Abstract
Recently, protocols using high-dose melphalan chemotherapy and autologous peripheral blood stem cell transplantation (HDM/SCT) have been developed for the treatment of patients with immunoglobulin light chain (AL) amyloidosis. Although peritransplantation mortality is greater than for other hematologic diseases, treatment leads to durable hematologic complete responses, improvements in organ function and quality of life, and extended survival in a substantial proportion of patients. To determine whether this treatment can be applied to older patients, we have analyzed HDM/SCT treatment outcomes for 65 patients (aged 65 years or older) with AL amyloidosis compared with outcomes for 280 younger patients. For patients over age 65 years who meet the same eligibility criteria as younger patients, toxicity, hematologic remission rate, and survival were not significantly different from those observed in younger patients, indicating that older patients should not be excluded a priori from consideration for HDM/SCT treatment.
Introduction
Treatment with high-dose melphalan chemotherapy and autologous peripheral blood stem cell transplantation (HDM/SCT) is effective therapy for patients with immunoglobulin light chain (AL) amyloidosis, leading to complete hematologic responses in about 40% of patients,1 with concomitant improvements in both organ function,1,2 and quality of life.3 Comparable results have been obtained at other treatment centers,4,5 and a case-control study has demonstrated the benefit of aggressive treatment for patients younger than 70 years of age.6 However, in amyloidosis, impaired organ function due to deposition of amyloidogenic light chains in tissues leads to much higher treatment-related morbidity and mortality than in other hematologic diseases. Thus, one of the most important goals of current clinical research in the field is to refine the selection criteria for patients undergoing this treatment. An unanswered question is the role of chronological age in predicting outcome. Here, we present a detailed analysis of outcomes in patients aged 65 years and older versus outcomes in those younger than 65 years, with up to 10 years of follow-up (median, 4.84 years).
Patients, materials, and methods
Patients
All studies were carried out with approval of the Institutional Review Board at Boston University Medical Center, in accordance with the Declaration of Helsinki. The data set includes 701 consecutive patients with AL amyloidosis evaluated at Boston Medical Center between July 1994 and June 2002, and aggregate data have been previously reported.1 For this analysis, the survival data were updated from June 2003, in the initial report, to January 15, 2006, and detailed comparisons were made between the cohort of patients younger than 65 years of age, and those aged 65 and older. The criteria for diagnosis of AL amyloidosis and evaluation of organ involvement have been described previously.1
Treatment
Patients were enrolled in a series of clinical trials using varying doses of intravenous melphalan and autologous SCT. Melphalan dosing was determined by protocol and depended on the patient's age and cardiac function. Generally, a dose of 200 mg/m2 was administered to patients younger than 65 years with a cardiac ejection fraction of 45% or greater and a stem cell yield of 2.5 × 106 CD34+ cells/kg or higher. A dose of 140 mg/m2 was administered to patients aged 65 and older, patients with a reduced cardiac ejection fraction (40%-45%), or patients with 2.0 to 2.5 × 106 CD34+ cells/kg. The melphalan dose was divided over 2 consecutive days, with stem cell infusions (day 0) 24 to 72 hours after the last dose of melphalan. Patients with an ejection fraction less than 40%, diffusing capacity of the lung for carbon monoxide (DLCO) less than 50%, symptomatic pleural effusions, uncontrolled congestive heart failure (CHF) or arrhythmias, supine BP less than 90 mm Hg, or Southwest Oncology Group (SWOG) performance status score greater than 2 (unless due to peripheral neuropathy) were excluded from HDM/SCT.
Responses
Hematologic and organ responses were evaluated annually. A hematologic complete response (CR) was defined as absence of clonal plasma cells in the bone marrow by immunohistochemical staining and of monoclonal gammopathy by immunofixation electrophoresis of serum and urine. More recently, examination of free light-chain levels has been done, providing a quantitative assessment of hematologic response, but CRs were highly concordant with the qualitative techniques.7 Clinical responses required objective improvement in organ function or performance status, as described.1
Statistics
Clinical and demographic factors were compared among subgroups using t tests for continuously measured variables and the χ2 test for dichotomous variables. Kaplan-Meier survival plots were used to display survival distributions. The 5-year survival rates were estimated by the actuarial life-table method and compared using the z test. Differences between survival distributions for subgroups were characterized using hazard ratios. The likelihood ratio test was used to test the significance of unadjusted and adjusted hazard ratios. Analyses were conducted by using SAS software, version 8.2 (SAS Institute, Cary, NC).
Results and discussion
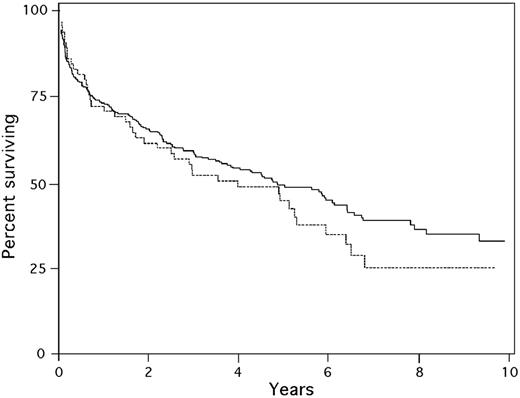
A total of 345 patients began granulocyte colony-stimulating factor (G-CSF) mobilization for HDM/SCT, of whom 65 (19%) were aged 65 years or older (median age, 68 years; range, 65-79 years); 72% were men and 28% were women. For the 280 patients younger than 65, the median age was 55 years (range, 29-64 years); 57% were men and 43% were women. Although the sex distribution was statistically different (P = .022), there was no difference in the time to diagnosis or referral, number of involved organs or cardiac involvement, performance status, or light-chain isotypes (Table 1). Patients for whom stem cell collection was complicated by severe CHF, arrhythmia, hypotension, hypoxemia, or death did not proceed with HDM/SCT. There was no difference in the rates of these serious complications during stem cell collection in the younger and older groups of patients because similar proportions of patients did not proceed to transplantation (12.3% in those 65 and older versus 10.4% in those younger than 65; P = .647). A total of 19% of patients 65 years and older received 200 mg/m2 melphalan, the remainder being treated with 100 or 140 mg/m2, whereas 65% of those younger than 65 received 200 mg/m2 (P < .001). There was no difference in early treatment-related mortality (10.3% in patients 65 years or older versus 13.4% in patients younger than 65; P = .665) or 1-year mortality (27.6% for patients 65 and older versus 26.8% for those younger than 65; P = .882). There was a nonsignificant trend toward a lower rate of hematologic CR in the older patients (32% versus 43%, P = .191). However, the median survival after HDM/SCT was no different (4.0 years for patients 65 and older versus 4.85 years for those younger than 65; P = 0.28 by log-rank test; Figure 1).
Clinical features in 345 patients, 1994-2002
. | Younger than 65 y . | 65 y old or older . | P . |
|---|---|---|---|
| No. patients (%) | 280 (81) | 65 (19) | — |
| Median age, y (range) | 55 (29-64) | 68 (65-79) | < .001 |
| Time from symptoms to diagnosis, mo | 12.4 ± 20.4 | 11.7 ± 15.9 | .807 |
| Time from diagnosis to referral, mo | 6.0 ± 13.8 | 7.0 ± 19.3 | .614 |
| Male, % | 57 | 72 | .022 |
| λ, % | 84 | 82 | .640 |
| Median no. of organs | 2.3 | 2.4 | .715 |
| Cardiac involvement, % | 44.3 | 42.4 | .837 |
| Performance status | 1.1 | 1 | .690 |
| Eligible for HDM/SCT, %* | 68.4 | 34.3 | < .001 |
| Collection complications, % | 10.4 | 12.3 | .647 |
| Received 200 mg/m2 melphalan, % | 65.0 | 19.0 | < .001 |
| Treatment-related mortality, % | 13.4 | 10.3 | .665 |
| 1-y mortality, % | 26.8 | 27.6 | .882 |
| Hematologic CR, % | 43.5 | 32.0 | .191 |
| Median survival, y | 4.85 | 3.98 | .28 |
. | Younger than 65 y . | 65 y old or older . | P . |
|---|---|---|---|
| No. patients (%) | 280 (81) | 65 (19) | — |
| Median age, y (range) | 55 (29-64) | 68 (65-79) | < .001 |
| Time from symptoms to diagnosis, mo | 12.4 ± 20.4 | 11.7 ± 15.9 | .807 |
| Time from diagnosis to referral, mo | 6.0 ± 13.8 | 7.0 ± 19.3 | .614 |
| Male, % | 57 | 72 | .022 |
| λ, % | 84 | 82 | .640 |
| Median no. of organs | 2.3 | 2.4 | .715 |
| Cardiac involvement, % | 44.3 | 42.4 | .837 |
| Performance status | 1.1 | 1 | .690 |
| Eligible for HDM/SCT, %* | 68.4 | 34.3 | < .001 |
| Collection complications, % | 10.4 | 12.3 | .647 |
| Received 200 mg/m2 melphalan, % | 65.0 | 19.0 | < .001 |
| Treatment-related mortality, % | 13.4 | 10.3 | .665 |
| 1-y mortality, % | 26.8 | 27.6 | .882 |
| Hematologic CR, % | 43.5 | 32.0 | .191 |
| Median survival, y | 4.85 | 3.98 | .28 |
— indicates not applicable.
Percent eligible of 450 patients younger than 65 y and 251 patients at least 65 y of age screened for HDM/SCT.
Hematologic malignancies and other clonal blood disorders, including monoclonal gammopathies, occur with increasing frequency with age. As new treatments are developed, it is important to evaluate response rates and toxicities in older as well as younger patients with such disorders. In multiple myeloma, 2 early studies suggested a progression-free benefit8 or even a survival benefit9 of transplantation in older patients. A small retrospective series compared outcome for 17 patients aged 65 years or older with otherwise matched controls and found no difference in toxicity, treatment-related mortality, or survival.10 The University of Arkansas program reviewed their experience and found that for 49 patients over age 65 (median, 67 years; range, 65-76 years), there was a slightly higher rate of peritransplant mortality compared with 50 patients younger than 65 years (8% versus 2%), but there were no differences in event-free survival, overall survival, toxicity, or ability to undergo a tandem transplantation,11 and they have continued to effectively treat older patients.12
Here, we have analyzed treatment outcomes in patients with AL amyloidosis, over a 10-year period, for patients age 65 and older compared with those under age 65. Although, as previously reported, fewer of the older patients met the eligibility criteria for HDM/SCT,1 for those who did, the rate of serious complications during stem cell mobilization and collection, and in the peritreatment period, was no different in the older and younger patients. Although the proportion of patients receiving modified dosing was significantly greater in the older patients (81% versus 35%, P < .001), the difference in hematologic CR rate was not statistically lower nor was the median survival. We also have not observed a significant difference in neutrophil or platelet engraftment.13 Our results strongly support the use of HDM/SCT in suitable patients over age 65 with AL amyloidosis.
Kaplan-Meier survival of patients younger than 65 (solid line) and 65 years or older (dashed line) from initiation of G-CSF mobilization of stem cells.
Kaplan-Meier survival of patients younger than 65 (solid line) and 65 years or older (dashed line) from initiation of G-CSF mobilization of stem cells.
Of note, there was a higher percentage of men in the older patient group. Interesting, senile systemic amyloidosis, the deposition of wild-type transthyretin, primarily in the heart, is much more common in older men than women. The reasons for these sex differences in the amyloidoses are not known.
Authorship
Contribution: D.C.S., J.J.A., M.S., K.M., D.G.W., K.Q., K.F., and V.S. designed the research; D.C.S., J.J.A., M.S., K.M., D.G.W., K.Q., K.F., B.O., and V.S. performed the research; D.C.S., J.J.A., M.S., V.S., and K.F. analyzed the data; D.C.S., J.J.A., M.S., D.G.W., and V.S. wrote the paper; and K.M., K.Q., K.F., and B.O. reviewed and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 22, 2006; DOI 10.1182/blood-2006-06-029728.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We gratefully acknowledge the participation of patients of all ages in clinical trials and research at Boston University Medical Center. We acknowledge the assistance of previous staff of the Stem Cell Transplant Program and clinical fellows from the Section of Hematology-Oncology, the past and current staff of the Clinical Trials Office, the superb inpatient and outpatient hematology-oncology nurses, and the research assistants in the Gerry Amyloidosis Research Laboratory.
This work was supported by grants from the National Institutes of Health (HL68705), Food and Drug Administration (FD-R-001346), Gerry Foundation, Young Family Amyloid Research Fund, Sue Sellors Finley Cardiac Amyloid Research Fund, and Amyloid Research Fund at Boston University.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal