hours with peptide-loaded T2 cells and subsequently stained with the following mAbs: CD69, HLA-DR, CD71, CD95, and TCRαβ. FITC-conjugated mAbs were combined with annV-PE and PE-conjugated mAbs with annV-FITC. Fluorescence intensity on gated CD8+, annVint CTLs are presented by the solid line. As a control annVneg CTLs from an unstimulated control are shown (dashed line). Gray histograms show isotype controls. (B) PI staining of annVint and annVhigh CTLs. The Melan-A–specific CTL clone (S2Cl14) was labeled with the membrane dye PKH-26 and stimulated with peptide-loaded T2 cells for 24, 48, 72, and 96 hours. PI staining was performed in parallel. Dot plots show annV-FITC and PI expression on gated annVint (middle column) and annVhigh (right column) T cells. The left column shows gating of cells. (C) Melan-A–specific CTLs were stimulated overnight with Melan-A–loaded T2 cells at a ratio of 3:1. The TUNEL assay was performed with FACS-sorted annVint and annVhigh CTLs. Representative experiments are shown.
Ag-primed annVint CTLs are activated but nonapoptotic T cells. (A) Phenotypic analysis of annVint cells by flow cytometry. Melan-A–specific CTLs were stimulated for 4 hours with peptide-loaded T2 cells and subsequently stained with the following mAbs: CD69, HLA-DR, CD71, CD95, and TCRαβ. FITC-conjugated mAbs were combined with annV-PE and PE-conjugated mAbs with annV-FITC. Fluorescence intensity on gated CD8+, annVint CTLs are presented by the solid line. As a control annVneg CTLs from an unstimulated control are shown (dashed line). Gray histograms show isotype controls. (B) PI staining of annVint and annVhigh CTLs. The Melan-A–specific CTL clone (S2Cl14) was labeled with the membrane dye PKH-26 and stimulated with peptide-loaded T2 cells for 24, 48, 72, and 96 hours. PI staining was performed in parallel. Dot plots show annV-FITC and PI expression on gated annVint (middle column) and annVhigh (right column) T cells. The left column shows gating of cells. (C) Melan-A–specific CTLs were stimulated overnight with Melan-A–loaded T2 cells at a ratio of 3:1. The TUNEL assay was performed with FACS-sorted annVint and annVhigh CTLs. Representative experiments are shown.
PL transport activity in Ag-specific CTLs
It has been reported that PL transport proteins regulate distribution of PS between the inner and the outer leaflet of the plasma membrane. To answer the question whether an increased outward or a decreased inward transfer of PS is responsible for the accumulation of PS molecules on the cell surface, we used fluorescent NBD-PS to follow its transport.
Fluorescence microscopy of stimulated Melan-A–specific CTLs demonstrate colocalization of exposed PS with lipid rafts. (A) Exposed PS is distributed nonhomogenously in patches. Melan-A–specific CTLs were stimulated for 4 hours with anti-CD3/CD28–coated beads (middle image) and isolated via magnetic depletion of the beads. Activated CTLs were stained with annV-FITC for 30 minutes at RT. CTLs were fixed with 2% PFA in annV-binding buffer and transferred onto poly-l-lysin–coated slides. Control CTLs were treated equally without stimulation (left image). Anti-fas mAb (clone CH-11) treated apoptotic/necrotic annVhigh CTLs are shown on the right image. (B) To detect colocalization of PS with membrane lipid rafts, CTLs were labeled with CTx-B and an anti–CTx-B–Alexa594-conjugated rabbit serum prior to annV-FITC staining. CTLs were fixed with 2% PFA in annV-binding buffer and transferred to poly-l-lysin–coated slides. Transmission light and emitted fluorescence was detected with a Zeiss Axioscope2plus microscope. An overlay of green and red fluorescence (yellow) exhibits a colocalization of PS with raft domains. (C) Melan-A–specific CTLs were preincubated with anti–MHC-I (blue) and CTx-B (lipid rafts, red) and stimulated with Melan-A–loaded T2 cells for 2 hours. AnnV-FITC (green) staining was performed and PS exposure at the immunologic synapse was visualized by overlay of fluorescence emission (yellow).
Fluorescence microscopy of stimulated Melan-A–specific CTLs demonstrate colocalization of exposed PS with lipid rafts. (A) Exposed PS is distributed nonhomogenously in patches. Melan-A–specific CTLs were stimulated for 4 hours with anti-CD3/CD28–coated beads (middle image) and isolated via magnetic depletion of the beads. Activated CTLs were stained with annV-FITC for 30 minutes at RT. CTLs were fixed with 2% PFA in annV-binding buffer and transferred onto poly-l-lysin–coated slides. Control CTLs were treated equally without stimulation (left image). Anti-fas mAb (clone CH-11) treated apoptotic/necrotic annVhigh CTLs are shown on the right image. (B) To detect colocalization of PS with membrane lipid rafts, CTLs were labeled with CTx-B and an anti–CTx-B–Alexa594-conjugated rabbit serum prior to annV-FITC staining. CTLs were fixed with 2% PFA in annV-binding buffer and transferred to poly-l-lysin–coated slides. Transmission light and emitted fluorescence was detected with a Zeiss Axioscope2plus microscope. An overlay of green and red fluorescence (yellow) exhibits a colocalization of PS with raft domains. (C) Melan-A–specific CTLs were preincubated with anti–MHC-I (blue) and CTx-B (lipid rafts, red) and stimulated with Melan-A–loaded T2 cells for 2 hours. AnnV-FITC (green) staining was performed and PS exposure at the immunologic synapse was visualized by overlay of fluorescence emission (yellow).
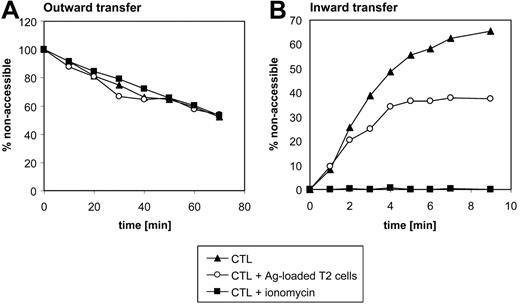
Outward and inward transport of NBD-PS in Melan-A–specific CTLs. (A) Melan-A–specific CTLs were intracellularly loaded with NBD-PS, washed, and then incubated at 37°C in complete medium in the absence (▴) or presence (○) of Ag-loaded T2 cells at a CTL/T2 ratio of 3:1. Alternatively, CTLs were stimulated with ionomycin (▪). To trap NBD lipids appearing on the cell surface, incubation was performed in the presence of 1% HSA. Aliquots of cells were removed at various time points and analyzed by flow cytometry after addition of 10 mM dithionite to reduce the NBD moiety of analogues in the medium. Only PI– CTLs were included for analysis. Relative NBD-PS fluorescence intensities at the start of the experiment were about 2400 arbitrary units. Shown are representative data of 3 independent experiments. (B) Ag-specific CTLs were stimulated with T2 cells (37°C for 45 minutes, ○) or 5 μM ionomycin (37°C for 15 minutes, ▪), or left untreated (▴), labeled with NBD-PS on ice, and incubated at 37°C to allow PS inward transfer. At times indicated, the fraction of internalized label was determined (in gated CD8+ PI–) CTLs by flow cytometry using back-exchange to HSA and treatment with dithionite as described in “Materials and methods.” The relative fluorescence protected against dithionite is shown for one representative experiment of 3. In panels A and B the percentage of nonaccessible fluorescence was normalized to fluorescence at time point zero. The absolute values of nonaccessible fluorescence at time point zero ranged from 10% to 20%.
Outward and inward transport of NBD-PS in Melan-A–specific CTLs. (A) Melan-A–specific CTLs were intracellularly loaded with NBD-PS, washed, and then incubated at 37°C in complete medium in the absence (▴) or presence (○) of Ag-loaded T2 cells at a CTL/T2 ratio of 3:1. Alternatively, CTLs were stimulated with ionomycin (▪). To trap NBD lipids appearing on the cell surface, incubation was performed in the presence of 1% HSA. Aliquots of cells were removed at various time points and analyzed by flow cytometry after addition of 10 mM dithionite to reduce the NBD moiety of analogues in the medium. Only PI– CTLs were included for analysis. Relative NBD-PS fluorescence intensities at the start of the experiment were about 2400 arbitrary units. Shown are representative data of 3 independent experiments. (B) Ag-specific CTLs were stimulated with T2 cells (37°C for 45 minutes, ○) or 5 μM ionomycin (37°C for 15 minutes, ▪), or left untreated (▴), labeled with NBD-PS on ice, and incubated at 37°C to allow PS inward transfer. At times indicated, the fraction of internalized label was determined (in gated CD8+ PI–) CTLs by flow cytometry using back-exchange to HSA and treatment with dithionite as described in “Materials and methods.” The relative fluorescence protected against dithionite is shown for one representative experiment of 3. In panels A and B the percentage of nonaccessible fluorescence was normalized to fluorescence at time point zero. The absolute values of nonaccessible fluorescence at time point zero ranged from 10% to 20%.
To study outward transport, Melan-A–specific CTL lines (> 90% Melan-A tetramer positive) were intracellularly labeled with NBD-PS and its transport back to the exoplasmic leaflet at 37°C was followed by continuous incubation in HSA-containing medium. HSA acts as an extracellular sink for short-chain lipids by rapidly extracting NBD-lipid analogues, thereby excluding analogues from inward transport via the APTL. The kinetics of outward redistribution (Figure 5A) revealed that about 50% of analogues originally located intracellularly became accessible to HSA within 70 minutes. However, stimulation with T2 cells or ionomycin (Figure 5A) in the presence of Ca2+ ions did not accelerate PS exposure, ruling out that an increased outward transfer by a Ca2+-dependent scramblase is the main reason for PS exposure in Ag-stimulated CTLs.
Next, we investigated the inward NBD-PS transport. After a preincubation with T2 cells or ionomycin, NBD-PS was introduced into the outer membrane leaflet and the amount of inward transported NBD-PS, which is protected from HSA extraction and dithionite-mediated quenching, was determined flow cytometrically at various times of incubation at 37°C. CTLs stimulated with ionomycin in the presence of Ca2+ exhibited an abrogated PS inward transfer (Figure 5B). Stimulation of Ag-specific CTLs with Ag-loaded T2 cells induced a 50% inhibition of the flippase activity (Figure 5B). These findings clearly indicate that Ag stimulation affects CTLs in their ability to restrict PS molecules to the inner membrane leaflet by inhibition of the flippase activity.
Blocking of exposed PS by unconjugated annV
Next, we attempted to elucidate the biologic relevance of PS exposure and to define functional consequences of PS exposure in Ag-specific CTLs. Melan-A–pulsed T2 cells were used as stimulators for Melan-A–specific CTLs in the presence or absence of 50 μg/mL unconjugated blocking annV. Because unconjugated annV is present during the whole coincubation period, PS molecules arising straight after Ag recognition on the cell surface were blocked. We found that the blockade of exposed PS resulted in a significant reduction of secreted Th1 cytokines (IFN-γ, IL-2, TNF-α; Figure 6A), whereas secretion of IL-5 and IL-4 was only marginally inhibited (Figure 6A). Moreover, blocking of exposed PS on CTLs during Ag recognition led to a diminished TCR internalization. (Figure 6B). In contrast, the activation-induced up-regulation of CD69 expression is not influenced by the blockage (data not shown).
Of interest, we found noticeable differences in the formation of cell clusters between annV-treated and untreated cocultures after 24 hours of incubation (Figure 6C). Although widespread light impermeable clusters could be observed in cocultures of CTLs with Melan-A–pulsed T2 cells, the cluster formation was inhibited in annV-treated cocultures. These data indicate that PS may significantly modulate the intercellular contact formation between T cells and APCs.
Discussion
Here we show that human CD8+ Ag-specific CTLs transiently expose PS on the cell membrane after cognate Ag recognition. The exposure of PS on T cells, a PL being restricted to the inner membrane leaflet under vital conditions, is Ag-dependent. Of note, Ag-independent APC contact leads to a background PS exposure on Ag-specific CTLs. We ascribe this finding to the phenomenon of Ag-independent synapse formation and intracellular Ca2+ responses.33-35
Inhibition of exposed PS with unconjugated annV modulates clustering of T cells. Melan-A–specific CTLs were stimulated in Ca2+-containing medium with Melan-A–loaded T2 stimulator cells in the presence or absence of 50 μg/mL unconjugated blocking annV during the assay. (A) After 5 hours of coculture, supernatants were harvested and the amount of indicated cytokines was determined by a cytokine bead array. Shown are mean values of annV-blocked samples normalized to unblocked control ± SEM; n = 5; **P < .001. (B) Cells were harvested at indicated time points, stained with Melan-A tetramer (TM), CD8-APC, annV-FITC and analyzed by FACS. Shown are mean values of blocked and unblocked CTL normalized to unstimulated CTL ± SEM; n = 4; *P < .08, **P < .01. (C) After a coculture of 24 hours, images were taken from representative unblocked (middle images) or annV-blocked (lower images) cell pellets in 96-well round bottom plates. Unstimulated CTLs (upper images) served as a control. One representative experiment of 5 is shown. Images were taken on an Axiovert25 microscope at a × 20 (insert) and × 50 magnification.
Inhibition of exposed PS with unconjugated annV modulates clustering of T cells. Melan-A–specific CTLs were stimulated in Ca2+-containing medium with Melan-A–loaded T2 stimulator cells in the presence or absence of 50 μg/mL unconjugated blocking annV during the assay. (A) After 5 hours of coculture, supernatants were harvested and the amount of indicated cytokines was determined by a cytokine bead array. Shown are mean values of annV-blocked samples normalized to unblocked control ± SEM; n = 5; **P < .001. (B) Cells were harvested at indicated time points, stained with Melan-A tetramer (TM), CD8-APC, annV-FITC and analyzed by FACS. Shown are mean values of blocked and unblocked CTL normalized to unstimulated CTL ± SEM; n = 4; *P < .08, **P < .01. (C) After a coculture of 24 hours, images were taken from representative unblocked (middle images) or annV-blocked (lower images) cell pellets in 96-well round bottom plates. Unstimulated CTLs (upper images) served as a control. One representative experiment of 5 is shown. Images were taken on an Axiovert25 microscope at a × 20 (insert) and × 50 magnification.
It is a well-known phenomenon that mature T cells can undergo apoptosis by activation-induced cell death. Besides death receptor-dependent cell death (eg, TNFR/fas), the TCR/MHC interaction can trigger diverse apoptotic programs and plays a crucial role in the control of immune homeostasis.36-38 We demonstrate here that activated human CTLs with an annVint phenotype are nonapoptotic. Using 3 independent assays for apoptosis we found that annVint T cells are PI–, do not exhibit DNA strand breaks, and contain no active caspase-3 (data not shown). In contrast, annVint CTLs revealed a strongly activated phenotype indicated by an up-regulation of CD69 expression and down-regulation of the TCRαβ chain expression.
Moreover, we found that activation-induced PS exposure as well as the recovery from PS exposure in annVint CTLs strongly depend on the amount of presented Ag. Whereas high amounts of Ag led to extensive PS exposure with a prolonged recovery period (3-4 days), low amounts of Ag induced a reduced PS exposure with fast recovery in the range of 1 to 2 days. Comparison of PS exposure and TCR internalization determined by Melan-A–MHC tetramer staining revealed similar kinetics. However, it seems as if PS exposure on Ag-specific CTLs reflects more sensitively the activation state of CTL than TCR down-regulation (Figure 2A).
To confirm that PS exposure is a characteristic feature of CTL activation and not the result of overstimulation by peptide-pulsed APCs, we used Melan-A–expressing tumor cell lines as stimulator cells and found that also endogenously processed and presented Melan-A peptide induces PS exposure on Melan-A–specific CTLs. Together with our data from CD4+ T-cell lines, which also expose PS on Ag recognition (data not shown), we demonstrate for the first time that PS exposure on Ag-specific CTLs is linked to T-cell activation and not an exclusive marker for early apoptosis.
Activation-induced PS exposure has been described for different immune cells such as stimulated platelets,13 B cells after anti-IgM cross-linking,15 fMLP-stimulated neutrophils,14 erythrocytes,16 mast cells during IgE signaling,17 and murine T-cell hybridoma cells and Jurkat T cells after ionophore treatment.18 Adequate stimulation of the particular cell type leads to an elevated cytoplasmic Ca2+ level followed by an rapid trans-bilayer redistribution of PL. By using a fluorescent lipid analogue, we determined a constitutive outward transfer of PS molecules in Melan-A–specific CTLs, reflecting the effector-memory phenotype of CTLs that had been restimulated on a weekly basis. Due to slow spontaneous transmembrane diffusion of PL in biologic membranes,1 we suggest that outward transport proteins in effector-memory CTLs are already active. The constitutive PS outward transport is not further enhanced after ionomycin treatment or Ag stimulation, indicating that in CTL the scramblase activity is not further inducible or another type of floppase activity might be present. In sharp contrast, the inward transporting flippase is strongly inhibited in stimulated CTLs. We postulate that both inward and outward PS transfer processes contribute to activation-induced PS exposure. The constitutive outward PS transfer in CTLs is normally antagonized by efficient inward-directed flippases. During TCR ligation and the concurrent rise of the intracellular Ca2+ level,39 inward-directed flippases become heavily suppressed, a process that results in an accumulation of PS molecules on the cell surface. For apoptotic cells, it has been reported that an increased outward PS transfer occurs in parallel to a decreased inward PS transfer, which are both responsible for the strong PS exposure during cell death.7-9 We assume that one reason for the moderate PS exposure in annVint CTLs is the lack of an additional increase of the outward PS transfer compared to apoptotic cells.
One interesting point that needs to be considered is the question whether activated annVint CTLs can be recognized by phagocytes via their PS receptors. It is a widely accepted phenomenon that macrophages engulf annV+ apoptotic immune cells well before the final stages of apoptosis via PS receptors.40-42 So far, such a new mechanism of down-regulating adaptive immune responses by cells of the innate immune system cannot be excluded. Nevertheless, suggestions from the literature indicate that a threshold for PS recognition as an engulfment signal does exist. In this case the amount of exposed PS on annVint T cells may be too low for clustering PS receptors on phagocytes.43,44
Signaling by the TCR is orchestrated at specific plasma-membrane microdomains, referred to as lipid rafts, which contain a multitude of signaling molecules such as Src, Lck, and LAT.45 Most notably, fluorescence microscopic analyses of anti-CD3/28-stimulated CTLs revealed a patchy membrane distribution of exposed PS colocalizing with the lipid raft marker CTx-B. A predominant occurrence of PS molecules in lipid rafts has been reported elsewhere.15,46 These data indicate the spatial proximity of exposed PS to signaling molecules. Indeed, analysis of the immunologic synapses between CTLs and APCs revealed an accumulation of externalized PS on CTLs at the APC contact side. These findings raise the question whether exposed PS may contribute significantly to events at the immunologic synapse, for example, by influencing membrane fluidity or cell-to-cell adhesion. The observation that cluster formation is diminished during PS blockade supports the assumption that exposed PS may be involved in T cell-APC interactions. Accordingly, Elliot et al defined a role of exposed PS in adhesion and migration processes in murine CD4+ T cells.24
Moreover, blocking of exposed PS during Ag recognition resulted in a significant inhibition of IFN-γ, IL-2, and TNF-α secretion as well as TCR internalization. Of note, because unconjugated annV binds not only to exposed PS on CTLs but also to PS on Ag-loaded T2 cells after cytotoxic attack by the T cells, we cannot exclude that PS molecules on APCs are also involved in T-cell activation and cell clustering. Nevertheless, these data suggest that exposed PS is somehow necessary for a profound CTL activation. One possible explanation is the existence of APC-resident PS-binding receptors that sustain T-cell activation. The question whether PS exposure influences signal transduction in human Ag-specific CTLs, as suggested for murine CD4+ T cells, remains to be elucidated.24
Together, these data suggest that PS may play a crucial role in T-cell immunology. Basic knowledge about the impact of PLs on adaptive immune responses will open new routes for membrane-targeted therapies that aim at the down-regulation of T-cell responses.
Authorship
Contribution: K.F., S.V., T.P., and A.M. contributed to the study concept and design; K.F., S.V., and J.B. did research and analyzed data; R.A. provided administrative and material support; and all authors participated in the analysis and interpretation of data and the drafting and critical revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 15, 2006; DOI 10.1182/blood-2006-03-011742.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported in part by the Deutsche Forschungsgemeinschaft (MA 1351/5-1) and the German José Carreras Leukemia Foundation.
We gratefully thank Dr Petra Hoffmann and Dr Krishna Mondal for critically reading the manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal