Abstract
BCR-mediated antigen processing occurs at immunologically relevant antigen concentrations and hinges on the trafficking of antigen-BCR (Ag-BCR) complexes to class II–containing multivesicular bodies (MVBs) termed MIICs. However, the molecular mechanism underlying the trafficking of Ag-BCR complexes to and within MIICs is not well understood. In contrast, the trafficking of the epidermal growth factor receptor (EGFR) to and within MVBs occurs via a well-characterized ubiquitin-dependent mechanism, which is blocked by acute inhibition of proteasome activity. Using a highly characterized antigen-specific model system, it was determined that the immunoglobulin heavy chain subunit of the IgM BCR of normal (ie, nontransformed) B cells is ubiquitinated. Moreover, acute inhibition of proteasome activity delays the formation of ubiquitinated ligand–BCR complexes, alters the intracellular trafficking of internalized Ag-BCR complexes, and selectively blocks the BCR-mediated processing and presentation of cognate antigen, without inhibiting the endocytosis, processing, and presentation of non–cognate antigen internalized by fluidphase endocytosis. These results demonstrate that the trafficking of Ag-BCR complexes to and within MVB-like antigen processing compartments occurs via a molecular mechanism with similarities to that used by the EGFR, and establishes the EGFR as a paradigm for the further analysis of Ag-BCR trafficking to and within MIICs.
Introduction
For MHC class II–expressing antigen presenting cells (APCs), exogenous antigen processing involves antigen internalization, proteolytic processing, the formation of antigenic peptide–class II complexes, and transport of these peptide–class II complexes to the cell surface.1 Moreover, the conversion of internalized antigen to peptide–class II complexes occurs within MHC class II–enriched endocytic compartments (MIICs) that have a multivesicular structure similar to that of multivesicular bodies (MVBs) found in many cell types.2 While B cells, like dendritic cells and macrophages, can process and present non–cognate antigens internalized via fluid-phase (F-P) endocytosis, BCR-mediated antigen processing is the most efficient form of processing and presentation carried out by this APC. BCR-mediated antigen processing and presentation also allow for subsequent cognate B-cell–T-cell interactions, which result in affinity maturation and immunoglobulin class switching.3,4 Moreover, the BCR-mediated processing of cognate antigen results in the formation of peptide–class II complexes with unique biologic properties.5 Therefore it is likely that one or more aspects of the molecular mechanism underlying BCR-mediated antigen processing and presentation differ from the processing and presentation of non–cognate antigen internalized via F-P endocytosis.
The binding of antigen to the BCR results in the efficient uptake and processing of antigen at immunologically relevant antigen concentrations6-8 as well as the accelerated delivery of antigen-BCR (Ag-BCR) complexes to antigen processing compartments.9,10 In addition, internalized Ag-BCR complexes can persist for a prolonged period of time within late endocytic compartments enriched in the class II peptide loading facilitator HLA-DM but that contain lower levels of the HLA-DM regulator HLA-DO.11 Antigen-induced BCR signaling can also result in the aggregation of class II–positive intracellular compartments and modulate the delivery of internalized Ag-BCR complexes to these remodeled late endocytic compartments.12,13 However, the observation that BCR signaling fails to alter the efficiency of processing and presentation of non–cognate antigen internalized by F-P endocytosis14 suggests that one or more novel aspects of Ag-BCR trafficking to or within multivesicular MIICs might act to focus the molecular machinery for antigen processing and the generation of peptide–class II complexes onto BCR-internalized cognate Ag.
Presently, the trafficking of Ag-BCR complexes to and within MIICs is an understudied area of BCR biology. However, the trafficking of other membrane proteins, such as the epidermal growth factor receptor (EGFR), to and within MVBs is a more highly characterized process.15-19 For the EGFR, trafficking to and within MVBs involves ubiquitination of the cytoplasmic tail of the receptor by the ubiquitin ligase Cbl, followed by the entry of the ubiquitinated receptor into the ESCRT protein sorting complex that resides on the limiting membrane of MVBs (reviewed in Dikic15 ; Babst et al17 ; Marmor and Yarden20 ). Ultimately, the receptor is incorporated into membrane buds that will form intraluminal vesicles (ILVs) that will eventually be delivered to terminal lysosomes. In this model system, it has been well established that acute inhibition of proteasome activity inhibits this sorting process, possibly by decreasing the levels of cytosolic-free ubiquitin.19,21 Therefore, a similar experimental approach was used to determine if the trafficking of Ag-BCR complexes to or within MVB-like MIICs occurs via a similar mechanism. The results of this analysis demonstrate that the heavy chain of the IgM BCR is ubiquitinated and that acute proteasome inhibition blocks BCR heavy chain ubiquitination and alters the intracellular trafficking of Ag-BCR complexes, resulting in a selective inhibition of BCR-mediated antigen processing and presentation. These results highlight the similarities in the molecular mechanisms responsible for the trafficking of Ag-BCR complexes and the EGFR to and within MVB-like late endocytic compartments, and establish the EGFR as a paradigm for the further investigation of this aspect of Ag-BCR biology.
Materials and methods
Cells and in vitro culture
Splenic B cells from either the MD4.B10.Br (expressing a transgenic, hen egg lysozyme [HEL]–specific IgMa BCR and I-Ak MHC class II molecules) or B10.Br (expressing a non–HEL-specific IgMb BCR and I-Ak MHC class II molecules) mouse were isolated and maintained in tissue culture as previously reported.5,11,14 All reported protocols have been reviewed and approved by the appropriate institutional review committee.
Reagents
The following reagents were used for this study: hen egg lysozyme (HEL, catalog no. L-6876; Sigma, St Louis, MO), MG-132 (Calbiochem, San Diego, CA), lactacystin (Calbiochem), puromycin (Sigma), and brefeldin A (Sigma).
Detection of ubiquitinated cellular proteins
Splenic B cells were treated with 10 μM MG-132 or lactacystin as noted, washed, and lysed by resuspension in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors (1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM PMSF, and Complete, Mini, EDTA-free protease inhibitor cocktail; Roche). After incubation for 10 minutes on ice, the lysates were cleared by centrifugation for 15 minutes at 16 000g at 4°C. Cleared lysates were analyzed by reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting as previously described,11,22 using either of 2 different antiubiquitin mAbs: 6C123 (Sigma) or FK224 (BioMol, Plymouth Meeting, PA), followed by goat anti–mouse IgG (heavy and light chain–specific) peroxidase conjugate (Calbiochem). Blots were developed with SuperSignal West Femto chemiluminescent peroxidase substrate (Pierce Biotechnology, Rockford, IL), using Blue Sensitive X-ray film (Laboratory Product Sales, Rochester, NY).
Analysis of BCR ubiquitination
p62 UBA binding. Splenocytes from MD4.B10.Br or B10.Br mice were isolated and pulsed with 10 μg/mL anti–IgMa-btn (BD Pharmingen, San Diego, CA) or anti–IgMb-btn (BD Pharmingen) as indicated. Cells were then washed and lysed at 2 × 107 viable cells/mL in RIPA buffer for 10 minutes on ice. Lysates were clear by centrifugation for 15 minutes at 16 000g at 4°C. The ubiquitinated proteins in 250 μL cleared lysate were precipitated by incubation for 2 to 3 hours with 10 μL p62 UAB-agarose beads (BioMol). Samples were washed and analyzed by reducing SDS-PAGE, Western blotting with streptavidin-HRP (Pierce Biotechnology), and SuperSignal West Femto ECL substrate, using Blue Sensitive X-ray film.
Antiubiquitin Western blotting. Splenocytes from MD4.B10.Br or B10.Br mice were isolated and lysed in RIPA buffer at a concentration of 5 × 107 viable cells/mL. Lysates were cleared by centrifugation for 15 minutes at 16 000g at 4°C. Lysate (100 μL) was precleared with 2 μg goat anti–murine IgM (Jackson Immunologicals, West Grove, PA) or goat anti–murine IgG (heavy + light chain specific; Jackson Immunologicals) and 10 μL protein G–sepharose (PGS; Pierce Biotechnology) as indicated. Lysates were then immunoprecipitated with 2 μg goat anti–murine IgM and 10 μL PGS. Immunoprecipitates were analyzed by reducing SDS-PAGE and Western blotting with either 6C1 (1:10:000) or FK2 (1:10:000) followed by goat anti–murine IgG (H+L)–HRP (1:25 000; Calbiochem) and SuperSignal West Femto ECL substrate, using Blue Sensitive X-ray film.
Analysis of the degradation of internalized anti-BCR monoclonal antibodies
MD4.B10.Br splenocytes (± 10 μM proteasome inhibitor for 1 hour at 37°C) were pulsed with 10 μg/mL anti–IgMa-btn mAb. The cells were then incubated at 37°C for the indicated time before harvesting, lysing in RIPA buffer, and analyzing whole-cell lysates (derived from the same number of total cells) by SDS-PAGE and Western blotting with streptavidin-HRP as described above.
Ag-BCR persistence
The intracellular persistence of internalized HEL-BCR complexes was analyzed by staining HEL-pulsed B cells with the 2D1 anti-HEL mAb (which recognizes HEL whether or not it is bound to the MD4 BCR) and analysis of the samples by immunofluorescence microscopy as previously reported,11,14 using an Olympus BX50 microscopy, equipped with a UplanFl 60×/1.25 NA objective and Optronix CCD camera mounted with a 0.63× adaptor. In some cases, samples were also stained with anti–LAMP-2 mAb (BD Pharmingen), followed by fluorochrome-labeled secondary antibody.22 To quantitate the distribution of persisting intracellular antigen, 100 cells were observed and scored as to whether persisting antigen was detectable within 1, 2, 3, or all 4 quadrants of the cell (cells were always divided into quadrants by horizontal and vertical lines that intersect at the center of the cell).
Kinetics of Ag-BCR internalization
The kinetics of HEL-BCR internalization were determined as previously reported.22
Ag-BCR dissociation
The dissociation of internalized HEL-BCR complexes was analyzed as previously reported by staining HEL-pulsed MD4.B10.Br B cells with the HyHEL10 anti-HEL mAb, which recognizes HEL only after dissociation from the MD4 BCR.11
Flow cytometric analysis of peptide–class II expression
For experiments involving the use of proteasome inhibitors, B cells were pretreated with the indicated concentration of inhibitor for 1 hour at 37°C, before a 4-hour pulse with antigen (100 nM HEL for MD4.B10.Br cells and 100 μM HEL for B10.Br cells). The cells were then washed (to remove both antigen and proteasome inhibitor) and then returned to culture in complete media for an additional 18 to 20 hours. For experiments involving the use of puromycin, leupeptide, or brefeldin A, the cells were pretreated with inhibitor for 1 hour at 37°C before addition of antigen and cultured overnight (in the continued presence of both inhibitor and HEL). The level of expression of HEL46-61–I-Ak complexes was determined by staining of the antigen-pulsed cells with the HEL46-61–I-Ak–specific mAb C4H325 plus fluorochrome-labeled secondary antibody, followed by analysis of the samples by flow cytometry as previously reported.5,11,14
Results
The heavy chain of the IgM BCR is ubiquitinated
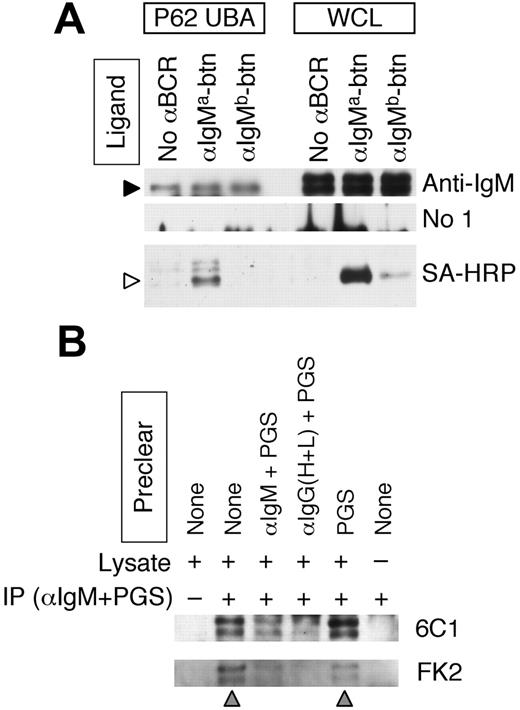
The sorting of the EGFR to and within MVBs involves the recognition of a mono/multiubiquitin tag on the cytoplasmic domain of the EGFR.15,20 If Ag-BCR sorting occurs via a similar mechanism, then it would be expected that the cytoplasmic domain of the BCR would also be ubiquitinated. The BCR is a multisubunit complex consisting of an IgH/L antigen-binding subunit and CD79A/B signaling subunit. While the KVK cytoplasmic domain of the IgH/L subunit of the IgM BCR is relatively small when compared with the cytoplasmic domain of the CD79A/B signaling complex, it does contain 2 highly conserved lysine residues that could be targets for ubiquitination. To determine whether the IgH polypeptide of the IgM BCR is ubiquitinated, ubiquitinated proteins from whole-cell lysates (WCLs) of splenic B cells were isolated by adsorption to p62 UBA–agarose. (p62 is a phosphotyrosine-independent ligand of p56lck, which also binds ubiquitin in vivo.26 p62 UBA is the isolated ubiquitin-binding domain of p62, which retains ubiquitin-binding activity.) The p62 UBA precipitates and WCLs were then probed for BCR IgM heavy chain (Figure 1A, anti-IgM). The results demonstrate the presence of 2 different molecular weight forms of IgM heavy chain in the WCLs (which we [J.R.D., unpublished results, August 2006] and others27 have determined represent different glycoforms of the IgM heavy chain) and show that the p62 UBA ubiquitin–binding protein selectively binds to the lower molecular weight heavy chain glycoform.
To determine whether the p62 UBA–binding glycoform of the IgM heavy chain is expressed at the surface of the B cell where it could bind antigen (as opposed to being sequestered within intracellular compartments), the p62 UBA precipitates were probed for the presence of biotinylated anti-IgM antibody that had been bound to the cells before lysis (Figure 1A, SA-HRP). The results show that the p62 UBA–binding IgM is capable of binding extracellular antigen, demonstrating that at least a portion of these BCR molecules is present at the surface of the cell.
To confirm the p62 UBA–binding results, which suggest that at least some of the IgM heavy chains of the cell surface BCR molecules are ubiquitinated, it was determined whether IgM BCR molecules immunoprecipitated from lysates of splenic B cells are recognized by 2 different antiubiquitin monoclonal antibodies (mAbs) (ie, 6C124 and FK223 ). Consistent with the p62 UBA–binding results, IgM BCR molecules immunoprecipitated from splenic B-cell lysates were recognized by both antiubiquitin mAbs (Figure 1B lane 2). Moreover, the identity of the proteins recognized by both the 6C1 and FK2 mAbs as IgM heavy chain is indicated by the finding that these bands are not detected if the WCL is immunoprecipitated with protein G–sepharose (PGS) only (which does not bind murine IgM heavy chain, Figure 1B lane 1), or if WCL is omitted from the protocol (lane 6). Furthermore, the ubiquitinated IgM heavy chains are depleted/removed if the WCL is precleared with either goat anti–murine IgM antibody plus PGS (Figure 1B lane 3) or goat anti–murine IgG heavy and light chain antibody plus PGS (which would bind the light chain of the IgM BCR, lane 4), but not if the WCL is precleared with PGS only (which does not bind murine IgM, lane 5).
The immunoglobulin heavy chain of the IgM BCR is ubiquitinated. (A) MD4.B10.Br B cells (expressing an IgMa BCR) were incubated with either no antibody, anti–IgMa-btn, or anti–IgMb-btn as indicated. Cells were then lysed and ubiquitinated proteins precipitated with p62 UBA–agarose. Ubiquitinated p62 UBA–binding proteins (P62 UBA) as well as whole-cell lysates (WCLs) were analyzed by reducing SDS-PAGE and Western blotting. Anti-IgM indicates goat anti–murine IgM plus rabbit anti–goat IgG–HRP (to detect BCR IgM heavy chain); No 1°, rabbit anti–goat IgG–HRP only (negative control); and SA-HRP, streptavidin-HRP (to detect biotinylated anti-BCR antibody). The anti-IgM and No 1° blots were treated identically except for the omission of the primary antibody. The molecular weights of the 2 bands in the WCLs are 70 and 80 kDa. The filled arrowhead indicates the 70-kDa BCR IgM heavy chain protein of the endogenous BCR detected in the p62 UBA precipitates. The open arrowhead indicates the position of the heavy chain of the anti–IgMa-btn antibody. Shown are representative results from 1 of 3 independent experiments. (B) Splenocytes were lysed in RIPA buffer and the lysates precleared as indicated (αIgM + PGS indicates goat anti–murine IgM plus PGS; αIgG(H+L) + PGS, goat anti–murine IgG (H+L) plus PGS). The murine IgM BCR was then immunoprecipitated from the cleared lysates as indicated (+ indicates goat anti–murine IgM and PGS; –, PGS only). Immunoprecipitates were probed for the presence of ubiquitinated IgM by reducing SDS-PAGE and Western blotting with antiubiquitin mAbs (either the 6C1 or FK2, as indicated). The gray arrowheads below the blot indicate the lanes in which the IgM BCR was not precleared. The molecular masses of the 2 bands in the blots are 70 and 80 kDa. Shown are representative results from 1 of 3 independent experiments.
The immunoglobulin heavy chain of the IgM BCR is ubiquitinated. (A) MD4.B10.Br B cells (expressing an IgMa BCR) were incubated with either no antibody, anti–IgMa-btn, or anti–IgMb-btn as indicated. Cells were then lysed and ubiquitinated proteins precipitated with p62 UBA–agarose. Ubiquitinated p62 UBA–binding proteins (P62 UBA) as well as whole-cell lysates (WCLs) were analyzed by reducing SDS-PAGE and Western blotting. Anti-IgM indicates goat anti–murine IgM plus rabbit anti–goat IgG–HRP (to detect BCR IgM heavy chain); No 1°, rabbit anti–goat IgG–HRP only (negative control); and SA-HRP, streptavidin-HRP (to detect biotinylated anti-BCR antibody). The anti-IgM and No 1° blots were treated identically except for the omission of the primary antibody. The molecular weights of the 2 bands in the WCLs are 70 and 80 kDa. The filled arrowhead indicates the 70-kDa BCR IgM heavy chain protein of the endogenous BCR detected in the p62 UBA precipitates. The open arrowhead indicates the position of the heavy chain of the anti–IgMa-btn antibody. Shown are representative results from 1 of 3 independent experiments. (B) Splenocytes were lysed in RIPA buffer and the lysates precleared as indicated (αIgM + PGS indicates goat anti–murine IgM plus PGS; αIgG(H+L) + PGS, goat anti–murine IgG (H+L) plus PGS). The murine IgM BCR was then immunoprecipitated from the cleared lysates as indicated (+ indicates goat anti–murine IgM and PGS; –, PGS only). Immunoprecipitates were probed for the presence of ubiquitinated IgM by reducing SDS-PAGE and Western blotting with antiubiquitin mAbs (either the 6C1 or FK2, as indicated). The gray arrowheads below the blot indicate the lanes in which the IgM BCR was not precleared. The molecular masses of the 2 bands in the blots are 70 and 80 kDa. Shown are representative results from 1 of 3 independent experiments.
While only the lower molecular weight glycoform of IgM heavy chain was detected in the p62 UBA–bound proteins, both forms of IgM are recognized by the 2 different antiubiquitin mAbs. However, since recognition of the IgH protein is occurring under 2 dramatically different sets of conditions in these 2 protocols (ie, binding of p62 UBA to nondenatured protein in aqueous solution versus the binding of 2 different antiubiquitin mAbs to SDS-denatured protein adsorbed to nitrocellulose), it is reasonable to expect that differences in the recognition of different forms of ubiquitinated IgM may occur. Therefore, the results presented in Figure 1 demonstrate that the heavy chain subunits of at least a portion of cell surface IgM BCR molecules are ubiquitinated, placing a tag on the BCR that could be used to support the intracellular sorting of internalized Ag-BCR complexes. Similar results were obtained whether these experiments were performed with cells from MD4.B10.Br BCR transgenic mice or nontransgenic B10.Br mice.
Proteasome inhibition selectively alters antigen-BCR trafficking and degradation
Previous reports have demonstrated that the trafficking of the EGFR to and within MVBs occurs via a ubiquitin-dependent mechanism, involving proteins of the ESCRT protein complex.28 Moreover, it has been reported that acute inhibition of proteasome activity profoundly alters the trafficking of the EFGR to and within MVBs.19,21 Therefore, to test the hypothesis that the trafficking of Ag-BCR complexes to or within MVB-like MIICs occurs via a similar mechanism, we determined whether acute inhibition of proteasome activity alters the intracellular trafficking or prolonged persistence of internalized Ag-BCR complexes.11
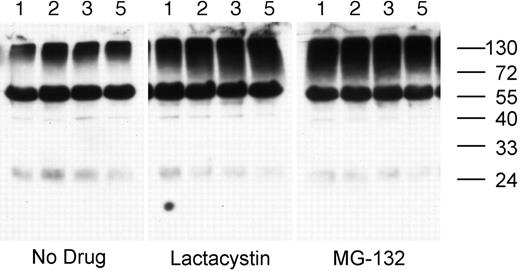
Treatment of splenic B cells with either lactacystin (a nonreversible proteasome inhibitor) or MG-132 (a reversible proteasome inhibitor) results in the accumulation of high-molecular-mass ubiquitinated cellular proteins in as little as 1 hour, demonstrating that the drugs inhibit proteasome activity in these cells (Figure 2). In addition to the smear of high-molecular-mass ubiquitinated proteins, the whole-cell lysates also contain a single predominant ubiquitinated protein of approximately 60-kDa molecular weight. While the identity of the 60-kDa ubiquitinated protein is presently unknown, it is not the heavy chain of the endogenous BCR, as it is not recognized by any anti–murine IgG or IgM antibodies tested and is not removed from the cell lysate by preclearing of the BCR (data not shown).
Proteasome inhibition results in the accumulation of ubiquitinated proteins in splenic B cells. MD4.B10.Br splenocytes were incubated at 37°C for 1, 2, 3, or 5 hours (as indicated above each lane) in media containing either no drug, 10 μM MG-132, or 10 μM lactacystin. The presence of ubiquitinated proteins in the cell lysates was determined by reducing SDS-PAGE and Western blotting with the 6C1 antiubiquitin mAb. The faint band at approximately 24 kDa is the light chain of the endogenous BCR, as it was detected in blots probed with secondary antibody alone (not shown). The prominent band of approximately 60-kDa molecular weight is a constitutively ubiquitinated protein (as opposed to detection of the endogenous BCR), as this band was not observed in blots probed with secondary antibody only. Similar results were obtained whether or not the B cells had been stimulated via the BCR (data not shown). Shown are representative results from 1 of 3 independent experiments.
Proteasome inhibition results in the accumulation of ubiquitinated proteins in splenic B cells. MD4.B10.Br splenocytes were incubated at 37°C for 1, 2, 3, or 5 hours (as indicated above each lane) in media containing either no drug, 10 μM MG-132, or 10 μM lactacystin. The presence of ubiquitinated proteins in the cell lysates was determined by reducing SDS-PAGE and Western blotting with the 6C1 antiubiquitin mAb. The faint band at approximately 24 kDa is the light chain of the endogenous BCR, as it was detected in blots probed with secondary antibody alone (not shown). The prominent band of approximately 60-kDa molecular weight is a constitutively ubiquitinated protein (as opposed to detection of the endogenous BCR), as this band was not observed in blots probed with secondary antibody only. Similar results were obtained whether or not the B cells had been stimulated via the BCR (data not shown). Shown are representative results from 1 of 3 independent experiments.
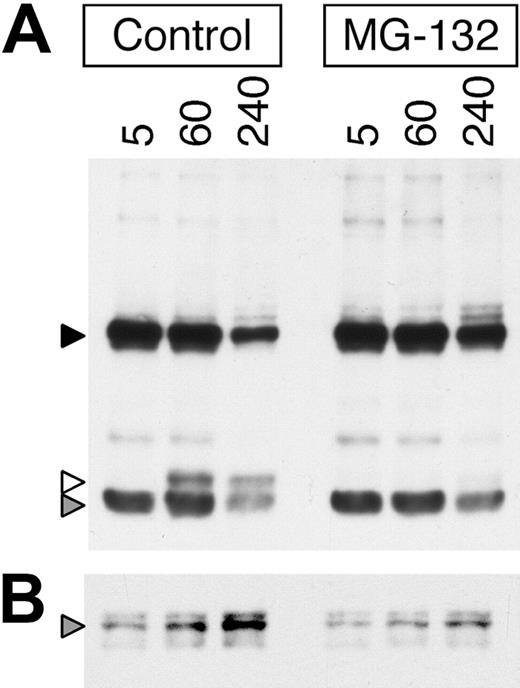
To determine if, similar to the EGFR,15,19,21,29 inhibition of proteasome activity alters the intracellular trafficking and degradation of internalized ligand-BCR complexes, the effect of the proteasome inhibition on the degradation of BCR-internalized anti-BCR antibody was determined. For these experiments, a biotinylated anti-IgM mAb (composed of a 55-kDa heavy chain and 23-kDa light chain) was used as a BCR ligand instead of HEL (which is composed of a single 15-kDa chain) to allow better resolution of the proteolytic processing of the BCR-associated ligand. Consistent with published observations in the EGFR system, inhibition of proteasome activity in MD4.B10.Br B cells both prolongs the persistence of intact BCR-internalized anti-IgM heavy and light chain protein (Figure 3A black and gray arrowheads, respectively) and delays the generation of proteolytic fragments of the anti-IgM heavy chain (Figure 3A white arrowhead). In addition, while there is a time-dependent increase in the level of ubiquitinated (ie, p62 UBA binding) ligand-BCR complexes in the non–proteasome-inhibited B cells (Figure 3B gray arrowhead), treatment with MG-132 inhibits/delays the generation of these ubiquitinated ligand–BCR complexes.
Proteasome inhibition slows the degradation of BCR-associated ligand and delays the increased formation of ubiquitinated ligand–BCR complexes. (A) MD4.B10.Br splenocytes were pretreated for 1 hour at 37°C with or without 10 μM MG-132 as indicated. The cells were then pulsed with 10 μg/mL anti–IgMa-btn for the indicated time (minutes) at 37°C in the continued presence of inhibitor. The ligand-pulsed cells were then washed and lysed, and the lysates cleared by centrifugation. The proteolytic degradation of the BCR-internalized anti–IgMa-btn mAb in the whole-cell lysate was analyzed by reducing SDS-PAGE and Western blotting with SA-HRP (upper panel, ligand). The high-molecular-weight band (55 kDa, black arrowhead) represents the intact heavy chain of the anti–IgMa-btn mAb. The prominent low-molecular-weight band (25 kDa, gray arrowhead) represents the intact light chain of the anti–IgMa-btn mAb. The band of 30-kDa molecular weight (white arrowhead) is a proteolytic fragment of the heavy chain of the anti–IgMa-btn mAb. Shown are representative results from 1 of 5 independent experiments. (B) A portion of the lysates analyzed in panel A was analyzed for the presence of anti–IgMa-btn–BCR-ubiquitin complexes by precipitation of ubiquitinated proteins (including ubiquitinated ligand–BCR complexes) with p62 UBA–agarose and analysis of the samples by reducing SDS-PAGE and Western blotting with SA-HRP. The gray arrowhead marks the position of intact 55-kDa heavy chain of the anti–IgMa-btn mAb, bound to the ubiquitinated BCR and thus precipitated by the p62 UBA–agarose. Shown are representative results from 1 of 3 independent experiments.
Proteasome inhibition slows the degradation of BCR-associated ligand and delays the increased formation of ubiquitinated ligand–BCR complexes. (A) MD4.B10.Br splenocytes were pretreated for 1 hour at 37°C with or without 10 μM MG-132 as indicated. The cells were then pulsed with 10 μg/mL anti–IgMa-btn for the indicated time (minutes) at 37°C in the continued presence of inhibitor. The ligand-pulsed cells were then washed and lysed, and the lysates cleared by centrifugation. The proteolytic degradation of the BCR-internalized anti–IgMa-btn mAb in the whole-cell lysate was analyzed by reducing SDS-PAGE and Western blotting with SA-HRP (upper panel, ligand). The high-molecular-weight band (55 kDa, black arrowhead) represents the intact heavy chain of the anti–IgMa-btn mAb. The prominent low-molecular-weight band (25 kDa, gray arrowhead) represents the intact light chain of the anti–IgMa-btn mAb. The band of 30-kDa molecular weight (white arrowhead) is a proteolytic fragment of the heavy chain of the anti–IgMa-btn mAb. Shown are representative results from 1 of 5 independent experiments. (B) A portion of the lysates analyzed in panel A was analyzed for the presence of anti–IgMa-btn–BCR-ubiquitin complexes by precipitation of ubiquitinated proteins (including ubiquitinated ligand–BCR complexes) with p62 UBA–agarose and analysis of the samples by reducing SDS-PAGE and Western blotting with SA-HRP. The gray arrowhead marks the position of intact 55-kDa heavy chain of the anti–IgMa-btn mAb, bound to the ubiquitinated BCR and thus precipitated by the p62 UBA–agarose. Shown are representative results from 1 of 3 independent experiments.
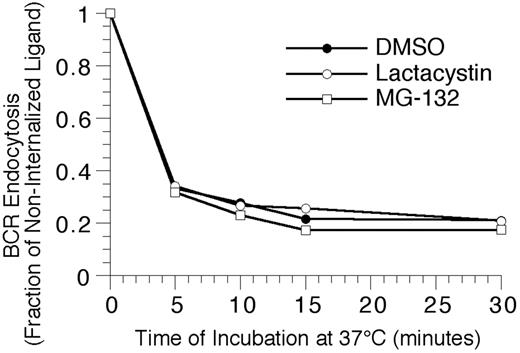
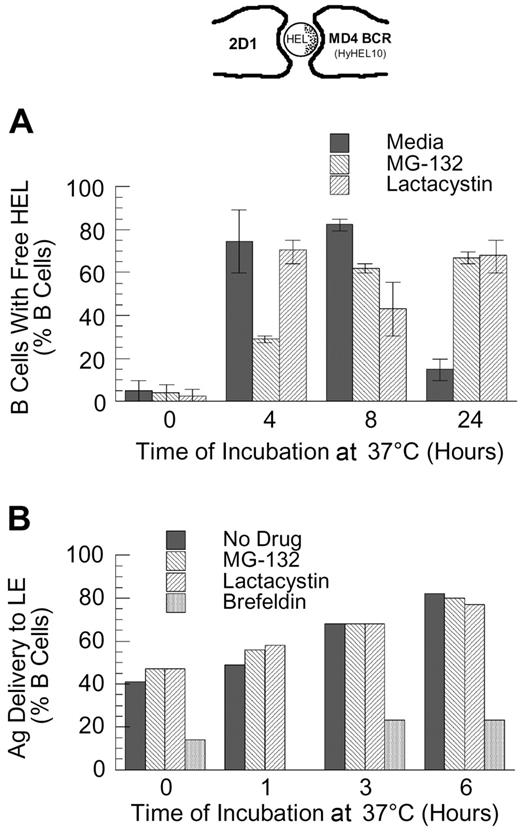
To extend the analysis to complexes of BCR and cognate antigen, the effect of proteasome inhibition on the endocytosis and degradation of HEL-BCR complexes in MD4.B10.Br BCR transgenic splenic B cells was determined. The first step in BCR-mediated antigen processing that could be altered by proteasome inhibition is antigen-BCR internalization. However, when the kinetics of HEL-BCR complex internalization were analyzed, it was determined that proteasome inhibition does not alter the rate of antigen-BCR internalization (Figure 4), suggesting that proteasome inhibition must be impacting a step in Ag-BCR trafficking subsequent to BCR internalization. Therefore, the intracellular dissociation of internalized HEL-BCR complexes was analyzed using a published immunofluorescence microscopy–based approach11,14 that relies on the selective recognition of BCR-dissociated HEL by the HyHEL10 mAb (which recognizes the same HEL epitope as the MD4 BCR, Figure 5). This particular approach was taken because it avoids the use of biotinylated HEL as a BCR ligand, since HEL that has been biotinylated on lysine residues binds to the HEL-specific transgenic BCR with altered characteristics (J.R.D., unpublished observation, December 1997).
Since the binding of HEL to the MD4 BCR is of high affinity30 and is relatively resistant to disruption by mild acidification (J.R.D., unpublished observation, February 1998), the intracellular dissociation of HEL-BCR complexes is likely to occur within deep endocytic compartments with high proteolytic activity and/or a highly acidic pH (eg, late endosomes [LEs]). Therefore, if the delivery of HEL-BCR complexes to MVB-like MIICs occurs via a ubiquitin-dependent mechanism, then inhibition of proteasome activity should slow the kinetics of Ag-BCR dissociation. As shown by the results presented in Figure 5A, while proteasome inhibition does not completely block the dissociation of intracellular Ag-BCR complexes at early times (ie, at 4 and 8 hours after antigen pulse, the percent of B cells possessing detectable levels of non–BCR-associated antigen is decreased but not brought to background levels), proteasome inhibition does result in the prolonged presence of BCR-dissociated antigen, such that the majority of proteasome-inhibited B cells possesses non–BCR-associated antigen 24 hours after antigen pulse.
Proteasome inhibition fails to alter the kinetics of BCR-mediated antigen internalization. Splenic B cells were pretreated with the indicated proteasome inhibitor for 1 hour at 37°C, before analyzing the BCR-mediated internalization of bound ligand by flow cytometry, as described in “Materials and methods.” The results demonstrate that inhibition of proteasome activity fails to alter the kinetics of BCR-mediated antigen internalization, even though the treatment resulted in the accumulation of high-molecular-mass ubiquitinated proteins (Figure 2). Shown are representative results from 1 of 4 independent experiments (4 investigating the effect of MG-132 on BCR internalization, and 2 analyzing the effect of lactacystin treatment on BCR internalization).
Proteasome inhibition fails to alter the kinetics of BCR-mediated antigen internalization. Splenic B cells were pretreated with the indicated proteasome inhibitor for 1 hour at 37°C, before analyzing the BCR-mediated internalization of bound ligand by flow cytometry, as described in “Materials and methods.” The results demonstrate that inhibition of proteasome activity fails to alter the kinetics of BCR-mediated antigen internalization, even though the treatment resulted in the accumulation of high-molecular-mass ubiquitinated proteins (Figure 2). Shown are representative results from 1 of 4 independent experiments (4 investigating the effect of MG-132 on BCR internalization, and 2 analyzing the effect of lactacystin treatment on BCR internalization).
Proteasome inhibition delays the dissociation of internalized antigen-BCR complexes. (A) MD4.B10.Br splenocytes were pretreated with proteasome inhibitor and pulsed with antigen as described in “Materials and methods.” The cells were then fixed and stained for BCR-dissociated antigen, using the HyHEL10 monoclonal antibody that recognizes the same HEL epitope as the MD4 BCR (see image at the top of the figure and Gondré-Lewis et al11 ). The cells were then analyzed by fluorescence microscopy, and the percent of B cells containing detectable BCR-dissociated antigen was determined. The results demonstrate that while BCR-dissociated antigen is readily detectable 4 hours after internalization under all conditions, BCR-dissociated antigen is present for a prolonged period of time in proteasome-inhibited B cells. Shown are average values from 2 independent experiments (bars). The error bars indicate the range of values obtained across both experiments. (B) B10.Br splenocytes were treated as indicated (no drug or 10 μM MG-132, lactacystin, or brefeldin A), and then allowed to internalize HEL by fluid-phase endocytosis for 30 minutes. The cells were then washed, chased for the indicated times, fixed, and stained for HEL and the LE/L marker LAMP. The percent of B cells in which HEL was detectable within LAMP+ LE/L is shown. The results demonstrate that unlike treatment with brefeldin A (which is known to inhibit trafficking between the earlier and later aspects of the endocytic pathway), treatment of the cells with the proteasome inhibitor fails to slow the delivery of fluid-phase markers to the later aspects of the endocytic pathway. It should be noted that at the 6-hour time point, the B cells contained low overall levels of detectable HEL. Shown are representative results from 1 of 3 independent experiments.
Proteasome inhibition delays the dissociation of internalized antigen-BCR complexes. (A) MD4.B10.Br splenocytes were pretreated with proteasome inhibitor and pulsed with antigen as described in “Materials and methods.” The cells were then fixed and stained for BCR-dissociated antigen, using the HyHEL10 monoclonal antibody that recognizes the same HEL epitope as the MD4 BCR (see image at the top of the figure and Gondré-Lewis et al11 ). The cells were then analyzed by fluorescence microscopy, and the percent of B cells containing detectable BCR-dissociated antigen was determined. The results demonstrate that while BCR-dissociated antigen is readily detectable 4 hours after internalization under all conditions, BCR-dissociated antigen is present for a prolonged period of time in proteasome-inhibited B cells. Shown are average values from 2 independent experiments (bars). The error bars indicate the range of values obtained across both experiments. (B) B10.Br splenocytes were treated as indicated (no drug or 10 μM MG-132, lactacystin, or brefeldin A), and then allowed to internalize HEL by fluid-phase endocytosis for 30 minutes. The cells were then washed, chased for the indicated times, fixed, and stained for HEL and the LE/L marker LAMP. The percent of B cells in which HEL was detectable within LAMP+ LE/L is shown. The results demonstrate that unlike treatment with brefeldin A (which is known to inhibit trafficking between the earlier and later aspects of the endocytic pathway), treatment of the cells with the proteasome inhibitor fails to slow the delivery of fluid-phase markers to the later aspects of the endocytic pathway. It should be noted that at the 6-hour time point, the B cells contained low overall levels of detectable HEL. Shown are representative results from 1 of 3 independent experiments.
Since the prolonged presence of BCR-dissociated HEL in the proteasome-inhibited MD4 B cells could be due either to an inhibition of degradation of the free HEL (after it has dissociated from the BCR) or to a slowing of the kinetics of Ag-BCR dissociation (followed by the normal degradation of the BCR-dissociated HEL), the effect of proteasome inhibition on the kinetics of degradation of free HEL internalized by F-P endocytosis was determined using the same IFM-based protocol.11 The results of this analysis demonstrated that proteasome inhibition fails to alter the rate of degradation of HEL internalized by F-P endocytosis (ie, no HEL was detectable 6 to 8 hours after pulsing non–BCR-transgenic B cells with 100 μM HEL,11,14 whether or not the cells were treated with proteasome inhibitor; data not shown). Nevertheless, to examine whether proteasome inhibition has a more subtle effect on the trafficking or degradation of non–BCR-associated HEL, the localization of F-P–internalized HEL with the late endosome/lysosome (LE/L) marker LAMP was determined. As shown by the result presented in Figure 5B, while addition of brefeldin A (a drug that, in addition to altering protein trafficking within the biosynthetic pathway, alters the trafficking of proteins between the early and latter aspects of the endocytic pathway31,32 ) resulted in a decrease in the percent of cells in which F-P–internalized HEL was delivered to LAMP+ endocytic compartments, acute inhibition of proteasome activity fails to alter the trafficking of non–BCR-associated HEL from early to late endosomes. These results suggest that the prolonged presence of BCR-dissociated HEL in proteasome-inhibited MD4 B cells (Figure 5A) is due to a delay in the dissociation of the internalized Ag-BCR complexes, followed by the normal degradation of the BCR-dissociated HEL.
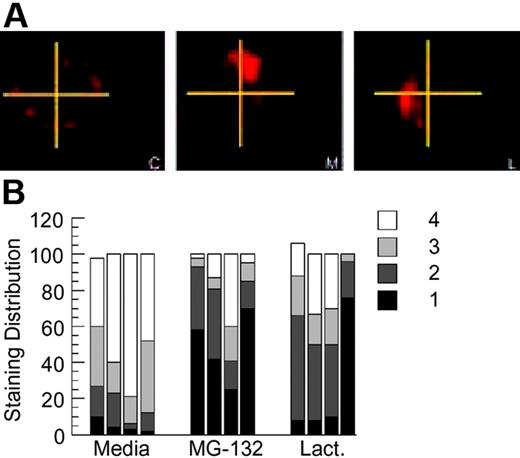
A novel aspect of BCR-mediated antigen processing that has been previously reported in this model system is the prolonged intracellular persistence of internalized HEL-BCR complexes within nonterminal late endocytic compartments.11,14 To determine whether BCR ubiquitination has a role in this aspect of BCR biology, HEL-specific B cells from MD4.B10.Br BCR transgenic mice were pretreated with proteasome inhibitor for 1 hour, pulsed with 100 nM HEL for 4 hours (in the continued presence of the proteasome inhibitor), then washed and returned to culture for 18 to 20 hours. (This strategy of acute proteasome inhibition has also been used in the analysis of EGFR trafficking and is thought to inhibit key ubiquitin-dependent events that occur soon after receptor internalization.15,19,21,29 ) The cells were then collected, and the presence and distribution of persisting Ag-BCR complexes was determined, using the anti-HEL mAb 2D1 that can bind HEL whether or not it is associated with the MD4 BCR. Analysis of the samples reveals that proteasome inhibition does not alter the percentage of B cells that possesses intracellular Ag-BCR complexes, as in all cases persisting Ag-BCR complexes can be detected in 80% to 90% of the B cells as previously reported.11 However, acute inhibition of proteasome activity does result in the altered distribution of persisting Ag-BCR complexes, with a large majority of the drug-treated cells exhibiting a more highly polarized distribution of persisting Ag-BCR complexes (Figure 6). Taken together, the results presented in Figures 1, 2, 3, 4, 5, 6 demonstrate that the immunoglobulin heavy chain of at least a portion of BCR molecules can undergo dynamic ubiquitination and that conditions that alter this process alter the degradation and intracellular trafficking of internalized Ag-BCR complexes.
Proteasome inhibition alters the intracellular distribution of persisting antigen-BCR complexes. (A) MD4.B10.Br splenocytes were pretreated with the indicated inhibitor for 1 hour at 37°C before pulsing with antigen (100 nM HEL) for 4 hours. The cells were then washed (to remove both antigen and drug) and cultured for an additional 18 to 20 hours at 37°C. The cells were then collected, fixed, and stained for persisting Ag-BCR complexes using the 2D1 anti-HEL mAb.11,14 C indicates control, non–drug-treated cells; M, MG-132–treated cells; and L, lactacystin-treated cells. Shown are representative images from 1 of 4 independent experiments. (B) For each experimental condition, 100 B cells were visualized and the distribution of persisting antigen was scored as described in “Material and methods.” Shown is the frequency of cells in which the persisting Ag-BCR complexes were detected in 1, 2, 3, or 4 quadrants of the cell. Each bar represents the results from a single independent experiment.
Proteasome inhibition alters the intracellular distribution of persisting antigen-BCR complexes. (A) MD4.B10.Br splenocytes were pretreated with the indicated inhibitor for 1 hour at 37°C before pulsing with antigen (100 nM HEL) for 4 hours. The cells were then washed (to remove both antigen and drug) and cultured for an additional 18 to 20 hours at 37°C. The cells were then collected, fixed, and stained for persisting Ag-BCR complexes using the 2D1 anti-HEL mAb.11,14 C indicates control, non–drug-treated cells; M, MG-132–treated cells; and L, lactacystin-treated cells. Shown are representative images from 1 of 4 independent experiments. (B) For each experimental condition, 100 B cells were visualized and the distribution of persisting antigen was scored as described in “Material and methods.” Shown is the frequency of cells in which the persisting Ag-BCR complexes were detected in 1, 2, 3, or 4 quadrants of the cell. Each bar represents the results from a single independent experiment.
Proteasome inhibition selectively inhibits BCR-mediated antigen processing and presentation
The results presented in Figures 1, 2, 3, 4, 5, 6, along with the previously published observation that the prolonged persistence of Ag-BCR complexes within LAMP+ late endocytic compartments allows for the prolonged expression of derivative antigenic peptide–class II complexes,11,14 suggest that inhibition of proteasome activity and the resultant change in Ag-BCR trafficking may selectively alter BCR-mediated antigen processing and presentation. To address this question, the effect of acute proteasome inhibition on F-P versus BCR-mediated antigen processing and presentation was determined, using a flow cytometry–based assay for the expression of HEL46-61–I-Ak peptide–class II complexes.5,11,14
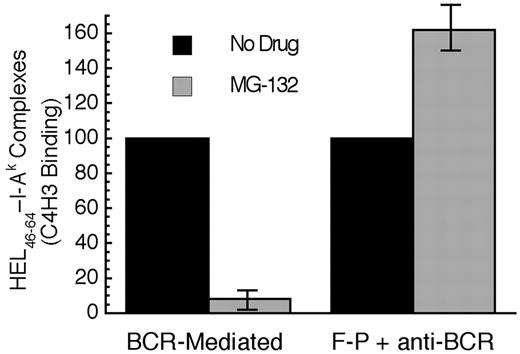
When the BCR-mediated processing and presentation of HEL in MD4.B10.Br B cells was analyzed, it was determined that acute proteasome inhibition profoundly inhibits BCR-mediated antigen processing and presentation (Figure 7). In contrast, acute proteasome inhibition has no inhibitory effect of the F-P processing of HEL in B10.Br B cells (stimulated with an anti-IgM antibody to mimic the BCR signaling events that occur in MD4.B10.Br B cells upon exposure to HEL). In fact, acute proteasome inhibition mildly augments F-P antigen processing, possibly due to subtle MG-132–induced changes in the trafficking of cellular proteins to antigen processing compartments or a slight shift in the balance between the complete degradation of antigen versus conversion to peptide–class II complexes. Nevertheless, when compared with the mild enhancing effect of proteasome inhibition on the F-P processing of non–cognate antigen, acute proteasome inhibition, which selectively alters the intracellular trafficking and degradation of Ag-BCR complexes, profoundly inhibits the BCR-mediated processing and presentation of cognate antigen.
Acute proteasome inhibition selectively inhibits BCR-mediated antigen processing and presentation. B cells were pretreated with the indicated drug for 1 hour at 37°C. The cells were then pulsed with antigen (BCR indicates MD4.B10.Br pulsed with 100 nM HEL; F-P, B10.Br pulsed with 100 μM HEL and 100 nM anti–murine IgM antibody) for 4 hours in the continued presence of drug before washing and an additional 18 to 20 hours of incubation at 37°C in the absence of drug or antigen. The cells were then harvested and the level of HEL46-61–I-Ak complexes expressed on the surface of the cell was determined by staining with the HEL46-61–I-Ak complex–specific mAb C4H3 and subsequent analysis by flow cytometry.5,11,14 Experiments performed with lactacystin gave similar results to those obtained with MG-132. However, this treatment resulted in a significantly greater level of B-cell death, presumably due to the irreversible nature of the inhibitor (data not shown). Parallel analysis of each sample for the expression of total I-Ak class II molecules (by staining with the panreactive mAb 11-5.2) revealed that none of the drug treatments resulted in a significant (ie, > 10%) decrease in the level of total I-Ak molecules expressed by the cells. Consistent with previous reports that HEL46-61 is presented exclusively on newly synthesized I-Ak molecules,5,14 treatment with either 10 μM puromycin or 10 μg/mL brefeldin A inhibited C4H3 binding by 80% to 90% under all conditions. The bars indicate the average level of HEL46-61–I-Ak complexes expressed under the indicated conditions, as a percent of the level observed in non–drug-treated cells. The error bars indicate the range of experimental values obtained under each condition. Shown are from results from 2 independent experiments.
Acute proteasome inhibition selectively inhibits BCR-mediated antigen processing and presentation. B cells were pretreated with the indicated drug for 1 hour at 37°C. The cells were then pulsed with antigen (BCR indicates MD4.B10.Br pulsed with 100 nM HEL; F-P, B10.Br pulsed with 100 μM HEL and 100 nM anti–murine IgM antibody) for 4 hours in the continued presence of drug before washing and an additional 18 to 20 hours of incubation at 37°C in the absence of drug or antigen. The cells were then harvested and the level of HEL46-61–I-Ak complexes expressed on the surface of the cell was determined by staining with the HEL46-61–I-Ak complex–specific mAb C4H3 and subsequent analysis by flow cytometry.5,11,14 Experiments performed with lactacystin gave similar results to those obtained with MG-132. However, this treatment resulted in a significantly greater level of B-cell death, presumably due to the irreversible nature of the inhibitor (data not shown). Parallel analysis of each sample for the expression of total I-Ak class II molecules (by staining with the panreactive mAb 11-5.2) revealed that none of the drug treatments resulted in a significant (ie, > 10%) decrease in the level of total I-Ak molecules expressed by the cells. Consistent with previous reports that HEL46-61 is presented exclusively on newly synthesized I-Ak molecules,5,14 treatment with either 10 μM puromycin or 10 μg/mL brefeldin A inhibited C4H3 binding by 80% to 90% under all conditions. The bars indicate the average level of HEL46-61–I-Ak complexes expressed under the indicated conditions, as a percent of the level observed in non–drug-treated cells. The error bars indicate the range of experimental values obtained under each condition. Shown are from results from 2 independent experiments.
Finally, since the processing and presentation of HEL46-61 is known to occur via newly synthesized I-Ak class II molecules loaded with peptide in deep endocytic compartments,5 the effects of classical inhibitors of this pathway on the generation of HEL46-61–I-Ak complexes from HEL internalized via both F-P and BCR-mediated endocytosis were determined. Treatment of B cells with 10 μM brefeldin A, which blocks both the trafficking of newly synthesized proteins within the early aspects of the biosynthetic pathway as well as trafficking of internalized molecules from early to late aspects of the endosomal/lysosomal pathway,31,32 inhibits both BCR-mediated and F-P antigen processing by more than 90% (BCR mediated: average inhibition, 94%; range, 87%-98%; n = 3; and F-P: average inhibition, 95%; range, 92%-100%; n = 3). Treatment of B cells with 2 mM leupeptin, which blocks the proteolytic processing of class II–associated invariant chain, inhibits both BCR-mediated and F-P antigen processing by more than 66% (BCR mediated: average inhibition, 86%; range, 78%-91%; n = 3; and F-P: average inhibition, 66%; range, 64%-76%; n = 3). Treatment of B cells with 10 μM puromycin, which blocks class II biosynthesis, inhibits both BCR-mediated and F-P antigen processing by more than 92% (BCR mediated: average inhibition, 100%; range, 99%-100%; n = 2; F-P: average inhibition, 96%; range, 92%-99%; n = 2). These results demonstrate that while the generation of peptide–class II complexes from HEL protein internalized by either F-P or BCR-mediated endocytosis occurs via a similar mechanism within deep elements of the endosomal/lysosomal pathway (eg, MIICs), the delivery of Ag-BCR complexes to MIICs occurs via a ubiquitin-dependent mechanism similar to the EGFR, establishing the EGFR as a paradigm for the further analysis of Ag-BCR trafficking to and within MIICs.
Discussion
The results presented in this report demonstrate that a portion of the heavy chains of the IgM BCR is ubiquitinated, and that conditions known to alter the intracellular trafficking of ubiquitinated receptors (eg, the EGFR) also alter the trafficking of internalized Ag-BCR complexes, selectively inhibiting the BCR-mediated processing and presentation of cognate antigen. These findings highlight the general similarities between the intracellular trafficking of the BCR and EGFR,15,19,21 and suggest that the intracellular trafficking of the 2 receptors to and within MVB-like MIICs occurs via a similar molecular mechanism.
Since the ubiquitination of lysine residues of target proteins is carried out by ubiquitin ligases residing within the cytosol of the cell (eg, Cbl-b, discussed in the next paragraph), the cytoplasmic domain of the heavy chain of the IgM BCR, which ends with the amino acids lysine-valine-lysine, represents the most likely site for BCR IgH ubiquitination. (This scenario is consistent with preliminary flow cytometry results showing that antiubiquitin antibodies bind only to detergent-permeabilized B cells, and not to nonpermeabilized B cells.) However, the precise form and extent of IgM heavy chain ubiquitination will require further analysis, which is presently in progress. In addition, while there are no publications reporting the direct ubiquitination of the CD79 signaling subunit of the BCR, which possesses a total of 5 cytoplasmic lysine residues, this possibility is also deserving of future study. Nevertheless, the results presented in this report demonstrate that at least one subunit of the BCR (ie, the IgM heavy chain) can be ubiquitinated, and that conditions that alter the level of ubiquitinated Ag-BCR complexes also selectively inhibit the BCR-mediated processing and presentation of cognate antigen.
In addition to the core IgH/L-CD79 BCR molecule, at least 2 BCR-associated signaling molecules (ie, lyn and syk) have been reported to be targets for ubiquitination by members of the Cbl family of BCR-associated ubiquitin ligases.33 Cbl family ubiquitin ligases are known to be phosphorylated upon BCR signaling,34 and have been shown to bind and ubiquitinate both lyn and syk.34-37 While a major impact of the ubiquitination of these BCR-associated signaling molecules is thought to be to target these enzymes for proteasome-dependent degradation, resulting in the down-regulation of BCR signaling, Cbl-mediated ubiquitination of these proteins would also place additional putative ubiquitin-based sorting motifs on the cytoplasmic domain of a subpopulation of Ag-BCR complexes (ie, those directly involved in BCR signaling). In addition, Cbl-b has been reported to modulate BLNK-dependent BCR signaling.38 Since the association of BLNK with internalized Ag-BCR complexes has been shown to be important for the delivery of Ag-receptor complexes to MVB-like MIICs,39,40 this suggests another mechanism by which the regulation of BCR ubiquitination, possibly via the activity of Cbl, may be important in controlling Ag-BCR trafficking. While each of these observations is consistent with the observed effect of acute proteasome inhibition on BCR-mediated antigen processing and presentation reported herein, the precise role of ubiquitination of the IgH subunit of the BCR, versus the role of the ubiquitination of these BCR-associated signaling molecules in the intracellular trafficking of Ag-BCR complexes, remains to be determined by future studies.
In conclusion, the results presented in this report highlight the unique cell biology underlying the BCR-mediated processing and presentation of cognate antigen. In addition, the results suggest that the regulated sorting of Ag-BCR complexes to and within MVB-like MIICs may represent a mechanism for the prolonged intracellular persistence of internalized Ag-BCR complexes11 and the subsequent formation of peptide–class II complexes with unique biologic properties.5 Future work will test these possibilities and provide a better understanding of this important aspect of B-cell biology.
Authorship
Contribution: L.D. designed research, performed research, and analyzed data; E.M.M.-B. designed research, performed research, and analyzed data; J.R.D. conceived the project, designed research, performed research, analyzed data, and wrote the paper.
Conflict of interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 24, 2006; DOI 10.1182/blood-2006-05-025338.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by an NIH grant (AI-46405) to J.R.D. E.M.M.-B. was supported by a T32 training grant (AI-49822) to the Center for Immunology and Microbial Disease.
The authors would like to thank Jamie van Ness for excellent technical assistance, as well as Drs Timothy Gondré-Lewis and Lisa Denzin for helpful discussions and critical reading of the paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal