Abstract
Von Willebrand factor (VWF) is an essential component of hemostasis. However, animal studies using VWF-deficient mice suggest that VWF may also contribute to inflammation. In the present study, we demonstrate that VWF was able to interact with polymorphonuclear leukocytes (PMNs) and monocytes under static and flow conditions. Adhesion under flow was dominated by short-lasting contact with resting PMNs, whereas adhesion of phorbol-12-myristate-13-acetate (PMA)–stimulated PMNs was characterized by firm adhesion. Transient binding of PMNs to VWF appeared to be mediated by P-selectin glycoprotein ligand-1 (PSGL-1). Moreover, recombinant PSGL-1 protein and cell surface–expressed PSGL-1 directly interacted with VWF. As for stable adhesion by PMA-stimulated PMNs, we observed that static adhesion and adhesion under flow were strongly inhibited (greater than 75%) by neutrophil-inhibitory factor, an inhibitor of β2-integrin function. In addition, the isolated I-domain of αMβ2 bound to VWF, and cell lines expressing αLβ2 or αXβ2 adhered efficiently to VWF. Taken together, our data showed that VWF can function as an adhesive surface for various leukocyte subsets (monocytes, PMNs). Analogous to VWF-platelet interaction, VWF provided binding sites for leukocyte receptors involved in rolling (PSGL-1) and stable (β2-integrins) adhesion. VWF is unique in its intrinsic capacity to combine the rolling and the stable adhesion step in the interaction with leukocytes.
Introduction
Recruiting leukocytes to sites of infection or tissue damage is a key element in the inflammatory response. However, these inflammatory cells are in a resting, low-adhesive state while circulating in blood or lymphatic vessels. Stimulatory signals are therefore required to promote the migration of leukocytes through the endothelial layer. This migration involves a complex process that distinguishes a number of distinct steps, including initial rolling to slow down the leukocytes and firm adhesion to allow transendothelial migration.1 Several endothelial and leukocyte receptors have been identified that contribute to these processes. For instance, leukocyte rolling over the endothelial layer is predominantly mediated through interactions between selectins that are exposed on the stimulated endothelial cell and the P-selectin glycoprotein ligand-1 (PSGL-1) on the leukocyte surface.2 These interactions result not only in the deceleration of cells but in the activation of signaling pathways that bring these leukocytes into the high-adhesive state required for stable adhesion.3 Important players in the stable adhesion step are the β2-integrins. This leukocyte-specific integrin family consists of 4 isotypes (αLβ2, αMβ2, αDβ2, and αXβ2), each of which has the intrinsic capacity to mediate stable adhesion.4 The major counterreceptors for the β2-integrin isotypes are the intercellular adhesion molecules (ICAMs).4 It has become clear, however, that many other proteins have the capacity to interact with this integrin family, including fibrinogen, vitronectin, junction-adhesion molecule-3, and glycoprotein (Gp)–1bα, to name a few.5-8 An interesting aspect of these ligands is that they are also present in platelet-rich thrombi and could, hence, promote the infiltration of leukocytes in these thrombi.
The formation of platelet-rich thrombi is greatly dependent on von Willebrand factor (VWF), particularly under conditions of high blood flow.9 VWF is a plasma protein that circulates as an array of multimerized subunits, with each subunit composing the domain structure D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-CK. The importance of VWF for the hemostatic system is illustrated by the severe bleeding tendency associated with its functional deficiency, both in patients and in animal models.10,11 Interestingly, studies using VWF-deficient mice revealed that VWF might also participate in inflammatory processes. For instance, when VWF–/– mice were stimulated in a cytokine-induced meningitis model (which represents a model for acute inflammation), reduced neutrophil infiltration compared with control mice was observed.12 VWF appeared to be associated with this effect in an indirect manner through P-selectin. P-selectin and VWF are stored together in Weibel-Palade bodies in endothelial cells,13 and the absence of VWF leads to the absence of these storage organelles.14 As such, the absence of VWF results in an impaired surface exposure of P-selectin upon stimulation of endothelial cells.
In a model for chronic inflammation, crossbred VWF–/– low-density lipoprotein receptor–/– mice were compared with low-density lipoprotein receptor–/– mice for the development of fatty streaks and early fibrous plaques. In this model, the absence of VWF was associated with smaller lesions after 8 and 22 weeks. Analysis of these lesions revealed fewer macrophages per lesion,15 pointing to a role for VWF in the recruitment of leukocytes to these lesions. Given that P-selectin exposure was normal in these lesions, the reduced leukocyte influx in the absence of VWF should be explained by alternative mechanisms. One possibility is a direct interaction between VWF and leukocytes. Indeed, VWF may contribute to leukocyte recruitment in a direct manner through its propeptide, which circulates in plasma independently of VWF. The propeptide has been reported to constitute a binding site for the leukocyte integrins α4β1 and α9β1.16,17 Koivunen et al18 have previously demonstrated that the monocytic THP-1 cell line adheres to VWF under static conditions in a β2-integrin–dependent manner, providing support for the possibility that VWF may directly interact with leukocytes.
These observations led us to address the hypothesis that VWF is capable of acting as an adhesive surface for blood-borne leukocytes (in particular, polymorphonuclear leukocytes [PMNs] and monocytes) under static and flow conditions. Furthermore, recombinant VWF fragments were used to determine which regions contributed to the interaction with these leukocytes. Finally, specific inhibitors and transfected cell lines were used to identify leukocyte elements that mediated binding to VWF. We concluded that multiple regions within the VWF molecule are able to support leukocyte adhesion. Adhesion to VWF is mediated by concerted actions of PSGL-1 and β2-integrins.
Materials and methods
Materials
Cell culture medium (RPMI 1640, DMEM/F-12, MEM-α medium), penicillin, streptomycin, and l-glutamine were obtained from Gibco Life Technologies (Paisley, United Kingdom). FCS was from Cambrex BioScience (Verviers, Belgium). Microtiter plates were from Costar (New York, NY) for protein assays or from Immulon 1B (Thermo Labsystem, Franklin, MA) for cell assays. Phorbol-12-myristate-13-acetate (PMA), methotrexate, P-nitrophenyl phosphate (PNP), and polyvinylpyrrolidone-360 (PVP) were purchased from Sigma (St Louis, MO). The Biacore2000 biosensor system and reagents, including streptavidin-sensor chips, were from Biacore AB (Uppsala, Sweden).
Proteins
Recombinant wild-type (wt)–VWF, VWF/ΔA1, VWF/ΔA2, VWF/ΔA3, VWF/D′-D3, and VWF/A1-A2-A3 were expressed and purified as described.19-21 Plasma-derived (pd)–VWF was a kind gift from C. Mazurier (LFB, Lille, France). The αM I-domain fused to glutathione-S-transferase (GST) was expressed and purified as follows: cDNA encoding residues Ser112-Gly321 of the αM subunit was obtained by standard molecular biology procedures using U937-derived mRNA as source material. The cDNA construct was cloned into the pGEX bacterial expression vector, to be expressed as a GST/αM I-domain fusion protein and purified as described.22 Recombinant PSGL-1/immunoglobulin fusion protein (rPSGL–immunoglobulin)23 was a generous gift from R. G. Schaub (Wyeth Research, Cambridge, MA). Purified recombinant neutrophil inhibitory factor (NIF) was a gift from D. I. Pritchard (University of Nottingham, Nottingham, United Kingdom). Fibrinogen was purchased from Enzyme Research Laboratories (South Bend, IN). Botrocetin was purified from crude Bothrops jararaca venom (Sigma), as described.24 Purified snake venom–derived Nk-protease was kindly provided by R. K. Andrews (Monash University, Clayton, Australia). Nk-protease was purified from Naja kaouthia venom and was similar to mocarhagin, a previously described snake venom protease that cleaves the amino terminal regions of GpIbα and PSGL-1.25,26 Monoclonal anti–PSGL-1 antibody KPL-1 was obtained from BD PharMingen (San Diego, CA). Isotype control antibody against C5aR (clone W17/1) was purchased from Serotec (Oxford, United Kingdom). Bovine serum albumin (BSA) fraction V (catalog number A2153) was purchased from Sigma.
Cell lines and culture conditions
Monocytic cell lines U937 and THP-1 were obtained from the American Type Culture Collection (Manassas, VA; CRL-1593.2) and were maintained in RPMI 1640, 10% FCS, 50 U/mL penicillin, 50 μg/mL streptomycin, and 50 μM β-mercaptoethanol in a humidified CO2 (5%) incubator at 37°C. CD18/CD11a- or CD18/CD11c-transfected L-cell lines were generous gifts from Y. van Kooyk (University Hospital, Nijmegen, The Netherlands) and were cultured in DMEM/F-12, 10% FCS, 50 U/mL penicillin, 50 μg/mL streptomycin, and 1 mg/mL G418. Chinese hamster ovary (CHO) cells expressing functionally active PSGL-1 have been described previously27 and were provided by R. G. Schaub and maintained in MEM-alpha medium, 10% FCS, 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM glutamine, 100 nM methotrexate, and 1 mg/mL G418. PMNs were freshly isolated by Ficoll-Paque (Amersham-Pharmacia, Uppsala, Sweden) density centrifugation from blood obtained from healthy volunteers. Erythrocytes were removed from the granulocyte fraction by ice-cold erythrocyte lysis buffer (0.155 mM NH4Cl, 7.4 mM KHCO3, and 0.1 mM EDTA (pH 7.4). Peripheral blood monocytes were isolated from the mononuclear cell fraction using CD14 microbeads and AutoMACS (Miltenyi Biotec, Bergisch-Gladbach, Germany). After isolation, cells were directly used for experiments. Cell purity was greater than 95% for PMNs and 90% for monocytes, as examined by CD15 and CD14 detection by flow cytometry analysis, respectively.
Static adhesion
Fibrinogen, BSA, or recombinant wild-type (WT) VWF, VWF/D′-D3, VWF/A1-A2-A3 (20 μg/mL) were immobilized in microtiter wells in Tris-buffered saline (TBS; pH 7.4) for 2 hours at 37°C and subsequently were blocked with 0.5% PVP for 2 hours at 37°C. In assays using PMNs, monocytes, or monocytic cell lines, cells were washed twice with PBS and subsequently incubated in DMEM/F-12 supplemented with 0.1% BSA/1 mM MnCl2/100 nM PMA for 15 minutes at a density of 2 × 106 cells/mL. CHO and L cells were resuspended in DMEM supplemented with 3% BSA to a similar cell density. Where indicated, cells were incubated for an additional 15 minutes in the presence of NIF (10 μg/mL). Cells (1 × 105/well) were added to protein-coated microtiter wells and incubated for either 60 minutes at 37°C (U937, CHO, L, and THP-1 cells) or 30 minutes at room temperature (PMNs and monocytes). Nonadherent cells were removed by gentle washing of wells with PBS. Cells were maintained in PBS, and images were acquired under light microscopy (Leitz Diaplan; Leica, Rijswijk, The Netherlands) using a 40×/1.00 numerical aperture objective lens, and computer-assisted image analysis was performed with Optimas 6.0 software (DVS, Breda, The Netherlands). Alternatively, adherent cells were determined by endogenous alkaline phosphatase activity with PNP as a substrate (3 mg/mL in 1% Triton-X100/50 mM acetic acid [pH 5]). Optical density (OD) was measured at 405 nm.
PMN adhesion under flow
Glass coverslips (0.17 mm × 60 mm × 24 mm) were coated with pd-VWF or wt-VWF (20 μg/mL in TBS) or with PVP (0.5% in PBS) for 2 hours at room temperature. After 1 rinse in TBS, coverslips were blocked by 15-minute incubation in 0.5% PVP/PBS. Before assembly into the flow chamber, coverslips were rinsed once more in DMEM/F-12 supplemented with 0.5% BSA. The flow chamber consisted of a rectangular cavity carved in an acrylic sheet (Plexiglas) block.28 Inner dimensions of the flow chamber were 0.2-mm depth, 29-mm length, and 5-mm width. Controlled flow rate was generated with an electric pump (PHD 2000; Harvard Apparatus, Les Ulis, France) using a 2-mL plastic syringe connected to the chamber through silicon tubings. PMNs were resuspended in DMEM/F-12 to a density of 5 × 105 cells/mL. Where indicated, cells were incubated with PMA (100 nM) for 15 minutes before perfusion. In inhibition experiments, an additional 15-minute incubation with NIF (10 μg/mL) was performed. All perfusions were executed at room temperature at a flow rate of 100 μL/min, corresponding to a wall shear rate of 50 s–1. The perfusion chamber was set on the stage of an inverted microscope (Axiovert 135; Carl Zeiss, Oberkochen, Germany) equipped with a 10 × Hoffman Modulation Contrast objective. A charge-coupled device camera (Sony, Tokyo, Japan) and a 20-inch monitor (PVM20N2E; Sony, Tokyo, Japan) allowed real-time visualization of flowing cells. Leukocyte interactions were visualized at 100 × magnification for 6 minutes and were recorded with a DVD recorder (RDR-GX7; Sony). Data were analyzed with Histolab 5.0.0 software (Microvision Instruments, Evry, France), allowing real-time analysis of transient and stable adhesion. Transient adhesion was defined as cells adhering for less than 3 seconds.
Surface plasmon resonance (SPR) analysis
SPR analysis was performed with a Biacore 2000 biosensor system (Biacore International Life Sciences, Uppsala, Sweden). Streptavidin sensor chips were loaded with biotinylated anti–Fcγ F(ab)2 fragments (Jackson ImmunoResearch Europe, Sirham, United Kingdom) until a density of 2.5 kRU was reached on 2 adjacent channels. The first of these channels (channel 1) was used as a control. Channel 2 was used to capture rPSGL–immunoglobulin fusion protein, which was passed at a concentration of 0.3 mg/mL in 100 mM NaCl, 0.005% Tween-20, 2.5 mM CaCl2, and 25 mM HEPES (pH 7.4) at a flow rate of 5 μL/min at 25°C to reach a density of 0.5 kRU. Subsequently, both channels were used for perfusion of the various proteins (wt-VWF, VWF/ΔA1, VWF/ΔA2, VWF/ΔA3, VWF/A1-A2-A3, VWF/D′-D3, botrocetin, VWF/botrocetin complex) at a flow rate of 5 μL/min in the same buffer. Binding to the rPSGL–immunoglobulin-coated channel was corrected for binding to the control channel (less than 5%). Regeneration was performed by perfusion with 50 mM EDTA/100 mM NaCl, 0.005% Tween-20, and 25 mM HEPES (pH 7.4) for 2 minutes. Channel 2 was reloaded with rPSGL–immunoglobulin after each regeneration step. Sensorgrams were analyzed with BIAevaluation software provided by the manufacturer (Biacore International Life Sciences).
Immunosorbent protein-binding assays
Recombinant wt-VWF was immobilized at indicated concentrations in microtiter wells in TBS for 2 hours at 37°C, and wells were then blocked with 3% BSA/0.1% Tween-20/TBS (pH 7.4) for 1 hour at 37°C. Subsequently, the GST/αM I-domain was diluted in TBS/2 mM MnCl2 and incubated with immobilized wt-VWF for 2 hours at 37°C. Binding of GST/αM I-domain to wt-VWF was detected using anti–αM I-domain antibody clone 44 (BD PharMingen) and a peroxidase-conjugated rabbit antimouse antibody (DakoCytomation, Glostrup, Denmark).
Statistical analysis
All data are expressed as mean ± SEM, unless stated otherwise. Between-group variations were examined with the Student t test. P values below .05 were considered statistically significant.
Results
Static adhesion of leukocyte subsets to immobilized VWF
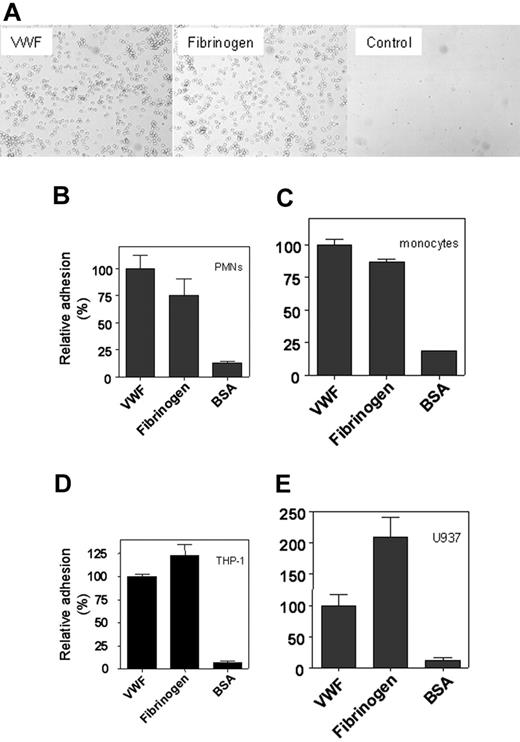
To investigate whether VWF was able to function as an adhesive surface for the various leukocyte subsets, the binding of PMNs and monocytes and the monocytic cell lines THP-1 and U937 to immobilized VWF was examined. For comparison, immobilized fibrinogen and BSA were used. When freshly isolated PMNs (5.0 × 105 cells/well) were added to microtiter wells coated with recombinant wt-VWF or fibrinogen, binding of cells was observed by light microscopy, whereas cells were virtually absent in noncoated or BSA-coated wells (Figure 1A). Similar results were obtained with monocytes and the monocytic cell lines THP-1 and U937 or when pd-VWF instead of recombinant wt-VWF was immobilized (not shown). Adhesion was quantified by measuring endogenous phosphatase activity of adhered cells. This revealed that PMNs, monocytes, and THP-1 cells adhered as efficiently to VWF as to fibrinogen, whereas U937 cells adhered 2-fold more efficiently to fibrinogen than to VWF (Figure 1B-E). Routinely, the number of adhered cells varied between 10% (U937 cells) and 40% (PMNs) of the total number of cells added (1.0 × 105 cells/well).
Static adhesion of leukocytes to immobilized VWF. (A) Freshly isolated PMNs (2 × 106 cells/mL) were incubated with PMA (100 nM) for 15 minutes and added to immobilized VWF, fibrinogen, or BSA at a concentration of 1 × 105 cells/well for 30 minutes at room temperature. After washing, adherent cells were visualized by light microscopy (original magnification, 400×). Freshly isolated PMNs (B), monocytes (C), THP-1 cells (D), and U937 cells (E) were stimulated with PMA for 15 minutes and added at a concentration of 1 × 105 cells/well to immobilized VWF, fibrinogen, or BSA for 30 minutes at room temperature (B-C) or for 60 minutes at 37°C (D-E). To quantify adhesion, bound cells were lysed using 1% Triton-X100 and 50 mM acetic acid (pH 5.0), and endogenous alkaline phosphatase activity was determined using PNP as a substrate. Relative adhesion is plotted with adhesion to VWF as a reference (100%) in each panel. The number of adhered cells was dependent on the cell type used and varied between 10% (U937 cells) and 40% (PMNs) of the total cells added. Data are corrected for adhesion to uncoated wells (less than 5% of VWF-coated wells) and represent the mean ± SEM of 3 to 8 experiments performed in duplicate.
Static adhesion of leukocytes to immobilized VWF. (A) Freshly isolated PMNs (2 × 106 cells/mL) were incubated with PMA (100 nM) for 15 minutes and added to immobilized VWF, fibrinogen, or BSA at a concentration of 1 × 105 cells/well for 30 minutes at room temperature. After washing, adherent cells were visualized by light microscopy (original magnification, 400×). Freshly isolated PMNs (B), monocytes (C), THP-1 cells (D), and U937 cells (E) were stimulated with PMA for 15 minutes and added at a concentration of 1 × 105 cells/well to immobilized VWF, fibrinogen, or BSA for 30 minutes at room temperature (B-C) or for 60 minutes at 37°C (D-E). To quantify adhesion, bound cells were lysed using 1% Triton-X100 and 50 mM acetic acid (pH 5.0), and endogenous alkaline phosphatase activity was determined using PNP as a substrate. Relative adhesion is plotted with adhesion to VWF as a reference (100%) in each panel. The number of adhered cells was dependent on the cell type used and varied between 10% (U937 cells) and 40% (PMNs) of the total cells added. Data are corrected for adhesion to uncoated wells (less than 5% of VWF-coated wells) and represent the mean ± SEM of 3 to 8 experiments performed in duplicate.
PMNs adhere to VWF under flow conditions
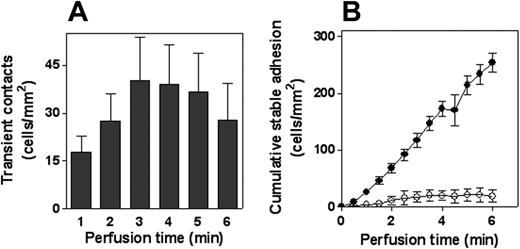
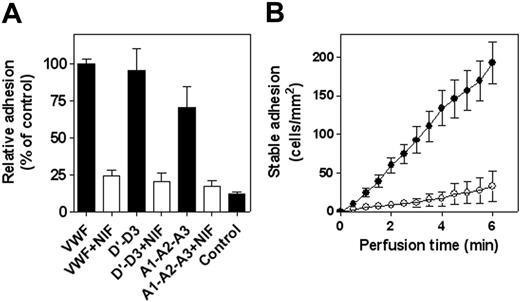
Adhesion of PMNs to VWF was examined in more detail under conditions of flow (shear rate, 50 s–1). Although little, if any, arrest of PMNs was observed to control coverslips during 6 minutes of perfusion (fewer than 2 cells/mm2; n = 7), distinct adhesion to immobilized recombinant wt-VWF was observed. Adhesion was predominated by short-lasting (less than 3 seconds) contact. Quantitative analysis revealed 31 ± 4 contacts/mm2 per minute (mean ± SEM) (Figure 2A and Videos S1 and S2, which are available on the Blood website; see the Supplemental Videos link at the top of the online article), resulting in a cumulative number of 188 ± 58 cells/mm2 of contacts during a 6-minute perfusion. A significant portion of the cells displayed stable adhesion, which added up to approximately 10% of the total contacts (19 ± 4 cells/mm2 [n = 7] after 6 minutes; Figure 2B). Similar data were obtained using coverslips coated with pd-VWF (not shown). The combination of short-lasting and stable contacts suggested that the adhesion process depended on the activation state of the leukocytes. This was further examined in experiments in which PMNs were stimulated with PMA for 15 minutes before perfusion. PMA stimulation resulted in similarly low background adhesion (fewer than 2 cells cells/mm2; n = 7), whereas firm arrest on VWF-coated coverslips was increased 10-fold after 6 minutes (255 ± 4 cells/mm2; n = 7; Figure 2B; Videos S1, S2). This increase in stable adhesion was accompanied by an almost complete lack of transient contacts. Furthermore, PMNs started to spread after adhesion. Thus, our data show that immobilized VWF provided an adhesive surface for leukocytes under static and flow conditions. Moreover, the adhesion process seemed to involve an initial step to decelerate the leukocytes and a secondary step that mediated firm adhesion.
Adhesion of PMNs to VWF under flow conditions. Freshly isolated PMNs (5 × 105 cells/mL) were perfused for 6 minutes over VWF-coated coverslips at a shear rate of 50 s–1. (A) Perfusion of nonstimulated PMNs was recorded for 6 minutes, and leukocyte interactions were analyzed to determine transient (less than 3 seconds) contact. Depicted are the numbers of contact for each minute. (B) Perfusion of nonstimulated (○) or PMA-stimulated (•) PMNs was recorded for 6 minutes and was analyzed to determine the amount of stable adherent cells. Shown is the cumulative number of stably adherent cells compared with time of perfusion. Data represent mean ± SEM of 7 to 8 experiments and are corrected for adhesion to PVP-coated coverslips (less than 2 cells/mm2 after 6 minutes).
Adhesion of PMNs to VWF under flow conditions. Freshly isolated PMNs (5 × 105 cells/mL) were perfused for 6 minutes over VWF-coated coverslips at a shear rate of 50 s–1. (A) Perfusion of nonstimulated PMNs was recorded for 6 minutes, and leukocyte interactions were analyzed to determine transient (less than 3 seconds) contact. Depicted are the numbers of contact for each minute. (B) Perfusion of nonstimulated (○) or PMA-stimulated (•) PMNs was recorded for 6 minutes and was analyzed to determine the amount of stable adherent cells. Shown is the cumulative number of stably adherent cells compared with time of perfusion. Data represent mean ± SEM of 7 to 8 experiments and are corrected for adhesion to PVP-coated coverslips (less than 2 cells/mm2 after 6 minutes).
VWF constitutes a binding site for PSGL-1
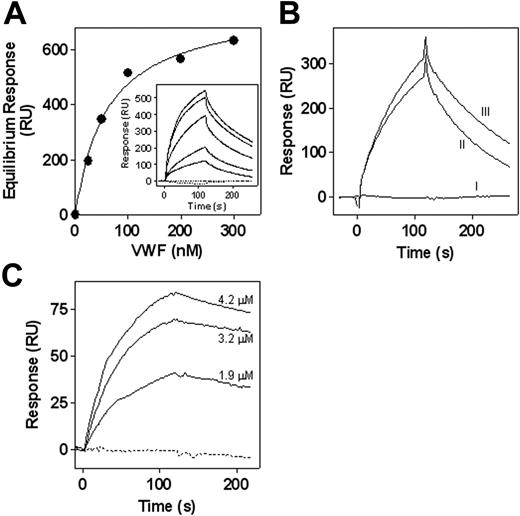
Primary contacts of leukocytes with the vascular wall are mediated by interactions between P- and E-selectins on the endothelial surface and their leukocyte counterreceptors, including PSGL-1. These interactions decelerate the cells and initiate intracellular signaling pathways that promote stable adhesion of the leukocyte. In view of the structural resemblance between the PSGL-1 and the VWF platelet receptor GpIbα, we considered the possibility that VWF was able to interact with PSGL-1. When subjected to SPR analysis, no association of soluble VWF to immobilized recombinant PSGL-1/immunoglobulin fusion protein (rPSGL–immunoglobulin) could be observed (Figure 3A, inset). Because binding of VWF to GpIbα required modulators such as botrocetin, we tested the binding of VWF to immobilized rPSGL–immunoglobulin in the presence of botrocetin. Although botrocetin itself was unable to associate to rPSGL–immunoglobulin, a dose-dependent binding of botrocetin-stimulated VWF (25-300 nM) to immobilized rPSGL–immunoglobulin could be observed (Figure 3A, inset). The response of the VWF/botrocetin complex at equilibrium was determined and plotted against the concentration applied (Figure 3A). Using the resultant binding isotherm, the apparent affinity constant was calculated to be 60 ± 10 nM. Given that the observed association was dependent on botrocetin, these data suggested that VWF binds to PSGL-1, as does GpIbα, in that it requires a conformational change within the VWF A1 domain. Indeed, VWF mutants with deletions of either the A2- or the A3-domain displayed normal botrocetin-dependent binding to rPSGL–immunoglobulin, whereas deletion of the A1-domain completely abolished this interaction (Figure 3B). These data do not exclude the possibility that binding to VWF opens a PSGL-1 binding site within botrocetin. Additional experiments were performed to examine whether VWF itself was able to bind PSGL-1. To this end, the recombinant truncated VWF fragment A1-A2-A3 was used and displayed spontaneous binding to GpIbα. Indeed, the isolated A1-A2-A3 fragment (1.9-4.2 μM) bound to immobilized rPSGL–immunoglobulin in the absence of any stimulators, whereas a control protein consisting of the D′-D3 domains was unable to interact with rPSGL–immunoglobulin (Figure 3C). It should be mentioned that binding of VWF/botrocetin or the A1-A2-A3 domains was completely inhibited in the presence of EDTA (not shown), suggesting that this interaction required the presence of metal ions.
Interaction of VWF with cell surface–exposed PSGL-1
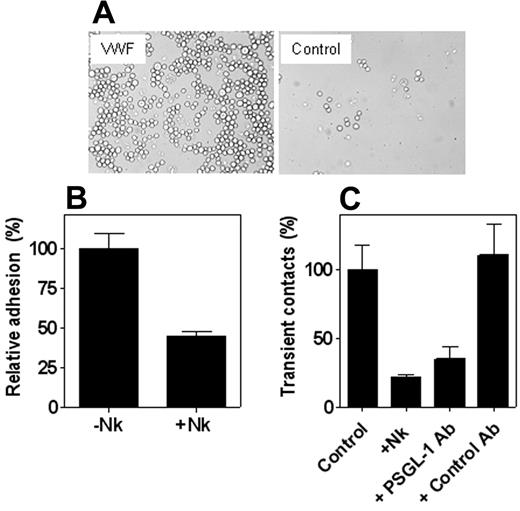
The interaction between VWF and PSGL-1 was further examined to investigate whether cell surface–expressed PSGL-1 was able to mediate adhesion to immobilized VWF. First, binding of PSGL-1–expressing CHO cells to immobilized VWF was tested in static adhesion assays. Visual inspection of the wells by light microscopy revealed that these cells bound to wells coated with VWF, whereas few cells were observed in control wells (Figure 4A). Quantification revealed that 15% of the added cells adhered to VWF. The specificity of this adhesion was examined by proteolytic removal of PSGL-1 from the cell surface with Nk-protease, a snake venom protease that cleaves the N-terminal part of PSGL-1. Preincubation of CHO-PSGL-1 cells with Nk-protease (5 μg/mL for 20 minutes at room temperature) reduced the amount of surface-exposed PSGL-1 by more than 80%, as assessed in flow cytometry experiments (not shown). This reduced PSGL-1 expression resulted in a significant (greater than 50%) reduction of adhesion to VWF (Figure 4B). A similar approach was used to test the contribution of PSGL-1 to the adhesion of PMNs to VWF under flow conditions. Freshly isolated PMNs were incubated with Nk-protease for 20 minutes. This procedure reduced PSGL-1 expression by more than 80%, whereas the expression of other leukocyte markers, such as β2-integrins, remained unaffected (not shown). Preincubation with Nk-protease was associated with 80% less transient contacts of nonstimulated PMNs to immobilized VWF (Figure 4C). In addition, preincubation of PMNs with the inhibiting monoclonal anti–PSGL-1 antibody KPL-1 was associated with a 65% reduction of transient contact, whereas no effect was observed in the presence of the isotype control antibody (Figure 4C). It should be noted that the decrease in transient contact resulted in a concordant decrease in the number of stably adhered cells (not shown). Taken together, these data support the possibility that PSGL-1 contributes to the initial step of adhesion of leukocytes to VWF.
Binding of VWF to rPSGL–immunoglobulin fusion protein. Biotinylated F(ab′)2 goat anti–human Fcγ antibodies were immobilized (2.5 kRU) onto 2 adjacent channels of a streptavidin-coated sensor chip. Purified recombinant rPSGL–immunoglobulin (0.3 mg/mL) was then applied to the second of these channels to reach a density of 0.5 kRU, whereas channel 1 was used as a reference. (A, inset) The various sensorgrams obtained with these channels are shown. Dotted lines indicate perfusion of recombinant wt-VWF (300 nM) or botrocetin (600 nM) alone. Solid lines represent sensorgrams of various concentrations of VWF/botrocetin complexes (25-300 nM). Complexes were obtained by incubating VWF with a 2-fold molar excess of botrocetin for 15 minutes before SPR analysis. (A) The response of the VWF/botrocetin complex at equilibrium was determined and plotted against the concentration applied. Using the resultant isotherm, the apparent affinity constant was calculated to be 60 ± 10 nM. (B) Various sensorgrams obtained through the perfusion of VWF deletion mutants (100 nM) complexed with botrocetin (200 nM). Line I, VWF/ΔA1. Line II, VWF/ΔA3. Line III, VWF/ΔA2. (C) Various concentrations of recombinant VWF/A1-A2-A3 fragment (0-4.2 μM; solid lines) or recombinant VWF/D′-D3 fragment (4.5 μM; dotted line) were passed over rPSGL–immunoglobulin, and resultant sensorgrams are depicted. Perfusions were performed in 100 mM NaCl, 0.005% Tween-20, 2.5 mM CaCl2, and 25 mM HEPES (pH 7.4) at a flow rate of 5 μL/min at 25°C. Association was allowed for 2 minutes, after which ligand solution was replaced by buffer. Dissociation was then followed for a 2-minute period. rPSGL–immunoglobulin dissociation during the association period was less than 5%. Before each ligand injection, channel 2 was reloaded with rPSGL–immunoglobulin to reach a density of 0.5 kRU.
Binding of VWF to rPSGL–immunoglobulin fusion protein. Biotinylated F(ab′)2 goat anti–human Fcγ antibodies were immobilized (2.5 kRU) onto 2 adjacent channels of a streptavidin-coated sensor chip. Purified recombinant rPSGL–immunoglobulin (0.3 mg/mL) was then applied to the second of these channels to reach a density of 0.5 kRU, whereas channel 1 was used as a reference. (A, inset) The various sensorgrams obtained with these channels are shown. Dotted lines indicate perfusion of recombinant wt-VWF (300 nM) or botrocetin (600 nM) alone. Solid lines represent sensorgrams of various concentrations of VWF/botrocetin complexes (25-300 nM). Complexes were obtained by incubating VWF with a 2-fold molar excess of botrocetin for 15 minutes before SPR analysis. (A) The response of the VWF/botrocetin complex at equilibrium was determined and plotted against the concentration applied. Using the resultant isotherm, the apparent affinity constant was calculated to be 60 ± 10 nM. (B) Various sensorgrams obtained through the perfusion of VWF deletion mutants (100 nM) complexed with botrocetin (200 nM). Line I, VWF/ΔA1. Line II, VWF/ΔA3. Line III, VWF/ΔA2. (C) Various concentrations of recombinant VWF/A1-A2-A3 fragment (0-4.2 μM; solid lines) or recombinant VWF/D′-D3 fragment (4.5 μM; dotted line) were passed over rPSGL–immunoglobulin, and resultant sensorgrams are depicted. Perfusions were performed in 100 mM NaCl, 0.005% Tween-20, 2.5 mM CaCl2, and 25 mM HEPES (pH 7.4) at a flow rate of 5 μL/min at 25°C. Association was allowed for 2 minutes, after which ligand solution was replaced by buffer. Dissociation was then followed for a 2-minute period. rPSGL–immunoglobulin dissociation during the association period was less than 5%. Before each ligand injection, channel 2 was reloaded with rPSGL–immunoglobulin to reach a density of 0.5 kRU.
PSGL-1–dependent adhesion to VWF. (A) CHO cells transfected to express functionally active PSGL-1 (1 × 105 cells/mL) were added to wells coated with VWF or BSA and incubated for 60 minutes at 37°C. After washing, adherent cells were visualized by light microscopy (original magnification, 400 ×). (B) CHO cells expressing PSGL-1 were incubated in the absence or presence of Nk-protease (5 μg/mL) for 20 minutes at room temperature and subsequently were added to immobilized VWF. After 60 minutes, wells were gently washed, bound cells were lysed with 1% Triton-X100/50 mM acetic acid (pH 5.0), and endogenous alkaline phosphatase activity was determined with PNP as a substrate. Relative adhesion was plotted with adhesion to VWF as a reference (100%, representing 15% of the total added cells). Data are corrected for adhesion to uncoated wells (less than 15% of VWF-coated wells) and represent the mean ± SEM of 3 experiments performed in duplicate. (C) Freshly isolated, nonstimulated PMNs were incubated in the absence or presence of Nk-protease (5 μg/mL) or antibody (10 μg/mL) for 20 minutes at room temperature. Cells (5 × 105 cells/mL) were then perfused over VWF-coated coverslips at a shear rate of 50 s–1. The amount of transient (less than 3 seconds) contact was determined by videoimaging analysis. Bars represent the relative numbers of transient contact (cumulative after 6 minutes) using non–Nk-treated PMNs as a reference (100%). Data are corrected for adhesion to PVP-coated coverslips (less than 2 cells/mm2 after 6 minutes) and represent the mean ± SEM of 5 experiments performed in duplicate.
PSGL-1–dependent adhesion to VWF. (A) CHO cells transfected to express functionally active PSGL-1 (1 × 105 cells/mL) were added to wells coated with VWF or BSA and incubated for 60 minutes at 37°C. After washing, adherent cells were visualized by light microscopy (original magnification, 400 ×). (B) CHO cells expressing PSGL-1 were incubated in the absence or presence of Nk-protease (5 μg/mL) for 20 minutes at room temperature and subsequently were added to immobilized VWF. After 60 minutes, wells were gently washed, bound cells were lysed with 1% Triton-X100/50 mM acetic acid (pH 5.0), and endogenous alkaline phosphatase activity was determined with PNP as a substrate. Relative adhesion was plotted with adhesion to VWF as a reference (100%, representing 15% of the total added cells). Data are corrected for adhesion to uncoated wells (less than 15% of VWF-coated wells) and represent the mean ± SEM of 3 experiments performed in duplicate. (C) Freshly isolated, nonstimulated PMNs were incubated in the absence or presence of Nk-protease (5 μg/mL) or antibody (10 μg/mL) for 20 minutes at room temperature. Cells (5 × 105 cells/mL) were then perfused over VWF-coated coverslips at a shear rate of 50 s–1. The amount of transient (less than 3 seconds) contact was determined by videoimaging analysis. Bars represent the relative numbers of transient contact (cumulative after 6 minutes) using non–Nk-treated PMNs as a reference (100%). Data are corrected for adhesion to PVP-coated coverslips (less than 2 cells/mm2 after 6 minutes) and represent the mean ± SEM of 5 experiments performed in duplicate.
Stable adhesion involves VWF/D′-D3 and A1-A2-A3 domains and leukocyte β2-integrins
Although PSGL-1 contributes to the initial step of leukocyte adhesion, the leukocyte-specific β2-integrin family is essential for the stable adhesion of these cells. The contribution of β2-integrins to the stable adhesion of PMNs to VWF was first tested in static adhesion assays with the β2-integrin inhibitor NIF. NIF is known to interfere with adhesion mediated by the various β2-integrin isotypes: αLβ2, αMβ2, and αXβ2.5,29 The presence of NIF (10 μg/mL) reduced the static adhesion of PMNs to immobilized wt-VWF by 75% ± 4% (Figure 5A), indicating that β2-integrins indeed contribute to the VWF-leukocyte interaction. To determine the location of the VWF regions involved in interaction with these integrins, we also tested adhesion to 2 separate recombinant VWF fragments, the monomeric A1-A2-A3 fragment and the dimeric D′-D3 fragment. As depicted in Figure 5A, both VWF fragments were as efficient as the full-length protein in acting as an adhesive surface for PMNs. Moreover, adhesion to both fragments was inhibited in the presence of NIF (Figure 5A). We further examined whether NIF also affected adhesion of PMNs under conditions of flow. These experiments revealed that in the presence of this β2-integrin inhibitor, the amount of stable adherent cells was reduced by more than 80% (Figure 5B). These data strongly support the possibility that β2-integrins indeed contribute to the interaction with VWF and that multiple regions in VWF comprise binding sites for these integrins.
VWF interacts with various β2-integrin isotypes
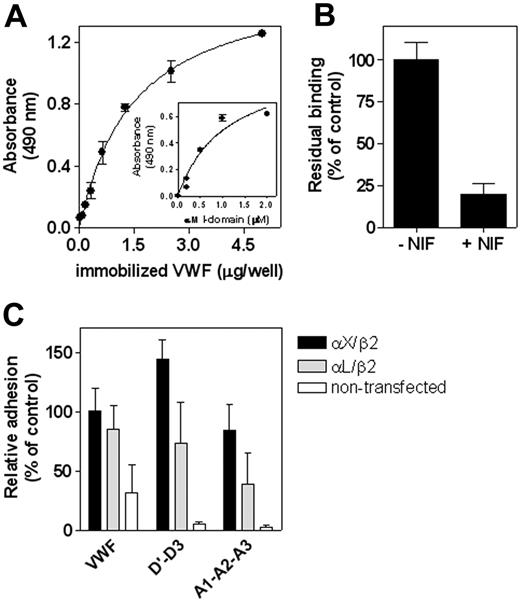
To obtain further support for the possibility that β2-integrins interact with VWF, a number of experiments were performed. First, the interaction with VWF was addressed in a system using purified components. The recombinant fusion protein GST/αM I-domain (1 μM) was incubated with immobilized wt-VWF (0-5 μg/well), and bound I-domain was monitored with an anti-αM antibody. This incubation resulted in a dose-dependent binding of GST/αM I-domain to VWF (Figure 6A). Similar dose dependency was observed when GST/αM I-domain concentrations were varied (0-2 μM) and wt-VWF was immobilized at a fixed concentration (1 μg/mL; Figure 6A, inset). This interaction could be inhibited by more than 80% in the presence of NIF (10 μg/mL; Figure 6B). In a second approach, we incubated fibroblastic L cells expressing the αLβ2 or αXβ2 integrin complex with immobilized full-length wt-VWF and with the truncated D′-D3 and A1-A2-A3 domain fragments. As illustrated in Figure 6C, control L cells displayed little adhesion to each of the fragments, whereas either αLβ2– or αXβ2–expressing L cells adhered efficiently to the immobilized proteins. In conclusion, our findings showed that both the D′-D3 and the A1-A2-A3 domains of VWF contained binding sites for the various β2-integrin complexes.
NIF inhibits PMN adhesion to VWF and its derivatives. Freshly isolated PMNs were stimulated with PMA (100 nM) for 15 minutes, followed by an additional 15-minute incubation in the absence or presence of NIF (10 μg/mL). (A) Static adhesion of PMNs to immobilized VWF, VWF/D′-D3, or VWF/A1-A2-A3 was performed and quantified as described in the Figure 1 legend. Relative adhesion is plotted with adhesion to VWF as a reference (100%) in each panel. Control represents adhesion to BSA-coated wells. Data represent mean ± SEM of 4 experiments performed in duplicate. (B) Perfusion of PMA-stimulated PMNs to immobilized VWF was performed and analyzed as described in the Figure 2B legend. Cumulative number of adherent cells was plotted in the absence (○) or presence (•) of NIF (10 μg/mL). Data represent mean ± SEM of 4 experiments and are corrected for adhesion to PVP-coated coverslips (less than 2 cells/mm2 after 6 minutes).
NIF inhibits PMN adhesion to VWF and its derivatives. Freshly isolated PMNs were stimulated with PMA (100 nM) for 15 minutes, followed by an additional 15-minute incubation in the absence or presence of NIF (10 μg/mL). (A) Static adhesion of PMNs to immobilized VWF, VWF/D′-D3, or VWF/A1-A2-A3 was performed and quantified as described in the Figure 1 legend. Relative adhesion is plotted with adhesion to VWF as a reference (100%) in each panel. Control represents adhesion to BSA-coated wells. Data represent mean ± SEM of 4 experiments performed in duplicate. (B) Perfusion of PMA-stimulated PMNs to immobilized VWF was performed and analyzed as described in the Figure 2B legend. Cumulative number of adherent cells was plotted in the absence (○) or presence (•) of NIF (10 μg/mL). Data represent mean ± SEM of 4 experiments and are corrected for adhesion to PVP-coated coverslips (less than 2 cells/mm2 after 6 minutes).
Interaction between VWF and β2-integrin isotypes. (A) Various concentrations of wt-VWF (0-5 μg/well) were immobilized in microtiter wells (2 hours at 37°C) and were then blocked with 3% BSA/0.1% Tween-20/TBS (pH 7.4) for 1 hour at 37°C. Subsequently, immobilized VWF was incubated with GST/αM I-domain (1 μM) in 2 mM Mn2+/TBS for 2 hours at 37°C. Bound GST/αM I-domain was detected using a monoclonal anti-αM antibody. Absorbance versus concentration of immobilized VWF was plotted (inset). Binding of various concentrations of GST/αM I-domain (0-2 μM) to immobilized VWF (1 μg/well) was determined, as described. (B) Binding of GST/αM I-domain (1 μM) to immobilized VWF (1 μg/mL) in the absence or presence of NIF (10 μg/mL) was assessed, as described for panel A. Relative binding was plotted with binding in the absence of NIF as a reference (100%). (C) Transfected fibroblastic L cells expressing αXβ2 or αLβ2 and their nontransfected counterparts (1 × 105 cells/well) were incubated with immobilized wt-VWF, VWF/D′-D3, or VWF/A1-A2-A3 for 60 minutes at 37°C. Wells were gently washed, and adhesion was quantified, as described in the Figure 1 legend. Relative adhesion was plotted with adhesion of αXβ2-expressing L cells to wt-VWF as a reference (100%). Data represent mean ± SEM of 3 to 6 experiments performed in duplicate.
Interaction between VWF and β2-integrin isotypes. (A) Various concentrations of wt-VWF (0-5 μg/well) were immobilized in microtiter wells (2 hours at 37°C) and were then blocked with 3% BSA/0.1% Tween-20/TBS (pH 7.4) for 1 hour at 37°C. Subsequently, immobilized VWF was incubated with GST/αM I-domain (1 μM) in 2 mM Mn2+/TBS for 2 hours at 37°C. Bound GST/αM I-domain was detected using a monoclonal anti-αM antibody. Absorbance versus concentration of immobilized VWF was plotted (inset). Binding of various concentrations of GST/αM I-domain (0-2 μM) to immobilized VWF (1 μg/well) was determined, as described. (B) Binding of GST/αM I-domain (1 μM) to immobilized VWF (1 μg/mL) in the absence or presence of NIF (10 μg/mL) was assessed, as described for panel A. Relative binding was plotted with binding in the absence of NIF as a reference (100%). (C) Transfected fibroblastic L cells expressing αXβ2 or αLβ2 and their nontransfected counterparts (1 × 105 cells/well) were incubated with immobilized wt-VWF, VWF/D′-D3, or VWF/A1-A2-A3 for 60 minutes at 37°C. Wells were gently washed, and adhesion was quantified, as described in the Figure 1 legend. Relative adhesion was plotted with adhesion of αXβ2-expressing L cells to wt-VWF as a reference (100%). Data represent mean ± SEM of 3 to 6 experiments performed in duplicate.
Discussion
The recruitment of platelets to sites of vascular injury to arrest bleeding and the recruitment of leukocytes to sites of inflammation to combat pathogens have traditionally been considered to involve separate cellular adhesive pathways. However, it has become apparent that these pathways are intricately linked by the adhesion molecules P-selectin, PSGL-1, and GpIbα.30 In the present study, we have identified a new link between the hemostatic and the inflammatory systems by establishing VWF as an adhesive component for PMNs and monocytes. Adhesion of these inflammatory cells involves various regions within the VWF molecule and is mediated by concerted actions of the PSGL-1 and β2-integrins.
The identification of PSGL-1 as a leukocyte receptor for VWF may seem surprising because VWF shares little homology with other PSGL-1–binding proteins. To date, few other proteins have been identified that are able to interact with PSGL-1. These obviously include P-, L-, and E-selectins,2 but they also include the human chemokine CCL27, the mouse chemokine KC (a homolog of human chemokine CXCL1), and the extracellular matrix proteoglycan PG-M/versican, which have been identified as counterreceptors for PSGL-1.31-33 However, the structure of PSGL-1 resembles that of the VWF platelet receptor GpIbα in that both single-membrane proteins contain a heavily glycosylated extracellular stack and an amino terminal cluster of sulfated tyrosines.30 The presence of these sulfated tyrosines is necessary for the optimal function of GpIbα and PSGL-1.34-38 Proteolytic treatment with the Nk-protease results in removal of the N-terminal part of PSGL-1, which includes these sulfated residues. Because this cleavage abolishes VWF binding (Figure 4), it seems conceivable that the sulfated tyrosines contribute to the interaction between PSGL-1 and VWF.
The physiological consequence of the P-selectin/PSGL-1 interaction is not only that resting leukocytes are brought into proximity with the activated endothelium but also that signaling pathways are initiated that convert leukocytes from a low-adhesive to a high-adhesive state.3 It is tempting to speculate that formation of the VWF/PSGL-1 complex exerts a similar effect. Stable adhesion of leukocyte involves β2-integrins, and we have obtained several lines of evidence in support of the involvement of these integrins in leukocyte adhesion to VWF (Figures 5, 6). The observation that VWF interacts with β2-integrins is in line with a previous report by Koivunen et al.18 It was shown that the monocytic THP-1 cell line adhered to VWF and that adhesion could be blocked by an anti–β2-integrin antibody or by β2-integrin–directed peptides. They further proposed that binding of β2-integrins to VWF could be mediated by 2 leucine-leucine-glycine motifs located in the VWF D3-domain (residues 965-967) and the connecting region between the A1 and A2 domains (residues1482-1484). The presence of distinct interactive sites for β2-integrins could be confirmed using truncated recombinant VWF fragments (Figures 5, 6). However, mutagenesis of the leucine-leucine-glycine motifs did not affect adhesion of PMNs or monocytes to VWF (data not shown), suggesting that these tripeptide motifs have a minor role in the interaction between VWF and β2-integrins expressed on blood-borne leukocytes.
VWF displayed binding to the purified I-domain of the αM-subunit (Figure 6), indicating that the I-domains of the α-subunits may contribute to the interaction with VWF. Indeed, NIF exerts its inhibitory capacity by interacting with the I-domain of the various subunits.5,29 The observation that VWF interacts with at least 3 isotypes (Figure 6) may point to a homologous structure in the subunits that mediates binding to VWF. In this regard, VWF is similar to ICAM-1, which is also able to interact with the same 3 isotypes.4 In contrast, most other ligands display a more specific interaction for 1 or 2 of the subtypes, such as GpIbα, which binds to αMβ2 but not to αLβ2.8,39
By identifying VWF as a ligand for PSGL-1 and β2-integrins, the network of adhesive interactions in the recruitment of leukocytes is becoming more intriguing. At the same time, it is interesting to consider how and when VWF would contribute to this process. We found that VWF is less efficient than P-selectin in mediating leukocyte rolling, as is illustrated by 3- to 6-fold more transient contact of PMNs on P-selectin–coated coverslips compared with VWF-coated coverslips (not shown). It seems unlikely, therefore, that regular leukocyte rolling on endothelium is mediated by VWF, as emphasized by the notion that this phenomenon is missing in P-selectin–deficient mice.40 That VWF is abundantly present in an active conformation in platelet-rich thrombi may point to a role of VWF in supporting the infiltration of leukocytes into these thrombi. P-selectin is relatively rapidly shed from activated platelets, leaving platelet-bound VWF as an alternative docking point for PSGL-1–expressing leukocytes. Within the platelet-rich thrombus, VWF may further facilitate leukocyte recruitment through its β2-integrin binding capacities. As such, VWF is complementary to a number of other β2-integrin ligands that are present in these thrombi, such as junction-adhesion molecule-3 and GpIbα. The fact that various ligands are present at the same time and place acknowledges the redundancy of a system that promotes leukocyte infiltration.
In conclusion, our data show that VWF has the capacity to act as an adhesive surface for various leukocyte subsets (PMNs, monocytes). Analogous with the VWF-platelet interaction, VWF provides binding sites for leukocyte receptors in rolling (PSGL-1) and stable adhesion (β2-integrins). VWF is unique in its intrinsic capacity to combine the rolling and stable adhesion steps in interaction with leukocytes.
Authorship
P.J.L. and C.V.D. conceived and designed the study, analyzed the data, and wrote the manuscript. R.P. and V.T. performed the research, analyzed the data, and approved the final manuscript. C.G.G. contributed reagents, revised the draft, and approved the final manuscript. O.D.C. and P.G.d.G. designed the study, revised the draft, and approved the final manuscript.
The authors declare no competing financial interests.
R.P. and V.T. contributed equally to this study.
Prepublished online as Blood First Edition Paper, August 22, 2006; DOI 10.1182/blood-2006-03-010322.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This study was supported by a European network grant (HPRN-CT-2002-00253), an INSERM–Netherlands Organization for Scientific Research/Netherlands Organization for Health Research and Development (NWO/ZonMW) exchange grant (910-48-603), a Groupe d'Etude sur l'Hémostase et la Thrombose (GEHT) grant, and the Academy of Finland.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal