Abstract
To explore the initial steps by which transplanted mesenchymal stem cells (MSCs) interact with the vessel wall in the course of extravasation, we studied binding of human MSCs to endothelial cells (ECs). In a parallel plate flow chamber, MSCs bound to human umbilical vein ECs (HUVECs) similar to peripheral-blood mononuclear cells (PBMCs) or CD34+ hematopoietic progenitors at shear stresses of up to 2 dynes/cm2. This involved rapid extension of podia, rolling, and subsequent firm adhesion that was increased when ECs were prestimulated with TNF-α. MSC binding was suppressed when ECs were pretreated with function-blocking anti–P-selectin antibody, and rolling of MSCs was induced on immobilized P-selectin, indicating that P-selectin was involved in this process. Preincubation of HUVECs with anti–VCAM-1 or of MSCs with anti–VLA-4 antibodies suppressed binding of MSCs to HUVECs but did not enhance inhibition by anti–P-selectin, indicating that both P-selectin and VCAM-1 are equally required for this process. Intravital microscopy demonstrated the capacity of MSCs to roll and adhere to postcapillary venules in vivo in a mouse model in a P-selectin–dependent manner. Thus, MSCs interact in a coordinated fashion with ECs under shear flow, engaging P-selectin and VCAM-1/VLA-4.
Introduction
In recent years, mesenchymal stem cells (MSCs) have been characterized as adherent-cell populations originating from bone marrow, capable of expanding in vitro as undifferentiated cells or differentiating into osteocytes, chondrocytes, tenocytes, adipocytes, or smooth muscle cells.1-3 MSCs have been used in a number of preclinical models to mediate the regeneration of muscle, endothelial, neuronal, skin, or renal epithelial tissue.4-7 In vitro differentiation studies have demonstrated the potential of MSCs to also form alveolar and airway epithelial cells or cardiac pacemaker cells.8,9 Moreover, MSCs have been transplanted intravenously and shown to distribute to spleen, bone, lung, and cartilage in several rodent models.10-12 Intravenously injected MSCs have already been used in patients to accelerate hematopoietic reconstitution after hematopoietic stem cell transplantation, to overcome the molecular defect in children with osteogenesis imperfecta, or to alleviate the outcome after myocardial infarction.13-17
Transplantation experiments in mice and primates have shown that intravenously applied MSCs distribute to several tissues and may accumulate in the lungs.10,12,18,19 However, currently it is poorly understood to what degree MSCs use specific adhesion mechanisms for egress from the bloodstream and whether they home in a tissue-specific manner. To leave the bloodstream, mature leukocytes and hematopoietic progenitor cells (HPCs) have been shown to undergo a coordinated sequence of adhesion steps, initiated by tethering events, which are mainly mediated by selectins and their ligands.20,21 Subsequently, the captured cells roll and encounter chemokines, which eventually activate integrins, resulting in firm arrest and subsequent transendothelial migration.
To elucidate the potential of MSCs to undergo coordinated steps of interaction with endothelial cells (ECs), we investigated human MSCs under shear flow using a parallel plate flow chamber and by intravital microscopy in mice. We show here that human MSCs home to different tissues and display coordinated rolling and adhesion behavior on ECs. Although P-selectin glycoprotein ligand 1 (PSGL-1) is not expressed by MSCs, MSCs bind to ECs in a P-selectin–dependent manner in vitro and in vivo. Furthermore, our data indicate that rolling MSCs engage VLA-4/VCAM-1 to mediate firm adhesion on ECs.
Materials and methods
Isolation and characterization of cells
Bone marrow samples were obtained from patients undergoing hip joint replacement after informed consent in accordance with the Declaration of Helsinki. Then, 10 to 30 mL of aspirate from the femur cavity was anticoagulated with 500 IU/mL heparin. The light-density mononuclear-cell fraction was prepared by density gradient centrifugation (yield, 2 × 107–7.2 × 108 cells), seeded in T25 or T80 tissue culture plastic flasks at 1 × 106 to 3 × 106 cells/cm2 in low-glucose DMEM medium (PAA, Cölbe, Germany) supplemented with 20% tested FCS (PAN Biotech, Aidenbach, Germany) and 25 ng/mL basic FGF (Tebu-Bio, Offenbach, Germany). After 48 hours of incubation at 37°C and 5% CO2, nonadherent cells were removed and the remaining cells were cultured for another 7 to 10 days until reaching 70% to 80% confluence. The uniform spindle-shaped cells were passaged by trypsinization and their multipotency was confirmed by successful induction of osteoblastic, chondrogenic, and adipogenic differentiation under the conditions published.3 MSCs used for the assays were harvested when they had reached approximately 80% confluence, corresponding to 1.5 × 106 to 2 × 106 MSCs/T80 flask. MSC populations could be passaged 14 to 20 times; to avoid acquisition of unfavorable genetic alterations,22,23 MSCs used for the experiments were expanded for maximally 9 passages. PBMCs and mobilized CD34+ progenitor cells were isolated from voluntary donors after they gave informed consent in accordance with the Declaration of Helsinki as described.24 For flow cytometric analysis, MSCs were trypsinized using trypsin-EDTA (0.5% trypsin, 6.8 mM EDTA in PBS; Invitrogen, Karlsruhe, Germany) at 37°C for 5 minutes, suspended as single cells in PBS, and incubated with fluorescence-labeled anti-CD24 (clone ALB9; Beckman Coulter, Krefeld, Germany), anti-CD34 (clone 581), anti-CD45 (clone H130), anti-CD73 (clone AD2), anti-CD117 (clone YB5.B8), anti–VLA-4 (clone 9F10), anti-β1 integrin (clone HUTS-21), anti-β2 integrin (clone L130), anti-CXCR4 (clone 12G5; all 13 clones from BD PharMingen, Heidelberg, Germany); anti-PSGL1 (clone KPL-1; MoBiTec, Göttingen, Germany); and anti–L-selectin (clone FMC46; Dako Cytomation, Hamburg, Germany). MSCs were analyzed in a Coulter Epics XL MCL flow cytometer (Beckman Coulter). To control for a potential loss of adhesion molecules by trypsin treatment, expression of adhesion receptors was determined flow cytometrically after either mechanical disruption by rubber policeman, incubation with 6.8 mM EDTA in PBS for 5 minutes at 4°C, or trypsinization as described. This yielded comparable percentages of cells positive for CXCR4 and α4, α5, and β1 integrins (data not shown). Expression of CXCR4 was also determined after passage of MSCs (106 in 1 mL) over glass slides in the parallel plate flow chamber system described below (see “Flow chamber assay”). After passage through the chamber, MSCs were immediately collected in HEPES-buffered salt solution (HBSS; Invitrogen) buffer at 4°C and analyzed by flow cytometry within 1 hour. Control cells were stored analogously and kept at no agitation for the period of the assay.
Detection of mRNA transcripts for adhesion molecules was performed by reverse transcription–polymerase chain reaction (RT-PCR). Total RNA was extracted from MSCs using TRIzol (Invitrogen). RNA integrity was checked by gel electrophoresis, and 1 μg RNA of intact samples was reverse transcribed by using the Omniscript Reverse Transcription Kit (Qiagen, Hilden, Germany) at 42°C for 50 minutes. PCR amplification of the resulting cDNAwas performed by using the Taq PCR core kit (Qiagen). Primers (Biospring, Frankfurt, Germany) and corresponding annealing temperatures (Tm) were as follows: GAPDH: sense 5′-GAAGGTGAAGGTCGGAGTC-3′, antisense 5′-GAAGATGGTGATGGGATTTC-3′ (Tm 62°C); c-kit: sense 5′-GCTGAGCTTT TCTTACCAGGTGG-3′, antisense 5′-TATGTCATACATTTCAGCAGG TGC-3′ (Tm 60°C); CXCR4: sense 5′-CTGAGAAGCATGACGGACAAGTACAG-3′, antisense 5′-CAACAGCTTCCTTGGCCTCTGACT-3′ (Tm 60°C); PSGL-1: sense 5′-ATGCCTC TGCAACTCCTCCT-3′, antisense 5′-CTGCTGAATCCGTGGACAGGTT-3′ (Tm 61°C); L-selectin: sense 5′-TCACGTCGTCTTCTGTATACTGTGG-3′, antisense 5′-TTGCAGCTAGCATTTCAGTGATG-3′ (Tm 60°C); β2 integrin: sense 5′-ACCAGCCCAGAGGT GACTGT-3′, antisense 5′-CTGCTCCTGGATGCACTCTGT-3′ (Tm 59°C); α4 integrin: sense 5′-AGAGAGACAAT CAGTGGTTGG-3′, antisense 5′-TCAGTTCTGTTCGTAAATCAGG -3′ (Tm 57°C); CD24: sense 5′-CTCCTACCCACGCAGATTTATTC-3′, antisense 5′-AGAGTGAGACCACGAAGAGAC-3′ (Tm 59°C); CD44: sense 5′-TGCCGCTTTGCAGGTGTAT-3′, antisense 5′-TGGCACCCGCTATGTCCA-3′ (Tm 59°C). Expression was validated using RNA from the mesenchymal tumor cell line HOS (American Tissue Type Culture Collection [ATCC], Manassas, VA) or the leukemic cell line KG-1 (ATCC) as positive controls and RNA from the untranscribed MSC sample as negative control. PCR products were separated on a 2% agarose gel and stained with ethidium bromide, and images were recorded.
Flow chamber assay
Glass slides precoated with 0.1% (wt/vol) gelatin (Sigma, Taufkirchen, Germany) were seeded with human umbilical vein ECs (HUVECs; Cambrex Bio Science, Verviers, Belgium) in EGM-2 medium (BulletKit, Cambrex Bio Science, Cambridge, United Kingdom). In some experiments, glass slides were left uncoated or coated with 10 μg/mL human P-selectin (R&D Systems, Wiesbaden, Germany) as previously described.25 HUVECs were used when they reached more than 90% confluency. In part of the experiments, HUVECs were preincubated with TNF-α (10 ng/mL) for 4 to 6 hours prior to the assay. MSCs were trypsinized within 1 to 2 hours before use in the assay, resuspended at 106/mL in HBSS supplemented with 1% human plasma and 25 mM HEPES, and incubated for 30 minutes at 37°C, 5% CO2. In some experiments, function-blocking antibodies were added to HUVECs (10 μg/mL anti–P-selectin clone AK4; 10 μg/mL anti–VCAM-1 clone 1G11; both from Immunotech, Marseille, France) or MSCs (anti–PSGL-1: 10 μg/mL clone KPL-1 from BD PharMingen; anti–VLA-4: 10 μg/mL clone HP2/1 from Immunotech; anti-β2 integrin: 10 μg/mL clone 7E4 from Immunotech; anti-β1 integrin: 10 μg/mL clone Lia1/2 from Immunotech) 30 minutes prior to analysis in PBS/1% BSA. For some experiments, MSCs were also preincubated for 1 hour at 37°C with 50 mU/mL α-l-fucosidase (Sigma) or 25 μg/mL O-sialoglycoprotein endopeptidase (Biozol, Eching, Germany) dissolved in PBS/1% BSA. Efficiency of fucosidase treatment was controlled by staining of fucosidase-treated and, as a control, neuraminidase-treated MSCs with FITC-conjugated Ulex europaeus agglutinin I (Sigma) and subsequent flow cytometric analysis, leading to a 5- to 10-fold reduction in fluorescence intensity after fucosidase, but no change after neuraminidase-treatment of MSCs. A 35-mm circular parallel plate flow chamber containing a 5-mm wide and 0.01-inch high channel (GlycoTech, Gaithersburg, MD) was mounted on top of glass slides with pregrown confluent HUVECs, precoated P-selectin, or VCAM-1. Using a perfusor pump connected to the inlet port via a 1/16-inch diameter tube, a uniform laminar flow was applied, allowing regulation of calculated shear stresses between 0.1 and 4 dynes/cm2. Prewarmed MSCs, PBMCs, or CD34+ cells (5 × 106/mL) were flushed over HUVECs at 0.1 dynes/cm2 for 10 minutes. In some experiments, medium at room temperature was used. The flow rate was then increased to yield a shear stress of 2 dynes/cm2 for a further 10 minutes. Video recordings were performed using a CCD camera (D-73431; Sony, Cologne, Germany) mounted on an inverted-stage microscope (Axiovert 135; Zeiss, Oberkochen, Germany) equipped with a × 10 objective (Zeiss). In addition, numbers of adherent cells were recorded in 3 representative fields after each flow phase (0.1 and 2.0 dynes/cm2). Rolling velocities were recorded using real-time video imaging and a defined space grid in microscopic fields. Analysis of video sequences (for rolling) and single frames (for adhesion) used windows sized to correspond to an area of 15 mm2. MSCs were considered noninteracting when they moved at the velocity of the flow, whereas cells moving at lower velocities were defined as rolling.
Homing assay for MSCs and intravital microscopy
MSCs were fluorescence-labeled using preincubation with PKH-26 (Sigma) or carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) according to the manufacturer's instructions as described.26 For homing assays, 1 × 106 MSCs were injected into the tail veins of NOD/SCID mice preirradiated with 2.5 Gy γ rays. After 2, 6, or 24 hours, cell suspensions were prepared from tissues or blood of killed mice by mincing, filtrated through 100-μm pore filters, and analyzed by flow cytometry.27 Briefly, volumes and live-cell numbers were recorded, and from a subfraction of cells diluted in PBS/2% FCS, 100 000 events were analyzed within a live-cell gate for the presence of PKH+ (or CFSE+) cells. Control animals that received no cells were included. Absolute numbers of homed MSCs per organ were calculated according to the formula n = mg/ ma × v1/v2 × 100 000/x, with mg indicating total mass of the organ analyzed; ma, mass of the analyzed proportion of the organ; v1, total volume in which the entire organ-cell suspension was contained; v2, volume of sample aspirated/analyzed; x, number of positive events acquired per 100 000 events as described.27 Extravasation of transplanted MSCs was analyzed in mice injected with 5 × 106 MSCs, using frozen sections of spleens and lungs. Cryosections were prepared and stained with FITC-conjugated monoclonal anti–murine CD31 antibody MEC13.3 (Becton Dickinson, Heidelberg, Germany) and mounted using Vectashield medium (Vector Laboratories, Burlingame, CA). PKH, FITC, and nuclear (DAPI) fluorescence were visualized using a fluorescence microscope and imaging software (Zeiss). For intravital microscopy, wild-type or P-selectin–/– mice (in C57/BL6 background; kindly provided by Dr Lubor Borsig, Institute of Physiology, University of Zurich, Switzerland) were anesthetized by an intraperitoneal injection of ketamine (Schwabe-Curamed, Karlsruhe, Germany) and xylazine (Bayer, Leverkusen, Germany) and placed on a homothermic blanket. The right carotid artery was prepared microsurgically, and a catheter was inserted for injection of MSCs (5 × 106/mL in PBS/1% BSA). The left ear of the mouse was placed gently on a microscope slide and covered with glycerol and a coverslip. Vascular architecture and labeled cells were visualized during their passage through vessels under fluorescent epi-illumination using a multiband filter system (XF 53; Omega Optical, Brattleboro, VT). The microcirculation was continuously recorded using a 1/3-inch DSP 3-CCD camera (DXC-390; Sony) mounted on a modified Zeiss microscope (Axiotech Vario 100 HD; Zeiss) equipped with a × 10 immersion proof objective (Nikon, Düsseldorf, Germany). Images were digitally stored using Media Studio Pro 7.0 (Ulead, Kaarst, Germany) for later off-line analysis. Migration behavior of MSCs was determined in individual vessel segments of either wild-type or P-selectin–deficient mice. Cells were considered non-interacting when they moved at the velocity of the mean blood flow, whereas lower velocities were defined as rolling.28
Statistical analysis was performed using the Student t test.
Results
Expression of characteristic surface markers and homing receptors by MSCs
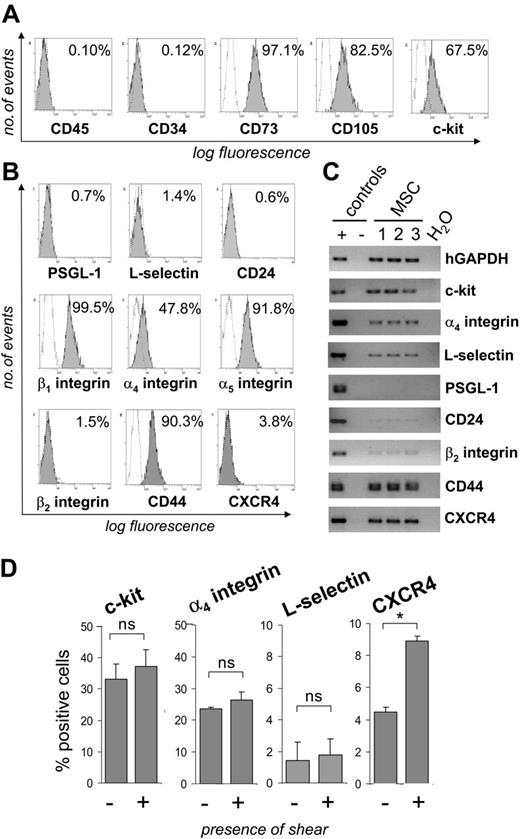
By flow cytometric analysis, MSCs were unequivocally negative for the hematopoietic markers CD45 and CD34, but expressed both the CD73/SH3 and CD105/SH2 antigens and, in part, c-kit (Figure 1A). MSCs did not express detectable levels of the adhesion molecules PSGL-1, L-selectin, the alternative P-selectin ligand CD24 or β2 integrin on their surface, but stained positive for α4, α5, and β1 integrins and CD44 (Figure 1B). Furthermore, a small percentage of MSCs expressed CXCR4 on the cell surface. RT-PCR analysis confirmed the absence of or only barely detectable expression of PSGL-1, CD24, and β2 integrin, and the presence of c-kit (Figure 1C). Interestingly, however, expression of L-selectin and CXCR4 was clearly detectable by RT-PCR. To investigate the possibility that shear stress may induce cell-surface expression of CXCR4 or L-selectin, MSCs were passed through parallel plate flow chambers at a calculated shear force of 0.5 dynes/cm2. Whereas the percentage of MSCs expressing L-selectin, α4 integrin, or c-kit remained largely constant, the percentage of CXCR4+ MSCs increased by approximately 2-fold after exposure of MSCs to shear stress (Figure 1D). Taken together, these data show that MSCs express several adhesion receptors shown to function as homing receptors in hematopoietic cells but lack expression of several selectins or their ligands.
Homing of MSCs in mice
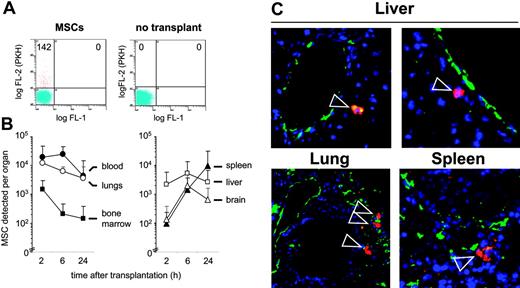
To investigate whether MSCs can undergo a regulated homing process in vivo, we transplanted MSCs into immunodeficient NOD/SCID mice. Flow cytometric analysis of organ-cell suspensions demonstrated time-dependent changes in the distribution of the MSCs. MSCs were detected predominantly in blood and lungs at 2 and 6 hours, and, at decreasing levels, at 24 hours after transplantation (Figure 2 A-B). Whereas relatively low numbers of MSCs were detected in femoral bone marrow, increasing amounts of MSCs were detected in spleen, liver, and brain. No MSCs were detected in muscle tissue in 6 of 6 mice in preparations of the muscularis quadriceps femoris (data not shown). Immunohistochemical analysis of lung, liver, and spleen sections was used to visualize the prelabeled transplanted cells by their PKH+ red fluorescence and blood vessels by staining with anti-CD31–FITC (Figure 2C). Whereas in lung, 25 of 25 identified PKH+ cells appeared to be contained within CD31+-stained vessel structures, in liver, 16 of 16 detected PKH+ cells were found clearly outside the vessel structure. In spleen, 12 of 19 identified PKH+ cells had extravasated, 4 remained intravascular, and for 3 this remained difficult to determine. These data indicate that the transplanted MSCs circulate in the blood after transplantation and are capable of extravasating into tissue.
MSCs express characteristic surface antigens and adhesion molecules. (A-B) MSCs were stained with monoclonal antibodies directed against the indicated antigens (filled areas) or isotype control antibodies (open areas). Inserted values represent percent positive cells. (C) RNA was extracted from 3 different MSC isolates and analyzed by RT-PCR for the presence of transcripts of the indicated adhesion receptors. (D) MSCs were passaged through a parallel plate flow chamber over uncoated glass slides at a calculated wall shear stress of 0.5 dynes/cm2. Controls were kept under static conditions during this time. Subsequently, MSCs were analyzed for the expression of adhesion receptors by flow cytometry. MSCs used for these experiments were from passages 5 to 9. *P < .05 (Student t test); ns indicates not significant.
MSCs express characteristic surface antigens and adhesion molecules. (A-B) MSCs were stained with monoclonal antibodies directed against the indicated antigens (filled areas) or isotype control antibodies (open areas). Inserted values represent percent positive cells. (C) RNA was extracted from 3 different MSC isolates and analyzed by RT-PCR for the presence of transcripts of the indicated adhesion receptors. (D) MSCs were passaged through a parallel plate flow chamber over uncoated glass slides at a calculated wall shear stress of 0.5 dynes/cm2. Controls were kept under static conditions during this time. Subsequently, MSCs were analyzed for the expression of adhesion receptors by flow cytometry. MSCs used for these experiments were from passages 5 to 9. *P < .05 (Student t test); ns indicates not significant.
MSCs interact with ECs under flow
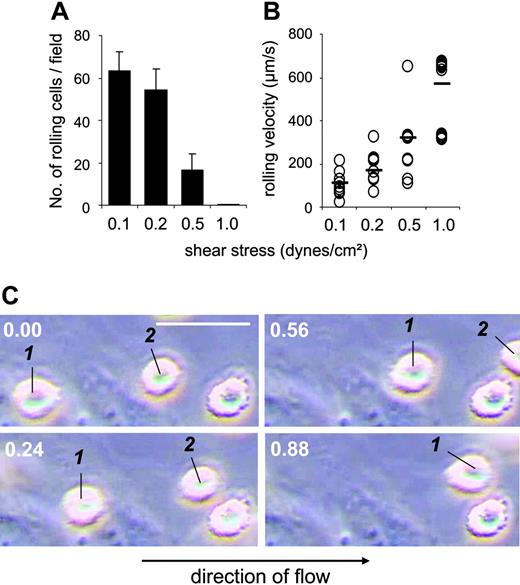
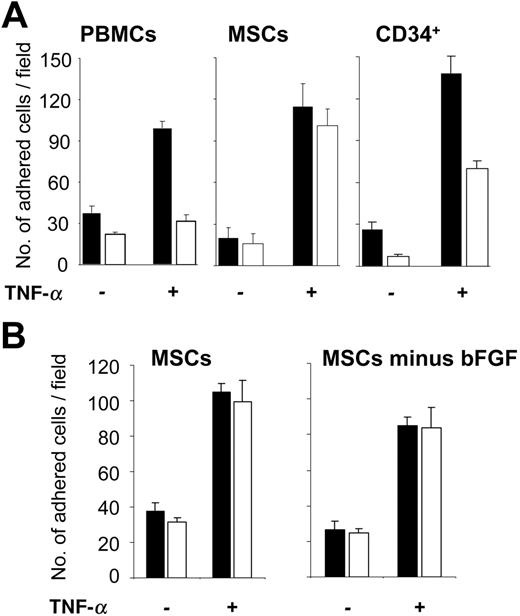
To investigate how MSCs interact with ECs under shear flow, a parallel plate flow chamber system was used with HUVECs as an endothelial-cell layer. As shown in Figure 3A, numbers of rolling MSCs were dependent on the applied shear force. Individual MSCs rolled on ECs with different velocities at low shear force (Figure 3B). When shear force was up-regulated, the frequency of rolling MSCs decreased, and their rolling velocity increased from approximately 100 μm/s to more than 500 μm/s. Rolling MSCs appeared as round cells, rapidly elaborating and retracting numerous podia that contacted the EC layer (Figure 3C; Video S1, available on the Blood website; see the Supplemental Video link at the top of the online article). We observed that only rolling MSCs eventually adhered. We then assessed adhesion of MSCs, which rolled on HUVECs at 0.1 dynes/cm2, and determined their capability to remain adherent both at low (0.1 dynes/cm2) and at increased (2 dynes/cm2) shear stress. Numbers of adherent cells were similar for all 3, MSCs, freshly isolated PBMCs, and hematopoietic CD34+ progenitor cells (Figure 4A). In addition, prestimulation of ECs with TNF-α, which is known to induce expression of adhesion molecules on HUVECs, increased numbers of adherent cells with all 3 cell types (Figure 4A). Notably, however, TNF-α–induced adhesion was more pronounced in MSCs at 2 dynes/cm2 compared with PBMCs or CD34+ cells, with only few adherent MSCs detaching at 2 dynes/cm2. Preculture of MSCs in the presence or absence of bFGF did not influence their adhesion behavior (Figure 4B). This also did not affect the ability of the MSCs to differentiate into chondrogenic, osteogenic, or adipogenic cells (data not shown). Binding of MSCs to TNF-α–pretreated HUVECs was not different at 37°C and room temperature in this assay (107.7 ± 10.2 versus 105.0 ± 17.7 cells binding at 0.1 dynes/cm2, and 98.0 ± 17.7 versus 93.3 ± 9.4 cells binding at 2 dynes/cm2 at room temperature versus 37°C, respectively). In contrast to MSCs, PBMCs, or CD34+ cells, no adhesion to the EC layer was observed with human erythrocytes or trypsinized HUVECs (data not shown). Thus, MSCs displayed coordinated, shear stress-dependent rolling and adhesion behavior on ECs in vitro, similar to peripheral-blood leukocytes or HPCs.
Tissue distribution of MSCs transplanted into NOD/SCID mice. (A-B) Frequency and distribution of fluorescence-labeled MSCs in murine organs. MSCs were labeled with the vital dye PKH-26 or CFSE and intravenously injected. At 2, 6, or 24 hours after transplantation with 1 × 106 MSCs, mice were killed, organs were minced, and single-cell suspensions were prepared. (A) The incidence of labeled cells was determined by flow cytometry by determination of the number of fluorescing cells per 100 000 cells, including non-transplanted controls. (B) Absolute numbers of MSCs homed per organ were determined as described in “Materials and methods.” Values are means ± SD from 4 (2 hours, 6 hours) or 8 (24 hours) mice. (C) Microscopic analysis of transplanted PKH-labeled MSCs. Cryosections from lung, liver, and spleen were derived from organs of mice given transplants 24 hours after transplantation of 5 × 106 PKH+ MSCs. ECs and vessels were stained with fluorescence-labeled anti-CD31 antibody (green), transplanted prelabeled MSCs were visualized by their PKH fluorescence (red; indicated by arrowheads), and nuclei were stained by DAPI (blue). Original magnification, × 200. MSCs used for these experiments were from passages 6 or 7.
Tissue distribution of MSCs transplanted into NOD/SCID mice. (A-B) Frequency and distribution of fluorescence-labeled MSCs in murine organs. MSCs were labeled with the vital dye PKH-26 or CFSE and intravenously injected. At 2, 6, or 24 hours after transplantation with 1 × 106 MSCs, mice were killed, organs were minced, and single-cell suspensions were prepared. (A) The incidence of labeled cells was determined by flow cytometry by determination of the number of fluorescing cells per 100 000 cells, including non-transplanted controls. (B) Absolute numbers of MSCs homed per organ were determined as described in “Materials and methods.” Values are means ± SD from 4 (2 hours, 6 hours) or 8 (24 hours) mice. (C) Microscopic analysis of transplanted PKH-labeled MSCs. Cryosections from lung, liver, and spleen were derived from organs of mice given transplants 24 hours after transplantation of 5 × 106 PKH+ MSCs. ECs and vessels were stained with fluorescence-labeled anti-CD31 antibody (green), transplanted prelabeled MSCs were visualized by their PKH fluorescence (red; indicated by arrowheads), and nuclei were stained by DAPI (blue). Original magnification, × 200. MSCs used for these experiments were from passages 6 or 7.
MSCs roll on HUVECs under shear flow. Analysis of numbers of rolling cells (A) and rolling velocities (B). MSCs (106, passage 9) were flushed at different shear stresses over a HUVEC layer in the parallel plate flow chamber, and rolling cells were determined as described in “Materials and methods.” Values are means ± SD; n = 7-9. (C) Lifted frames of a video clip with 2 moving cells and one stationary adherent MSC. Values in top left corners indicate time from video start in seconds; the white horizontal scale bar represents 50 μm.
MSCs roll on HUVECs under shear flow. Analysis of numbers of rolling cells (A) and rolling velocities (B). MSCs (106, passage 9) were flushed at different shear stresses over a HUVEC layer in the parallel plate flow chamber, and rolling cells were determined as described in “Materials and methods.” Values are means ± SD; n = 7-9. (C) Lifted frames of a video clip with 2 moving cells and one stationary adherent MSC. Values in top left corners indicate time from video start in seconds; the white horizontal scale bar represents 50 μm.
Involvement of adhesion molecules in the interaction of MSCs with ECs
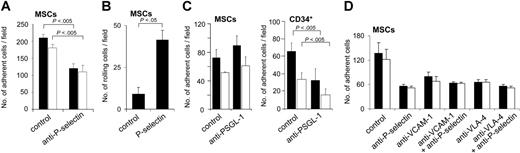
We next analyzed the influence of blocking antibodies against adhesion receptors expressed on MSCs or ECs. Preincubation of HUVECs with anti–P-selectin function-blocking antibody decreased numbers of MSCs bound to ECs to approximately 50% of controls (Figure 5A). Moreover, MSCs also rolled on immobilized P-selectin (Figure 5B). Interaction of MSCs with P-selectin was dependent on the presence of divalent cations, because the numbers of rolling cells decreased to 44.2% ± 13.5% of controls when the assay was performed in the absence of Ca2+ and Mg2+. Whereas preincubation of MSCs with function-blocking antibody against the P-selectin ligand PSGL-1 efficiently inhibited binding of CD34+ hematopoietic progenitors to ECs, it did not suppress binding of MSCs to ECs, in agreement with the absent expression of PSGL-1 on MSCs (Figure 5C). When MSCs were pretreated with either O-sialoglycoprotein endopeptidase or fucosidase, numbers of MSCs binding to HUVECS prestimulated with TNF-α were suppressed 2.7-fold (0.1 dynes/cm2) or 1.7-fold (2 dynes/cm2) compared to controls after O-sialoglycoprotein endopeptidase, and 54.4-fold (0.1 dynes/cm2) or 21.9-fold (2 dynes/cm2) after fucosidase. This indicates that MSCs may bind to P-selectin using a ligand that contains fucose and sialic acid residues, but that is different from PSGL-1.
MSCs adhere to ECs under shear flow. (A) MSCs from passage 9, PBMCs, or CD34+ HPCs (106/analysis) were flushed over a HUVEC monolayer in the parallel plate flow chamber and analyzed at a calculated shear stress of 0.1 dynes/cm2 (▪) or 2.0 dynes/cm2 (□). Numbers of adherent cells were determined in 3 representative fields. (B) MSCs (passage 8), pregrown in medium in the presence and absence of bFGF for 7 days, were analyzed as described in panel A. The analyses were performed either with or without prestimulation of HUVECs with 10 ng/mL TNF-α for 4 to 6 hours as indicated. Values are means ± SD; n = 3.
MSCs adhere to ECs under shear flow. (A) MSCs from passage 9, PBMCs, or CD34+ HPCs (106/analysis) were flushed over a HUVEC monolayer in the parallel plate flow chamber and analyzed at a calculated shear stress of 0.1 dynes/cm2 (▪) or 2.0 dynes/cm2 (□). Numbers of adherent cells were determined in 3 representative fields. (B) MSCs (passage 8), pregrown in medium in the presence and absence of bFGF for 7 days, were analyzed as described in panel A. The analyses were performed either with or without prestimulation of HUVECs with 10 ng/mL TNF-α for 4 to 6 hours as indicated. Values are means ± SD; n = 3.
Because both α4 and β1 integrins were expressed on MSCs, and because VLA-4 and its cognate ligand VCAM-1 have been shown to mediate adhesion of leukocytes and HPCs to ECs, we used function-blocking antibodies against these molecules. Preincubation of MSCs with anti–VLA-4, or of HUVECs with anti–VCAM-1, inhibited the binding of MSCs to ECs, indicating that MSCs bind to HUVECs by both a P-selectin and a VLA-4–dependent mechanism (Figure 5D). The simultaneous blockade of P-selectin on ECs and of either VCAM-1 on ECs or of VLA-4 on MSCs did not result in a further decrease of MSC adhesion (Figure 5D). These data indicate that binding via P-selectin and VLA-4/VCAM-1 are both equally required for binding of MSCs to HUVECs and that P-selectin and VCAM-1/VLA-4 act on different steps of the rolling and adhesion process.
MSCs display P-selectin–dependent rolling and adhesion behavior on ECs. (A-B) P-selectin–dependent interaction of MSCs under flow. (A) MSCs (106) were preincubated or not with anti–P-selectin antibody and analyzed in the parallel plate flow chamber at a calculated shear stress of 0.1 dynes/cm2 (▪) or 2.0 dynes/cm2 (□) on precoated HUVECs stimulated with TNF-α (10 ng/mL for 4-6 hours). Numbers of adhered cells were determined in 3 representative fields. (B) MSCs (106) were flushed over glass slides precoated with P-selectin or control slides coated with BSA-containing buffer only in the parallel plate flow chamber at a calculated shear stress of 0.2 dynes/cm2. Numbers of rolling cells were determined in 3 representative microscopic fields. (C) MSC binding to HUVECs is not blocked by anti–PSGL-1. MSCs or CD34+ progenitors (106) were flushed over HUVEC layers pretreated with 10 ng/mL TNF-α for 4 to 6 hours. Numbers of cells were determined after application of a calculated shear stress of 0.1 dynes/cm2 (▪) or 2.0 dynes/cm2 (□). Pretreatment of MSCs or CD34+ cells with anti–PSGL-1 antibody was performed as indicated. (D) Influence of VCAM-1/VLA-4 on adhesion of MSCs to HUVECs. MSCs were pretreated or not with anti–VLA-4 antibody, HUVECs with anti–P-selectin or anti–VCAM-1 (or both) antibody for 30 minutes prior to analysis as indicated. Subsequently, 106 MSCs were flushed through a parallel plate flow chamber and numbers of adherent cells were recorded after application of a calculated shear stress of 0.1 dynes/cm2 (▪) or 2.0 dynes/cm2 (□). HUVEC monolayers were pretreated with 10 ng/mL TNF-α for 4 to 6 hours prior to the experiments. MSCs used for the experiments were derived from passages 6 to 9. Values are means ± SD; n = 3.
MSCs display P-selectin–dependent rolling and adhesion behavior on ECs. (A-B) P-selectin–dependent interaction of MSCs under flow. (A) MSCs (106) were preincubated or not with anti–P-selectin antibody and analyzed in the parallel plate flow chamber at a calculated shear stress of 0.1 dynes/cm2 (▪) or 2.0 dynes/cm2 (□) on precoated HUVECs stimulated with TNF-α (10 ng/mL for 4-6 hours). Numbers of adhered cells were determined in 3 representative fields. (B) MSCs (106) were flushed over glass slides precoated with P-selectin or control slides coated with BSA-containing buffer only in the parallel plate flow chamber at a calculated shear stress of 0.2 dynes/cm2. Numbers of rolling cells were determined in 3 representative microscopic fields. (C) MSC binding to HUVECs is not blocked by anti–PSGL-1. MSCs or CD34+ progenitors (106) were flushed over HUVEC layers pretreated with 10 ng/mL TNF-α for 4 to 6 hours. Numbers of cells were determined after application of a calculated shear stress of 0.1 dynes/cm2 (▪) or 2.0 dynes/cm2 (□). Pretreatment of MSCs or CD34+ cells with anti–PSGL-1 antibody was performed as indicated. (D) Influence of VCAM-1/VLA-4 on adhesion of MSCs to HUVECs. MSCs were pretreated or not with anti–VLA-4 antibody, HUVECs with anti–P-selectin or anti–VCAM-1 (or both) antibody for 30 minutes prior to analysis as indicated. Subsequently, 106 MSCs were flushed through a parallel plate flow chamber and numbers of adherent cells were recorded after application of a calculated shear stress of 0.1 dynes/cm2 (▪) or 2.0 dynes/cm2 (□). HUVEC monolayers were pretreated with 10 ng/mL TNF-α for 4 to 6 hours prior to the experiments. MSCs used for the experiments were derived from passages 6 to 9. Values are means ± SD; n = 3.
MSCs roll and adhere on the vessel wall in vivo
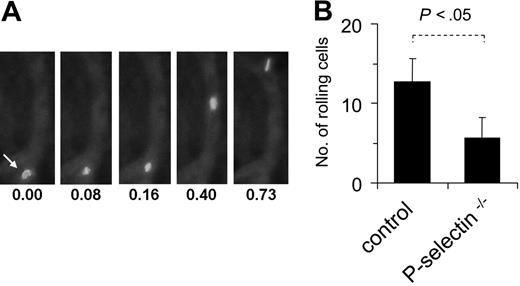
Finally, to investigate whether MSCs roll and adhere to ECs in vivo, intravital microscopy was applied in a murine model after intra-arterial injection of fluorescence-marked MSCs. Visualization of postcapillary venous blood flow in the ear veins of mice revealed that the labeled MSCs displayed the typical pattern of slowing down in postcapillary venules (Figure 6A-B). In addition, MSCs also adhered firmLy for short periods in some vessels. When MSCs were analyzed under the same conditions in P-selectin–/– mice, the number of rolling events was significantly reduced compared to analysis in wild-type mice (Figure 6B), demonstrating the involvement of P-selectin in this process. Together, these data indicate that MSCs are capable of coordinated rolling and adhesion behavior on ECs, both in vitro and in vivo.
Discussion
In this study, we have demonstrated that bone marrow–derived MSCs interact with ECs under shear flow, involving rolling with rapid extension of podia, and activation of both selectin-dependent and integrin-dependent binding.
Both culture-expanded as well as freshly isolated adult tissue-derived MSCs have so far been shown to circulate in the blood for limited time periods and to distribute to various organs including lung, liver, and spleen.10,29-31 Previous work has analyzed MSC homing at early time points (24 and 48 hours) mostly using PCR, or imaging techniques based on 111In labeling of injected cells, which cannot determine whether MSCs have egressed from the bloodstream. Our data show extravasation of transplanted MSCs into tissue as early as 24 hours after injection. Anjos-Afonso et al have analyzed the tissue distribution of transplanted murine culture-expanded MSCs.32 They analyzed histologic sections at 4 weeks after transplantation and found that MSCs can be trapped intravascularly in the lungs where they caused damage. Similar to our observations, these authors found that murine MSCs homed to liver, spleen, and brain, but that very few or none homed to muscle. With regard to brain homing of MSCs, additional or different mechanisms may be operative. Further histologic analysis will be required to demonstrate that MSCs can pass the blood–brain barrier, as has been suggested by recent data showing that transplanted MSCs ameliorate experimental encephalomyelitis.33 Our results show that detectable numbers of MSCs can be found in blood and in different organs as early as 2 hours after transplantation. The transplanted MSCs were detected at frequencies of between 10 and 200/100 000 cells, which is in the same range as HPCs, and they were found to distribute with similar kinetics to tissues as HPCs.27,34
MSCs interact with endothelium in vivo. MSCs (passages 6-9) were fluorescence-marked with PKH-26 as described in “Materials and methods,” injected intra-arterially into wild-type or P-selectin–/– mice and followed microscopically in an ear window. (A) Representative views of a fluorescing cell, rolling on a microvessel. Numbers represent time points in seconds after start of analysis. (B) The rolling fraction of all cells in murine skin postcapillary venules was calculated after video microscopic analysis in and MSCs. Values are means ± SD from 9 vessels/3 animals analyzed in wild-type mice and 6 vessels/2 animals analyzed in P-selectin–deficient mice.
MSCs interact with endothelium in vivo. MSCs (passages 6-9) were fluorescence-marked with PKH-26 as described in “Materials and methods,” injected intra-arterially into wild-type or P-selectin–/– mice and followed microscopically in an ear window. (A) Representative views of a fluorescing cell, rolling on a microvessel. Numbers represent time points in seconds after start of analysis. (B) The rolling fraction of all cells in murine skin postcapillary venules was calculated after video microscopic analysis in and MSCs. Values are means ± SD from 9 vessels/3 animals analyzed in wild-type mice and 6 vessels/2 animals analyzed in P-selectin–deficient mice.
Compared with circulating leukocytes or HPCs, MSCs only partially expressed adhesion molecules, which are known to serve as homing receptors in circulating hematopoietic cells. Although MSCs did not express PSGL-1, they clearly bound to ECs in a P-selectin–dependent manner. From our data it can be assumed that MSCs possess 1 or more functional P-selectin ligands, which are different from PSGL-1 and CD24. Dimitroff et al have reported that CD44 can mediate binding to E-selectin of hematopoietic progenitors.35,36 Although MSCs expressed CD44, we found that pretreatment of HUVECs by function-blocking anti–E-selectin antibody did not suppress binding of MSCs to ECs (B.R. and R.H., unpublished data, July 2005). L-selectin was expressed in MSCs, as assessed by RT-PCR. However, numbers of MSCs expressing L-selectin on the cell surface were low, and we did not observe increased expression of L-selectin after administration of shear force as observed with CXCR4. This indicates that L-selectin is also unlikely to serve as a major adhesion molecule in the MSCs. Thus, the main selectin ligands on MSCs remain to be determined.
We found expression of the β1-type integrin receptors VLA-4 and VLA-5 on MSCs, similarly as described by Bruder et al.37 Segers et al have shown that MSCs, injected intra-arterially into the heart, can strongly adhere to ECs in a VCAM-dependent fashion, indicating that MSCs may use VLA-4 as an adhesion molecule.29 In our flow chamber model, MSCs also adhered to ECs via VLA-4/VCAM-1. Interestingly, we observed differences in the ability of EC-bound PBMCs/HPCs and MSCs to detach at increased shear force. Unlike hematopoietic cells, only few bound MSCs detached at an increased shear stress of 2 dynes/cm2. It has been shown in vitro (eg, our study) and in vivo by Mazo et al38 that a substantial fraction of CD34+ HPCs, which become adherent to prestimulated ECs or to the vessel wall, will detach within seconds to minutes. A defect in the detachment process of MSCs from activated ECs may, at least in part, explain the protracted adhesion of MSCs as observed in pulmonary vessels after transplantation.
Unlike most mature hematopoietic-cell populations, MSCs did not express β2 integrin. Hematopoietic stem cells have also been described to lack expression of β2 integrin and to bind to ECs via VLA-4, for example, for homing to the bone marrow after transplantation.39 It is, however, possible that other integrins are used by MSCs to adhere to ECs, because binding to ECs was only partially blocked by both anti–VLA-4 and anti–VCAM-1 antibodies. We observed a variability of MSCs to adhere to HUVECs, with a 2- to 2.5-fold difference in numbers of adherent cells per field when analyzing a constant number of 106 MSCs. In part, this can be attributed to interassay variation, which we also observed when analyzing established cell lines or freshly isolated bone marrow cells in this system. In addition, different isolates of MSCs, derived from different patients, may bind with different efficiencies.
Activation through chemokine receptors has been described to enhance integrin binding. A major candidate molecule is the receptor for SDF-1, CXCR4.20,40 Our data showing that a subpopulation of MSCs express CXCR4 correspond to the findings of Wynn et al.41 The data suggest that MSCs can use this receptor for organ homing. However, the fraction of MSCs adhering to ECs in our experiments was higher than the fraction of MSCs detectably expressing CXCR4. Moreover, we observed that MSCs express CCR6 and that they are chemo-attracted by the CCR6 ligand, MIP-3α (B.R. and R.H., unpublished results, August 2004). Recently, Sordi et al observed expression of chemokine receptors CXCR4, CX3CR1, CXCR6, CCR1, and CCR7 on an MSC population that is able to migrate into pancreatic islets of mice.42 Similar to our study, chemokine receptors were expressed only in subpopulations of between 1.8% and 26% MSCs. Honczarenko et al have described the expression of only a limited number of chemokine receptors on MSCs, preferably at earlier passages, and have confirmed functionality of CXCR4 by the induction of specific signal transduction pathways.43 The data indicate that chemokines that are present on the luminal surface of ECs can activate cognate chemokine receptors on MSCs, thus enhancing stimulation of integrin receptors and subsequent firm adhesion of MSCs to ECs.
We have used an intravital microscopy system to directly observe transplanted MSCs after intra-arterial injection. When focusing on postcapillary venules of mice constitutively expressing P-selectin,44,45 fluorescence-labeled MSCs were readily seen to move within the blood flow. The numbers of rolling cells correspond to our previous findings using freshly isolated human peripheral-blood mononuclear cells.46,47 We confirmed our in vitro observations of MSC-EC interactions, shown to depend on P-selectin in vitro, by the use of P-selectin–deficient mice.28 These results also confirm the coordinated rolling and adhesion behavior of the MSCs observed on ECs, which includes induction by TNF-α and binding via P-selectin, in an in vivo situation.
Taken together, our results demonstrate that MSCs are capable of coordinated rolling and adhesion behavior on ECs. For this, MSCs bind to P-selectin and VCAM-1 on ECs, and a yet undefined lectin ligand and VLA-4 on MSCs. Thus, MSCs fulfill essential prerequisites for tissue-specific extravasation and homing. The data will be of further relevance for the engineering of MSCs for transplantation in the various clinical indications, where they have been described as promising cellular therapeutics.
Authorship
B.R., S.G., E.S., and R.H. designed research; B.R., S.G., R.B., and S.M. performed research; R.J.L. and J.G. contributed analytical tools; B.R., J.G., R.J.L., and R.H. analyzed data; and B.R. and R.H. wrote the paper.
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 8, 2006; DOI 10.1182/blood-2006-05-025098.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Sabrina Boehme for technical support, Andreas Bräuninger for help with fluorescence microscopy and Kristine Eschedor for secretarial assistance.
This work was supported by BMBF grants 0312683 and 01GN0525 (R.H.) and Deutsche Forschungsgemeinschaft SFB T/R23 project C3 (J.G. and R.H.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal