Abstract
Previous studies demonstrated that circulating dendritic cells (DCs) in myeloma patients were functionally abnormal. However, the phenotype and function of patients' monocyte-derived DCs (MoDCs), which are commonly used for immunotherapy, were poorly defined. This study was undertaken to examine the quality of MoDCs from myeloma patients compared with cells from healthy donors. We found that patient-derived MoDCs are phenotypically and functionally defective. Compared with their normal counterparts, patient-derived, mature MoDCs expressed significantly lower levels of CD1a, CD40, CD80, and HLA-DR and were poor at activating alloreactive T cells, presenting recall antigen, and activating autologous antigen- and myeloma-specific T cells. These abnormalities may be attributed to elevated production of autocrine cytokines such as IL-6, activated p38 and STAT3, and inhibited MEK/ERK signaling pathways in the progenitor cells. Treatment with neutralizing IL-6–specific antibody and, more importantly, p38 inhibitor, or both, could correct these abnormalities. Treating patient-derived cells with these agents not only significantly increased cell yield but also produced MoDCs that were as functional as their normal counterparts. Thus, this study has delineated the mechanistic defects of MoDCs from myeloma patients and identified ways for restoring the function of the cells to improve the efficacy of DC-based immunotherapy in this disease.
Introduction
Dendritic cells (DCs) are the sentinels of the immune system.1-3 In their immature state, DCs are distributed primarily in tissues where they efficiently survey for incoming pathogens. Encounter with pathogens leads to DC activation and migration to secondary lymphoid organs, and during the migration they undergo maturation. Mature DCs not only acquire the ability to stimulate quiescent, naive CD4+ and CD8+ T cells and B cells and initiate primary immune responses but can also induce a strong secondary immune response with relatively small numbers of DCs and low levels of antigen.2 Given their central role in controlling immunity, DCs are logical targets for many clinical situations that involve T cells, such as graft rejection, allergy, autoimmune diseases, resistance to infection and tumors, immunodeficiency, and vaccination.
DC-based immunotherapy holds great promise for treating malignancies4-6 including multiple myeloma (MM).7-10 However, preliminary reports of DC-based immunotherapy in human MM have demonstrated minor clinical responses.7-10 The lack of effectiveness of DC vaccines in tumor patients may be associated, at least in part, with defects in DCs.11-14 Indeed, previous studies showed that the numbers of circulating DCs were significantly lower in patients with MM than in healthy individuals,14 and the phenotype and function of these cells were also impaired.13,14 The underlying mechanisms are largely unknown. Using the 5T2 myeloma mouse model, we have recently shown that myeloma cells or tumor-culture conditioning medium (TCCM) were able to inhibit differentiation and function of murine bone marrow–derived DCs.15 However, the phenotypic and functional properties of monocyte-derived DCs (MoDCs) from myeloma patients were still poorly defined. This information is particularly important and relevant because MoDCs are commonly used as vaccines for immunotherapy in myeloma patients.7-10 Therefore the present study was undertaken to examine MoDCs from myeloma patients. We found that, compared with cells from healthy donors, MoDCs generated from myeloma patients were phenotypically abnormal and functionally impaired. These abnormalities may be attributed to elevated production of autocrine cytokines such as IL-6, activated STAT3 and p38, and inhibited Raf/MEK/ERK signaling pathways. Treatment with IL-6–neutralizing antibody and, more importantly, p38 inhibitor, or both, may correct these abnormalities.
Materials and methods
Reagents
PE-conjugated or FITC-conjugated monoclonal Abs (mAbs) against human CD1a, CD40, CD54, CD80, CD83, CD86, HLA-ABC, and HLA-DR and mouse IgG1 isotype control were purchased from BD PharMingen (San Diego, CA). Neutralizing antibodies against IL-6, IL-10, and TGF-β1 were purchased from R&D Systems (Minneapolis, MN). Recombinant IL-1β, IL-2, IL-4, IL-6, IL-7, IL-10, IL-15, GM-CSF, TGF-β1, and TNF-α were purchased from R&D Systems. Prostaglandin E2 (PGE2) was purchased from Sigma (St Louis, MO). Tuberculin-purified protein derivative (PPD) was purchased from Statens Serum Institute (Copenhagen, Denmark). p38 inhibitor III (specific p38 inhibitor) was purchased from Calbiochem-Novabiochem (La Jolla, CA). [3H]thymidine and Ficoll-Hypaque were purchased from Amersham Pharmacia Biotech (Piscataway, NJ).
Human myeloma cell lines and tumor-culture conditioning medium
The human myeloma cell line MM.1S was kindly provided by Dr Steven Rosen from Northwestern University (Chicago, IL). ARP-1 was established at the Arkansas Cancer Research Center from bone marrow aspirates of patients with MM,16 and U266 was purchased from American Type Culture Collection (Rockville, MD). These cells were cultured at a density of 1 × 106/mL in RPMI-1640 complete medium for 16 hours, and culture media were collected, pooled, and stored at 4°C for up to 1 week before use.
Samples from patients with MM and healthy blood donors
Peripheral blood from 12 patients with MM was used for this study. These patients were newly diagnosed patients with stage II to III MM. M.D. Anderson Cancer Center institutional review board–approved informed consent was obtained from all patients. As controls, buffy-coat blood from 10 healthy donors was obtained from the M. D. Anderson Cancer Center blood bank and used in this study.
Generation of MoDCs
MoDCs were generated from peripheral-blood mononuclear cells (PBMCs) using standard protocols.17,18 Briefly, PBMCs were isolated from myeloma patients and healthy donors using Ficoll-Hypaque gradient centrifugation. MoDCs were obtained by culturing the adherent cells in RPMI-1640 complete medium (RPMI-1640 supplemented with 10% fetal calf serum, 1 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin) with addition of GM-CSF (10 ng/mL) and IL-4 (10 ng/mL) in a humidified incubator at 37°C in 5% CO2, with further addition of cytokines by replacing medium with fresh medium containing the cytokines on day 3 and day 5. In some experiments, monocytes were purified from PBMCs by positive selection using a magnetic-activated cell separation (MACS) column with anti-CD14 antibody–conjugated microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The positively selected fraction contained more than 95% CD14+ monocytes. A cocktail of IL-1β, IL-6, TNF-α, and PGE2 was added on day 7, and matured DCs were harvested on day 9.
To examine the effects of myeloma-derived factors on the differentiation of MoDCs, we added freshly obtained TCCM to the cell cultures (50% TCCM to 50% fresh medium) on day 0 and on day 3 when medium was changed. No additional TCCM was added on other days. In some experiments, the adherent cells were cultured in complete medium containing GM-CSF and IL-4, with the addition of 20 ng/mL IL-6, IL-10, and TGF-β1 on day 0 and day 3. Medium changes and maturation induction were the same as normal MoDCs.
To examine the effects of p38 inhibition on DC differentiation, we added p38 inhibitor III (1 μM) to the cultures on day 0, and no additional p38 inhibitor was added at medium changes or during DC maturation. Cultures with addition of dimethylsulfoxide (DMSO; 0.1%; Sigma) served as control.
Flow-cytometry analysis
MoDCs or cultured cells were incubated with FITC-conjugated or PE-conjugated mAbs against CD1a, CD40, CD54, CD80, CD83, CD86, HLA-ABC, and HLA-DR (BD PharMingen) for 30 minutes at 4°C; washed twice; and resuspended in PBS. Analyses of fluorescence staining were performed using a Becton Dickinson FACScan (San Jose, CA).
Allogeneic MLR assay
To examine the capacity of MoDCs to activate alloreactive T cells, we used an allogeneic mixed-lymphocyte reaction (MLR). Briefly, allogeneic T cells (1 × 105 cells/100 μL/well) were seeded in 96-well, U-bottom tissue-culture plates and cultured with various numbers of MoDCs at 37°C in 5% CO2 for 6 days. Sixteen hours before harvest, 1 μCi (0.037 MBq)/well of [3H]thymidine was added to each well. Cells were harvested, and radioactivity was measured in a beta-liquid scintillation analyzer (Packard, Meriden, CT). Results are expressed as the mean count per minute (CPM) of triplicate cultures.
Presentation of soluble antigens by MoDCs
To examine the capacity of MoDCs to uptake and present soluble antigens and to activate autologous antigen-specific T cells, we performed an assay of T-cell response to recall antigen PPD. T cells were isolated from healthy blood donors or myeloma patients who had been immunized with bacillus Calmette-Guérin vaccines and showed a positive T-cell proliferative response against PPD in vitro. As we previously described,19 a stimulation index exceeding 3 measured by proliferation assay was considered a positive response to PPD. Immature MoDCs were pulsed with 2.5 μg/mL PPD and matured for 2 days, collected, washed 3 times with PBS, and cocultured with purified autologous T cells for 6 days at various T/MoDC ratios. T-cell proliferative response was measured by overnight incubation with [3H]thymidine (0.037 MBq/well), as described in the MLR assay.
In vitro induction of myeloma-specific CTLs
Cell lysate from autologous primary myeloma cells was prepared by 5 cycles of freezing and thawing as described previously.20 Nuclear debris was removed by centrifugation at 800 g for 10 minutes, and cell lysate was filtered. Lysate protein was quantified and added to immature MoDCs, and 4 hours later the cytokine cocktail was added to promote cell maturation. On day 9, lysate-pulsed, mature MoDCs were harvested and used as antigen-presenting cells. To compare the capacity of treated MoDCs for the induction of antimyeloma immunity, we induced tumor-specific cytotoxic T lymphocytes (CTLs) from myeloma patients' CD8+ T cells by repeated in vitro stimulation with the lysate-pulsed autologous MoDCs in the presence IL-2, IL-7, and IL-15, as described previously.20,21 After 4 cycles of stimulation, T cells were harvested and their cytotoxicity against lysate-pulsed or unpulsed (control) autologous MoDCs was examined by a standard 4-hour 51Cr-release assay. Results are shown as mean percentage of 51Cr release, which is calculated as follows: [(sample counts–spontaneous counts)/(maximum counts–spontaneous counts)] × 100%. Spontaneous release was less than 20% of the maximum 51Cr uptake.
RT-PCR for detection of cytokine mRNA
Reverse transcriptase–polymerase chain reaction (RT-PCR) was employed to detect cytokine mRNA expression by cultured differentiating cells. Purified monocytes were cultured in complete medium containing GM-CSF and IL-4, and on day 2, cells were harvested and total cellular RNA was extracted by Tri-Reagent (Molecular Research Center, Cincinnati, OH). Reverse transcription was performed using a Transcriptor First-Strand cDNA Synthesis Kit (Roche, Indianapolis, IN). Taq DNA polymerase was purchased from Roche, and PCR was performed according to the manufacturer's instruction. Primer sets used for these analyses were as follows: IL-6 forward, 5′-GAA AGC AGC AAA GAG GCA CT; reverse, 5′-GTT GGG TCA GGG GTG GTT AT; IL-10 forward, 5′-TCT GTT GCC TGG TCC TCC T; reverse, 5′-CCT TGA TGT CTG GGT CTT GG; TGF-β1 forward, 5′-GGA AAC CCA CAA CGA AAT CT; reverse, 5′-CTA AGG CGA AAG CCC TCAAT; and β-actin forward, 5′-CTC TTC CAG CCT TCC TTC CT; reverse, 5′-TCG TCA TAC TCC TGC TTG CT. Each of the primer sets was confirmed by running samples on agarose gels. β-actin transcript levels were used to normalize the amount of cDNA in each sample.
ELISA
Enzyme-linked immunosorbent assays (ELISAs) for IL-6, IL-10, and TGF-β1 were used to measure the secreted cytokines. Supernatants from cell cultures with or without the addition of the p38 inhibitor were collected on day 3, and the amounts of the cytokines in the supernatants were quantified using commercially available ELISA kits (R&D Systems).
Western-blot analysis
To examine intracellular signaling, we detected phosphorylated (p) p38 (pp38), pMEK1/2, pERK1/2, and pSTAT3 as previously described.15 In brief, purified monocytes from healthy donors or myeloma patients were cultured in complete medium, containing GM-CSF and IL-4 with or without IL-6–neutralizing antibody and/or p38 inhibitor, for 2 days. Cells were collected and lysed, and the samples were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis. After transfer to nitrocellulose membrane and subsequent blocking, the membranes were immunoblotted with respective antibodies (Cell Signaling, Beverly, MA) and visualized with alkaline phosphatase–conjugated secondary antibodies, followed by an enhanced chemiluminescence (Bio-Rad Laboratories, Hercules, CA) and autoradiography.
Phenotypic and functional properties of MoDCs from healthy donors (HD) and myeloma patients (MM). Representative histograms showing the expression of CD1a, CD40, CD80, and HLA-DR on (A) immature and (B) mature MoDCs generated from a healthy donor and a myeloma patient. Numbers inside the histograms represent the mean fluorescence intensity (MFI). (C) Pooled data depicting the expression (MFI) of CD1a, CD40, CD80, and HLA-DR on mature MoDCs from all tested healthy donors (n = 10) and myeloma patients (n = 12). Bars indicate the mean MFI. (D) Allostimulatory capacity of mature MoDCs in activating alloreactive T cells in an MLR assay. Shown are the mean ± SD of the results obtained with cells from 7 healthy donors and 7 myeloma patients. (E) Antigen-presentation capacity of MoDCs to autologous T cells. Shown are the mean ± SD of the results of cells presenting recalled antigen PPD to autologous T cells obtained from 5 healthy donors and 5 myeloma patients. In these experiments, different T cell/DC ratios (10:1, 100:1, and 1000:1; noted in the figure) were used. * P values less than .05.
Phenotypic and functional properties of MoDCs from healthy donors (HD) and myeloma patients (MM). Representative histograms showing the expression of CD1a, CD40, CD80, and HLA-DR on (A) immature and (B) mature MoDCs generated from a healthy donor and a myeloma patient. Numbers inside the histograms represent the mean fluorescence intensity (MFI). (C) Pooled data depicting the expression (MFI) of CD1a, CD40, CD80, and HLA-DR on mature MoDCs from all tested healthy donors (n = 10) and myeloma patients (n = 12). Bars indicate the mean MFI. (D) Allostimulatory capacity of mature MoDCs in activating alloreactive T cells in an MLR assay. Shown are the mean ± SD of the results obtained with cells from 7 healthy donors and 7 myeloma patients. (E) Antigen-presentation capacity of MoDCs to autologous T cells. Shown are the mean ± SD of the results of cells presenting recalled antigen PPD to autologous T cells obtained from 5 healthy donors and 5 myeloma patients. In these experiments, different T cell/DC ratios (10:1, 100:1, and 1000:1; noted in the figure) were used. * P values less than .05.
Statistical analysis
The Student t test was used to compare various experimental groups; significance was set at P values less than .05.
Results
Patient-derived MoDCs are phenotypically and functionally abnormal
We examined and compared MoDCs generated from patients with MM and healthy donors for their phenotype and functional capacity. For phenotypic and functional studies, MoDCs were generated from adherent cells. As shown in Figure 1A, the expression of DC-related surface molecules such as CD1a, CD40, and HLA-DR was lower on immature MoDCs from myeloma patients than on the cells from healthy donors (P < .05). We focused on mature MoDCs, since these cells are used for immunotherapy in myeloma patients. As shown by the representative histograms (Figure 1B) of mature MoDCs from a patient with MM and a healthy donor expressing DC-related surface molecules, and the pooled data from all 12 patients and 10 healthy donors depicted in Figure 1C, the expression levels (mean fluorescence intensity) of CD1a, CD40, CD80, and HLA-DR on mature MoDCs from myeloma patients were significantly lower than those from healthy donors (P < .05). The expression of HLA-ABC on these cells was also lower in some patients (Figure 1B), but no significant difference was observed when pooled data were used for comparison (not shown). CD54, CD83, and CD86 were not different between the 2 groups.
We next examined whether the functional capacity of MoDCs was different between patients with MM and healthy donors. As shown in Figure 1D, patient-derived mature MoDCs had significantly lower allostimulatory capacity compared with control cells in the MLR assay (P < .05). Patient-derived cells were also poor at presenting recall antigen PPD to and activating autologous PPD-specific T cells (Figure 1E; P < .05, compared with controls). Taken together, these results demonstrate that phenotype and antigen processing and/or presenting capacity of MoDCs generated from myeloma patients are impaired.
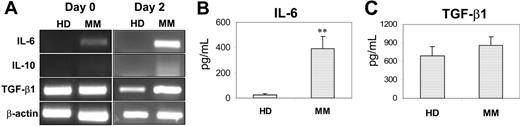
To determine the cause of the abnormalities in patient-derived MoDCs, we examined cytokine profiles of cells from myeloma patients and healthy donors. In these and cell-signaling studies, purified monocytes were used to generate MoDCs. We focused on autocrine production of IL-6, IL-10, and TGF-β1 because previous studies identified these cytokines as negative factors for DC differentiation and function.13,14,22-26 RT-PCR was used to detect cytokine mRNA expression in freshly isolated monocytes (day 0) and in day-2–cultured cells, and the results are shown in Figure 2A. Compared with control cells that expressed only TGF-β1, patient-derived cells expressed high levels of mRNA for IL-6 and TGF-β1 (day-2 cells) and low levels for IL-10. ELISA was used to measure the concentrations of these cytokines in cell-culture supernatants harvested on day 3, and, as shown in Figure 2B, significantly higher levels of IL-6 were detected in the supernatants of patient-derived cell cultures (P < .01). TGF-β1 was slightly increased, but the difference was not statistically significant (P > .05; Figure 2C). IL-10 was undetectable in the supernatants of the cultures.
Expression and production of cytokines by DC progenitor cells from healthy donors (HD) and myeloma patients (MM). (A) RT-PCR detecting mRNA expression for IL-6, IL-10, and TGF-β1 in freshly isolated monocytes (day 0) and day-2–cultured cells from a healthy donor and a myeloma patient. Representative results of 4 independent experiments are shown. ELISA detecting (B) IL-6 or (C) TGF-β1 in the supernatants of day-3 cultures of the cells. Shown are the mean ± SD of the results obtained from all tested healthy donors (n = 7 for IL-6, and n = 3 for TGF-β1) and myeloma patients (n = 7 for IL-6, and n = 3 for TGF-β1). ** P values less than .01.
Expression and production of cytokines by DC progenitor cells from healthy donors (HD) and myeloma patients (MM). (A) RT-PCR detecting mRNA expression for IL-6, IL-10, and TGF-β1 in freshly isolated monocytes (day 0) and day-2–cultured cells from a healthy donor and a myeloma patient. Representative results of 4 independent experiments are shown. ELISA detecting (B) IL-6 or (C) TGF-β1 in the supernatants of day-3 cultures of the cells. Shown are the mean ± SD of the results obtained from all tested healthy donors (n = 7 for IL-6, and n = 3 for TGF-β1) and myeloma patients (n = 7 for IL-6, and n = 3 for TGF-β1). ** P values less than .01.
Neutralizing IL-6 and/or inhibiting p38 restored the phenotype and function of patient-derived MoDCs
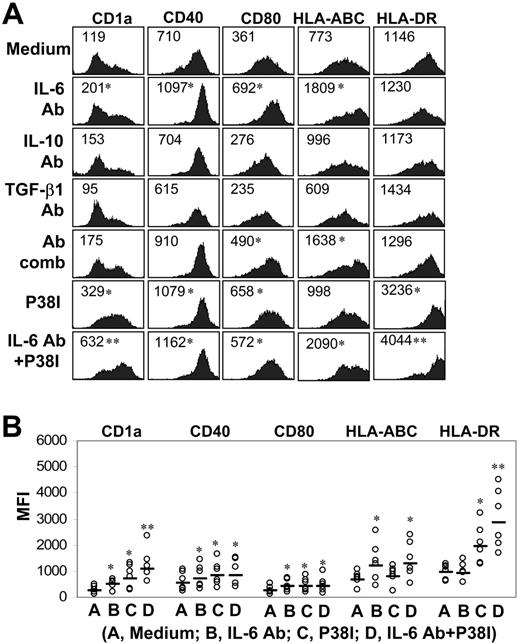
Our findings demonstrate an abnormal production of autocrine cytokines by patient-derived DC progenitor cells. To determine the role of these cytokines and to identify strategies to restore the function of MoDCs generated from myeloma patients, we used neutralizing antibodies against IL-6, IL-10, and TGF-β1. These antibodies (10 μg/mL), either individually or in a combination of all 3, were added to cultures of patient-derived adherent cells on day 0 and day 3, and no additional antibodies were added to the cells during the culture. As shown by representative results depicted in Figure 3A and pooled data in Figure 3B, neutralizing antibodies against IL-6, but not IL-10 or TGF-β1, improved the phenotype of the cells, evident by the up-regulated expression of CD1a, CD40, CD80, and HLA-ABC, compared with those cells obtained in complete medium without the addition of neutralizing antibodies. Combination of antibodies against all 3 cytokines had no additive effects, indicating that IL-6 was the major player. Furthermore, IL-6 antibody–treated, patient-derived MoDCs had improved allostimulatory capacity compared with untreated cells (P < .05; Figure 4A), although their ability to present PPD to autologous T cells (Figure 4B), and (DC) cell yields in cultures with the antibody (Figure 4D), counted as large, DC-like cells, were comparable to control cells or cultures. These data indicate that the differentiation and function of IL-6 antibody–treated MoDCs were only partially restored.
Effects of cytokine-neutralizing antibodies and p38 inhibitor on the phenotype of MoDCs from myeloma patients. (A) Representative histograms showing the expression of CD1a, CD40, CD80, HLA-ABC, and HLA-DR on patient-derived mature MoDCs generated in cultures with or without addition, individually or in a combination (Ab comb), of all 3 neutralizing antibodies (Ab; 10 μg/mL) to IL-6, IL-10, or TGF-β1; p38 inhibitor; or p38 inhibitor and IL-6 antibody. Numbers inside the histograms represent the MFI. (B) Pooled data depicting the expression (MFI) of CD1a, CD40, CD80, HLA-ABC, and HLA-DR on the cells from all tested myeloma patients (n = 6) treated with IL-6 antibody, p38 inhibitor, or both. Bars indicate the mean MFI. *P values less than .05; **P values less than .01.
Effects of cytokine-neutralizing antibodies and p38 inhibitor on the phenotype of MoDCs from myeloma patients. (A) Representative histograms showing the expression of CD1a, CD40, CD80, HLA-ABC, and HLA-DR on patient-derived mature MoDCs generated in cultures with or without addition, individually or in a combination (Ab comb), of all 3 neutralizing antibodies (Ab; 10 μg/mL) to IL-6, IL-10, or TGF-β1; p38 inhibitor; or p38 inhibitor and IL-6 antibody. Numbers inside the histograms represent the MFI. (B) Pooled data depicting the expression (MFI) of CD1a, CD40, CD80, HLA-ABC, and HLA-DR on the cells from all tested myeloma patients (n = 6) treated with IL-6 antibody, p38 inhibitor, or both. Bars indicate the mean MFI. *P values less than .05; **P values less than .01.
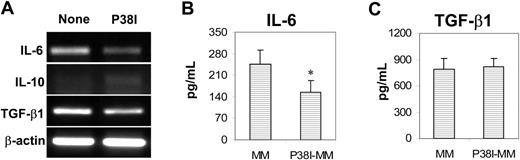
Our recent studies indicated that inhibiting p38 activity accelerated the differentiation and generation of normal human MoDCs in vitro27 and abrogated tumor-derived factor-mediated inhibition of murine bone marrow–derived DC differentiation.15 To examine the effects of p38 inhibition on patient-derived MoDCs, a specific p38 inhibitor (p38 inhibitor III) was used and added to cell cultures on day 0. The p38 inhibitor significantly increased the expression of DC-related surface molecules CD1a, CD40, CD80, and HLA-DR (Figure 3A-B; P < .05). Furthermore, p38 inhibitor–treated cells had improved capacity (P < .05; compared with untreated cells) to activate alloreactive T cells (Figure 4A) and present PPD to and activate PPD-specific autologous T cells (Figure 4B). We also examined and compared the capacity of these cells to stimulate myeloma-specific CTLs from patients.20 As shown in Figure 4C, patient-derived mature MoDCs were poor at priming myeloma-specific CTLs, whereas p38 inhibitor–treated cells displayed a significantly enhanced capacity to induce a myeloma-specific CTL response (P < .05; compared with untreated MoDCs). Furthermore, in cultures with addition of the p38 inhibitor, the yields of MoDCs were significantly higher than the control cultures (P < .05; Figure 4D). Inhibiting p38 also led to decreased mRNA expression for IL-6 and TGF-β1, but not IL-10 (Figure 5A), and reduced production of IL-6 (P < .05; Figure 5B), but not TGF-β (Figure 5C), in patient-derived cells. Again, IL-10 was undetectable in the supernatants of the cell culture.
Effects of cytokine-neutralizing antibodies and p38 inhibitor on functional properties and yield of MoDCs from myeloma patients. (A) Allostimulatory and (B) antigen-presentation capacities of myeloma patient–derived MoDCs generated in cultures treated with IL-6 antibody, p38 inhibitor, or both. Shown are the mean ± SD of results obtained with the cells from all tested patients (n = 6 for MLR, and n = 4 for PPD); a T/MoDC ratio of 100:1 was used. (C) Cytotoxicity of myeloma-specific CTLs induced by myeloma lysate–pulsed autologous MoDCs or p38 inhibitor–treated MoDCs from 2 myeloma patients (MM1 and MM2). Target cells were lysate-pulsed autologous MoDCs. Unpulsed MoDCs were used as controls. (D) Cell yields determined by microscopic cell count of large, DC-like cells in cultures treated with IL-6 antibody, p38 inhibitor, or both. Shown are the mean ± SD of the results obtained with the cells from 4 patients. In panels A, B, and D, pooled data of normal MoDCs from healthy donors (n = 7 for MLR, n = 5 for PPD, and n = 4 for cell yields) were included for comparison (▪). *P values less than .05; **P values less than .01.
Effects of cytokine-neutralizing antibodies and p38 inhibitor on functional properties and yield of MoDCs from myeloma patients. (A) Allostimulatory and (B) antigen-presentation capacities of myeloma patient–derived MoDCs generated in cultures treated with IL-6 antibody, p38 inhibitor, or both. Shown are the mean ± SD of results obtained with the cells from all tested patients (n = 6 for MLR, and n = 4 for PPD); a T/MoDC ratio of 100:1 was used. (C) Cytotoxicity of myeloma-specific CTLs induced by myeloma lysate–pulsed autologous MoDCs or p38 inhibitor–treated MoDCs from 2 myeloma patients (MM1 and MM2). Target cells were lysate-pulsed autologous MoDCs. Unpulsed MoDCs were used as controls. (D) Cell yields determined by microscopic cell count of large, DC-like cells in cultures treated with IL-6 antibody, p38 inhibitor, or both. Shown are the mean ± SD of the results obtained with the cells from 4 patients. In panels A, B, and D, pooled data of normal MoDCs from healthy donors (n = 7 for MLR, n = 5 for PPD, and n = 4 for cell yields) were included for comparison (▪). *P values less than .05; **P values less than .01.
Effects of p38 inhibitor on cytokine expression and production by myeloma patient–derived MoDC progenitor cells. (A) RT-PCR detecting mRNA expression for IL-6, IL-10, and TGF-β1 in day-2–cultured cells from a myeloma patient. Representative results of 4 independent experiments are shown. ELISA detecting (B) IL-6 or (C) TGF-β1 in the supernatants of day-3 cultures of the cells. Shown are the mean ± SD of the results obtained from tested myeloma patients (n = 5 for IL-6, and n = 3 for TGF-β1). *P values less than .05.
Effects of p38 inhibitor on cytokine expression and production by myeloma patient–derived MoDC progenitor cells. (A) RT-PCR detecting mRNA expression for IL-6, IL-10, and TGF-β1 in day-2–cultured cells from a myeloma patient. Representative results of 4 independent experiments are shown. ELISA detecting (B) IL-6 or (C) TGF-β1 in the supernatants of day-3 cultures of the cells. Shown are the mean ± SD of the results obtained from tested myeloma patients (n = 5 for IL-6, and n = 3 for TGF-β1). *P values less than .05.
As both IL-6–neutralizing antibody and the p38 inhibitor had positive effects on patient-derived MoDCs, we examined whether these 2 had synergistic or additive effects on the cells. As shown in Figure 3, addition of both the IL-6–neutralizing antibody and p38 inhibitor to the cultures further improved the phenotype (Figure 3A-B), antigen presentation, and activation of T cells (Figure 4A-B) and cell yields (Figure 4D) of patient-derived MoDCs, indicating that these 2 agents had additive effects on the cells. These cells were comparable to healthy donor–derived MoDCs in terms of phenotypic and functional capacities and cell yields.
Elucidation of signaling pathways involved in differentiation of patient-derived MoDCs
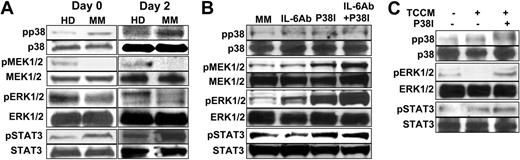
Abnormal signaling pathways were reported to be associated with tumor-mediated or tumor-derived factor–mediated inhibition of DC differentiation.15,28-30 To identify signaling pathways associated with the defective MoDC differentiation in MM, we analyzed protein levels of pp38, pMEK1/2, pERK1/2, and pSTAT3 in patient-derived differentiating progenitor cells. Purified monocytes were used to generate MoDCs. Cell lysates were prepared from freshly isolated monocytes (day 0) and day-2–cultured cells and used for the analyses. As shown by the representative data depicted in Figure 6A, increased levels of pp38 and pSTAT3 and decreased levels of pMEK1/2 and pERK1/2 were observed in patient-derived cells compared with cells from healthy donors. Protein levels of nonphosphorylated p38, STAT3, MEK1/2, and ERK1/2 remained the same. These results demonstrate the existence of activated p38 MAPK and STAT3 and inhibited Raf/MEK/ERK signaling pathways in the progenitor cells from myeloma patients.
Signaling pathways in the differentiating MoDCs obtained from healthy donors or myeloma patients. (A) Western-blot analysis showing protein levels of phosphorylated (p) and nonphosphorylated p38, MEK1/2, ERK1/2, and STAT3 in freshly isolated monocytes (day 0) and day-2–cultured cells from a healthy donor and a myeloma patient. (B) Effects of IL-6–neutralizing antibody (Ab), p38 inhibitor (P38I), or both on signaling pathways in myeloma patient–derived cells. (C) Effects of TCCM and p38 inhibitor on signaling pathways in normal MoDC progenitor cells from healthy donors. In all the experiments, day-2–cultured cells were examined. Representative results of 3 independent experiments are shown.
Signaling pathways in the differentiating MoDCs obtained from healthy donors or myeloma patients. (A) Western-blot analysis showing protein levels of phosphorylated (p) and nonphosphorylated p38, MEK1/2, ERK1/2, and STAT3 in freshly isolated monocytes (day 0) and day-2–cultured cells from a healthy donor and a myeloma patient. (B) Effects of IL-6–neutralizing antibody (Ab), p38 inhibitor (P38I), or both on signaling pathways in myeloma patient–derived cells. (C) Effects of TCCM and p38 inhibitor on signaling pathways in normal MoDC progenitor cells from healthy donors. In all the experiments, day-2–cultured cells were examined. Representative results of 3 independent experiments are shown.
To determine the effects of IL-6 neutralization and/or the p38 inhibitor on these signaling pathways, we examined protein levels of pp38, pMEK1/2, and pERK1/2 in patient-derived cells cultured with or without addition of the neutralizing antibody or p38 inhibitor. Cell lysates were prepared from day-2–cultured cells and used in the analyses. As shown in Figure 6B, neutralizing IL-6 in the cultures slightly up-regulated the expression of pERK and down-regulated pSTAT3. No effects were observed on pp38, pMEK, or nonphosphorylated kinases. Consistent with previous results that the p38 inhibitor inhibits the activity of p38, but does not prevent p38 phosphorylation,15,31,32 a band of pp38 was seen in patient-derived cells treated with the p38 inhibitor (Figure 6B). Nevertheless, the activity of p38 was indeed inhibited because its capacity to phosphorylate its substrate, activating transcription factor-2 (ATF-2), was abrogated by the treatment.15 Addition of the p38 inhibitor to the cultures drastically increased MEK1/2 and ERK1/2 phosphorylation and activation, whereas the levels of nonphosphorylated p38, MEK1/2, and ERK1/2 remained unchanged. Combining the IL-6–neutralizing antibody with the p38 inhibitor further up-regulated pMEK and pERK without affecting p38 or STAT3 activities. These results correlate well with the phenotypic and functional changes of the cells and suggest that p38 inhibition and IL-6 neutralization improved the quality and function of patient-derived MoDCs through inhibiting STAT3 and p38 and restoring the activity of Raf/MEK/ERK signaling pathways.
Next we examined the relationship of p38 and STAT3 in MoDC differentiation, since activation of STAT3 was reported to be another important signaling mechanism for defective DC differentiation mediated by tumor or tumor-derived factors.28-30,33 As shown in Figure 6B, addition of the p38 inhibitor slightly increased the phosphorylation of STAT3, indicating that inhibiting p38 did not down-regulate STAT3 activity. On the other hand, we used myeloma-derived TCCM or a combination of cytokines IL-6, IL-10, and TGF-β1 to treat progenitor cells from healthy donors, since previous studies have shown that TCCM or these cytokines were able to inhibit DC differentiation via activating STAT3.15,29,33-35
As shown in Figure 6C, TCCM or the cytokines (data not shown) added to the cultures activated STAT3 and p38 and inhibited ERK activity. The addition of the p38 inhibitor abrogated the inhibitory effect of TCCM or the cytokines (not shown) on ERK without affecting STAT3. Taken together, these results suggest that STAT3 activation was not a major contributor to impaired differentiation of patient-derived MoDCs, and p38 inhibitor improved the generation and function of the MoDCs via inhibiting p38 and activating Raf/MEK/ERK signaling pathways independent of the STAT3 pathway.
Discussion
We have recently shown that myeloma cells or TCCM were able to inhibit differentiation and function of murine bone marrow–derived DCs.15 The current study further demonstrated that patient-derived ex vivo–generated MoDCs were also functionally abnormal. MoDCs have been commonly used as vaccines for immunotherapy in patients with cancers including MM. In this study, we showed that myeloma patient–derived MoDCs were phenotypically and functionally impaired compared with their normal counterparts. Patient-derived cells expressed significantly lower levels of CD1a, CD40, CD80, and HLA-DR and were poor at activating alloreactive and autologous antigen-specific T cells. These cells were also less potent at inducing myeloma-specific CTLs in vitro. We further show that either neutralizing IL-6 or inhibiting p38 with a specific p38 inhibitor, or both, significantly increased the yields and improved the phenotype and function of patient-derived MoDCs. These treatments reduced the production of autocrine cytokines IL-6 and TGF-β1, inhibited p38 and STAT3 activities, and restored the MEK/ERK signaling. Therefore, our study elucidated the underlying molecular and signaling mechanisms and, more importantly, identified novel strategies to correct the defects and restore the function of these cells.
DC abnormalities have been described in patients with MM. The number of circulating DCs has been shown to be significantly lower in patients with MM than in healthy individuals,14 and the phenotype and function of these cells were also impaired.13,14 However, Ratta et al36 examined and compared MoDCs from myeloma patients and healthy individuals and found no difference in cell yield, phenotypic profile, and functional properties. These results appear to conflict with the current study. Although the reason for the discrepancy is unknown, it is possible that differences in the treatment of patients, stages of the disease, samples collected from G-CSF–mobilized patients, and culture conditions may be contributing factors. Further studies will be needed to determine whether myeloma treatment and G-CSF mobilization36 may positively affect generation of MoDCs from patients.
It is well documented that differentiation and function of DCs in cancer patients are impaired due to tumor cells or tumor-derived cytokines or other factors.13,14,22-26 However, this is not the case for ex vivo–generated MoDCs, since tumor cells or tumor-derived factors were not present during the cultures. Therefore, the defective differentiation and generation of patient-derived MoDCs could be the result of defects in the progenitor monocytes from the patients. Indeed, we show that patient-derived progenitor cells expressed and/or produced higher amounts of autocrine cytokines IL-6, IL-10, and TGF-β1 and displayed activated p38 and STAT3 and inhibited MEK/ERK signaling pathways, which were not observed in cells from healthy donors. Furthermore, treatment of patient-derived cells with neutralizing antibody against IL-6, with p38 inhibitor, or a combination of the two, restored the function of the cells, indicating that these changes were contributing factors to the abnormalities. Although the causes of defects in patient-derived monocytes, it is possible that cytokines such as IL-6, IGF-1, VEGF, and TGF-β, which are secreted either by myeloma cells or bone marrow stromal cells and are elevated in patients' serum,37 may be responsible for the elevated expression of IL-6 and TGF-β, constitutive activation of p38 and STAT3, and inhibited MEK/ERK pathways in the progenitor cells. Indeed, it is known that these cytokines activate p38 and STAT315,29 and TCCM inhibited ERK phosphorylation15 (Figure 6C). In line with this notion, we show that treatment of normal monocytes from healthy donors with TCCM or the cytokines impaired the generation of MoDCs.
In this study, we demonstrate that autocrine cytokines, especially IL-6, and constitutive activation of p38 and inhibition of MEK/ERK may be responsible for defective MoDC differentiation. However, the causal relationship between the abnormal signaling pathways and cytokine expression and production in patient-derived progenitor cells is undetermined. We found that, despite the presence of p38 inhibitor in the cultures, patient-derived cells continued to express and/or produce, although slightly reduced, higher amounts of IL-6, IL-10, and TGF-β1 compared with normal cells, indicating that p38 inhibitor could not completely inhibit the cytokine production. This suggests that, in the presence of p38 inhibitor, these cytokines could still be produced and exert their inhibitory effects on DC differentiation in an autocrine fashion. These results provide further explanation for the observation that p38 inhibitor and IL-6–neutralizing antibody had additive effects on improving the quality of patient-derived MoDCs, even though p38 inhibitor was much more potent than the antibody on the cells. However, our study cannot exclude the possibility that cytokines other than IL-6, IL-10, and TGF-β1 and other factors may also have contributed to the abnormalities.
Activated Jak/STAT3 signaling was reported to be associated with defective DC differentiation mediated by tumor cells or tumor-derived factors.28,29,33 Indeed, in our study, Jak/STAT3 signaling pathway was activated in patient-derived cells and also in normal progenitor cells treated with TCCM or cytokines IL-6, IL-10, and TGF-β, suggesting that activation of STAT3 may be involved in the defective differentiation of MoDCs from myeloma patients. It is plausible that elevated expression and production of autocrine cytokines including IL-6 activated STAT3 in the progenitor cells, and neutralizing IL-6 partially reduced STAT3 activation and led to partially restored differentiation of functional MoDCs. However, we believe that activation of p38 and inhibition of MEK/ERK signaling pathways were major contributors to the abnormalities because addition of the p38 inhibitor, via inhibiting p38 and restoring MEK/ERK signaling, significantly improved the differentiation and function of patient-derived MoDCs. Thus, as we showed in this study, functional MoDCs, as potent as those generated from healthy donors, could be generated from myeloma patients using both IL-6–neutralizing antibody and p38 inhibitor. These strategies have important implications for DC-based immunotherapy in MM.
In conclusion, this study demonstrated that MoDCs generated from myeloma patients were phenotypically and functionally defective. Underlying mechanisms associated with these abnormalities were elevated expression and production of autocrine cytokines, particularly IL-6, activation of p38 and STAT3, and inhibition of MEK/ERK signaling pathways. More importantly, treatment of patient-derived cells with p38 inhibitor alone, or with p38 inhibitor and IL-6–neutralizing antibody, was able to correct the abnormalities and generate potent MoDCs from myeloma patients. Considering the inefficiency of DC-based vaccination in tumor immunotherapy clinical trials, our study identified novel strategies to improve the quality of DC vaccines, which will benefit ongoing and future clinical trials of DC-based immunotherapy in cancer patients.
Authorship
Contribution: S.W. and Q.Y. designed the study; S.W., S.H., J.Y., J.Q., and X.Z. performed the research and analyzed the data; E.S. and L.W.K. provided patient samples and critical suggestions; and S.W. and Q.Y. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 17, 2006; DOI 10.1182/blood-2006-04-016980.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by institutional start-up funds from the University of Texas M. D. Anderson Cancer Center and grants from the National Cancer Institute (R01 CA96569 and R01 CA103978), the Leukemia and Lymphoma Society (6041-03), and Common-wealth Foundation for Cancer Research. We thank Alison Woo for editorial assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal