Abstract
Chemokines play a role in regulating hematopoietic stem cell function, including migration, proliferation, and retention. We investigated the involvement of CCL18 in the regulation of bone marrow hematopoiesis. Treatment of human long-term bone marrow cultures (LTBMCs) with CCL18 resulted in significant stimulation of hematopoiesis, as measured by the total number of hematopoietic cells and their committed progenitors produced in culture. Monocytes/macrophages, whose survival was almost doubled in the presence of CCL18 compared with controls, were the primary cells mediating this effect. Conditioned media from CCL18-treated mature monocytes fostered colony-promoting activity that increased the number of colonies formed by hematopoietic progenitor cells. Gene expression profiling of CCL18-stimulated monocytes demonstrated more than 200 differentially expressed genes, including those regulating apoptosis (caspase-8) and proliferation (IL-6, IL-15, stem cell factor [SCF]). Up-regulation of these cytokines was confirmed on the protein expression level. The contribution of SCF and IL-6 in CCL18-mediated stimulatory activity for hematopoiesis was confirmed by SCF- and IL-6–blocking antibodies that significantly inhibited the colony-promoting activity of CCL18-stimulated conditioned medium. In addition to the effect on monocytes, CCL18 facilitated the formation of the adherent layer in LTBMCs and increased the proliferation of stromal fibroblast-like cells.
Introduction
Pulmonary and activation-regulated chemokine (CCL18/PARC)1 —also referred to as DC-CK-1,2 AMAC-1, and MIP-4—is a member of the CC-chemokine family most closely related to MIP-1α and with which it shares 61% sequence identity. However, it does not activate the same receptors as does MIP-1α, which is a ligand for both CCR1 and CCR5. Thus far, the identity of the CCL18 receptor has not been elucidated, though CCL18 is a noncompetitive inhibitor for CCL11/eotaxin binding to the CCR3.3
In spite of various reports suggesting a role for CCL18 in various chronic, primarily TH2-mediated diseases,4-8 assessing its function experimentally has been difficult because it appears to be present in primates only, obviating small animal models.
In vitro findings show that CCL18 is chemotactic for lymphocytes,1 particularly naive T cells2 and B cells,9 and for immature dendritic cells.10 Although freshly isolated monocytes do not respond to CCL18, maturing monocytes/macrophages cultured for 2 to 4 days acquire transient responsiveness to CCL18.11 More unusually for a chemokine, CCL18 activates fibroblasts, leading to increased proliferation and collagen production by these cells.12
CCL18 is produced by a number of leukocytes. It has been detected in high concentrations in the lung, especially in alveolar macrophages,1 and in lower concentrations in various lymphatic tissues,1 dendritic cells,2 adherent monocytes, and eosinophils.11 Although CCL18 expression has been demonstrated in bone marrow,1,11 the role of CCL18 in the regulation of bone marrow hematopoiesis is largely unknown; however, a negative effect of CCL18 on hematopoietic progenitors cultured in the presence of optimal growth supplements has been observed.13
Precedence exists for the involvement of chemokines in the regulation of hematopoietic stem/progenitor cell (HSPC) behavior, notably for SDF-1/CXCL12, which regulates the proliferation, extravasation, and retention of HSPCs within the bone marrow microenvironment.14-16 Like stromal cell–derived factor-1 (SDF-1), CCL18 is present in high concentrations in plasma. According to published accounts17 and our own ELISA determinations, plasma concentrations of CCL18 can be as high as approximately 20 ng/mL, or 2.5 nM, and one report claims even higher concentrations (approximately 80 ng/mL).18 In certain pathologic conditions, such as in childhood acute lymphoblastic leukemia, plasma CCL18 concentrations can increase considerably.17
Here we demonstrate that CCL18 induces the expression of factors in mature monocytes—among them stem cell factor (SCF), IL-15, and IL-6—that stimulate the proliferation of committed hematopoietic progenitors in vitro. Thus, CCL18 contributes to the structure of the bone marrow hematopoietic niche by regulating the hematopoiesis-supportive function of mature bone marrow monocytes.
Materials and methods
Reagents
Recombinant CCL18 was purified as previously described, with minor modifications.11 It was expressed with the plasmid pGEX5X-3 (Amersham, Piscataway, NJ) in Escherichia coli strain DH5α as a chimera consisting of glutathione-S-transferase, the major acidic polypeptide (MAP) of eosinophil pro–major basic protein, followed by CCL18. After induction by 0.1 M IPTG, the cells were harvested and lysed, and the chimera was purified on glutathione-Sepharose.19 A thrombin-susceptible bond was engineered between MAP and CCL18, allowing the release of CCL18 after digestion by thrombin (1:1000 ratio of weight of enzyme to substrate) for 15 hours at 23°C. CCL18 could then be purified by passage through DEAE–Sephacel (GE Healthcare Life Sciences, Giles, Bucks, United Kingdom) followed by chromatography with CM–Sephadex (GE Healthcare Life Sciences). Commercial CCL18 was obtained from R&D Systems (Minneapolis, MN). CCL18 was heated to 70°C for 30 minutes to achieve heat inactivation. Endotoxin was determined with the limulus amebocyte assay (Cambrex, East Rutherford, NJ) and measured less than 0.1 ng/μg protein in all CCL18 preparations used.
Mouse anti–CD14-FITC was purchased from BD Biosciences (San Diego, CA), anti–IL-6 and anti-SCF Abs were obtained from PeproTech (Rocky Hill, NJ), and anti–IL-15 was obtained from R&D Systems.
Cell isolation and culture
Monocytes were purified from fresh human blood by Ficoll and Percoll gradient centrifugation followed by adhesion to tissue culture plastic, as previously described,11,20 and they were cultured in RPMI 1640 containing 10% FCS. CCL18-conditioned medium was obtained by the addition of 40 nM CCL18 to monocyte cultures for 20 hours on day 3 in the presence of serum-free RPMI 1640. For RNA isolation and detection of surface-bound IL-15, cells were stimulated with CCL18 for 3.5 and 4 hours, respectively.
Monocyte adhesion was determined as described.21 In short, microtiter plates (96-well; Nunc, Rochester, NY) were coated with fibronectin or vitronectin (each at 50 μg/mL) and were blocked with 0.5% human serum albumin (HSA). Cultured monocytes were fluorescence labeled with 1 μg/mL calceinAM reagent (Molecular Probes, Eugene, OR), treated with enzyme-free cell dissociation buffer on ice, centrifuged, and allowed to settle on ice in the wells of the coated plate in Hanks balanced salt solution containing 10 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, and 0.5% HSA (5 × 104 cells/well). CCL18 (20 and 100 nM) was added, and the plate was incubated for 20 minutes at 37°C. To remove nonadherent cells, the plate was centrifuged upside down, and the fluorescence of adherent cells was detected on a fluorescence plate reader (Packard Fluorocount; Packard Bioscience, Meriden, CT). Results are expressed relative to nonstimulated cells.
Phase-contrast images were taken through a DM IRBE microscope (Leica, Wetzlar, Germany) with a Hamamatsu (Hamamatsu City, Japan) camera, processed with OpenLab Improvision (Basel, Switzerland) software, and transferred to Photoshop (Adobe Systems, San Jose, CA) TIFF files.
Long-term bone marrow cultures
Myeloid long-term bone marrow cultures (LTBMCs) were established as described.16 In brief, human bone marrow cells (106 cells/mL; Cambrex) were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 20% horse serum (StemCell Technologies, Vancouver, BC, Canada) and 10–6 M hydrocortisone (Sigma, St Louis, MO) in 6-well plates at 37°C in a humid atmosphere containing 5% CO2. Cultures were fed weekly by exchange of half the culture medium. Nonadherent cells were collected from the culture medium, counted, and assayed for colony-forming units (CFUs). Viability of cells was monitored by trypan blue exclusion and was greater than 99% in all experiments.
CFU assay
Human bone marrow cells or nonadherent cells from LTBMCs were plated at a concentration of 104 cells/mL on semisolid methylcellulose supplemented with 30% FCS, 1% bovine serum albumin (BSA), 10–4 M 2-mercaptoethanol, 2 mM l-glutamine, 200 μg/mL human transferrin, 50 ng/mL SCF, 20 ng/mL IL-6, 20 ng/mL IL-3, 3 U/mL erythropoietin, 20 ng/mL G-CSF, and 20 ng/mL GM-CSF (StemCell Technologies). Plates were cultured at 37°C in 5% CO2 atmosphere. Colonies were counted under the microscope after 7 days of culture.
In some experiments, CFUs were determined using human bone marrow cells (104/mL) or the mononuclear fraction of peripheral blood (2 × 105/mL) in methylcellulose medium (Stem Cell Technologies). These cultures were set up in the presence or absence of 40 nM CCL18 or with a 1:10 dilution of monocyte-conditioned media from unstimulated monocytes or from cells stimulated with 40 nM CCL18.
FACS analysis
FACS detection of IL-15 followed a general FACS protocol, as previously described, using anti–human IL-15 antibody (PeproTech) and PE-conjugated anti–rabbit IgG as the secondary antibody (Upstate, Charlottesville, VA). FITC-stained antibodies against various leukocytic cell surface markers were used to identify specific cell populations. FACS analysis was performed on a FACScan (BD Biosciences) with CellQuest Pro (BD Biosciences) software.
For the detection of apoptosis of monocytes, 3-day-old monocytes were incubated in full media in the presence or absence of 40 nM CCL18. Twenty hours later, the cells were harvested with a cell scraper, and an annexin V–propidium iodide staining kit (Calbiochem, La Jolla, CA) and FACS analysis were used to detect live cells (unstained), early apoptotic cells (annexin V positive), or late apoptotic cells (annexin V and propidium iodide positive).
Gene array analysis and RT-PCR
Total RNA was isolated from monocytes with or without CCL18 using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's instructions, ethanol precipitated, and dried. RNA (5-10 μg) was resuspended in DEPC-treated water, and oligo-dT18VN primer (6 μg) was added. The solution was denatured at 70°C for 10 minutes and cooled on ice. Subsequently, 7 μL of 5 × first-strand buffer (Invitrogen, Carlsbad, CA), 0.5 μL 25 mM dNTP (dCTP only, 10 mM), 3 μL of 0.1 M DTT, 2 μL SuperScript (Invitrogen), 1 μL RNAsin (Promega, Madison, WI), and 3 μL Cy-Dye–labeled dCTP (Amersham) were added on ice. The reaction was incubated for 1 hour at 42°C, and 1 μL Superscript was added, followed by another hour of incubation at 42°C. NaOH (3 μL, 0.1 N) was added to hydrolyze the RNA. After a 15-minute incubation at 70°C, the reaction was neutralized with 3 μL 0.1 N HCl. Finally, the labeled cDNA was purified with QiaQuick PCR purification columns (Qiagen, Valencia, CA).
cDNA micoarrays representing 42 000 genes were obtained from the Stanford Functional Genomics Facility. Prehybridization was performed with a solution of 25% formamide, 5 × SSC, 0.1% SDS, and 0.1 mg/mL BSA at 42°C for 45 minutes. After washing the slides twice in water and drying them by centrifugation, labeled cDNAs were hybridized to the array in a solution of 25% formamide, 5 × SSC, 0.1% SDS, and 33 μg/mL each of poly-dT and Cot-1-DNA to block unspecific binding. The probe was heated to 95°C for 5 minutes to denature the cDNA before it was added to the arrays. Slides were covered with a coverslip and incubated overnight at 42°C. Washes were performed as follows: 2 × SSC, 0.1% SDS at 42°C for 5 minutes, 0.1 × SSC, 0.1% SDS at room temperature for 10 minutes, and 0.1 × SSC for 1 minute (4 times). Then the slides were rinsed in 0.01 × SSC and in water. Finally, slides were spun down and scanned in a ScanArray Express (PerkinElmer, Wellesley, MA) microarray scanner. Analysis was performed using the Limma package (R project, http://www.r-project.org/).
For semiquantitative RT-PCR, RNA was reverse transcribed using the Omniscript (Qiagen) reverse transcription kit. PCR was performed with the following primers: forward 5′-AACTGGGTGAATGTAATAAGTGATTTG-3′ and reverse 5′-TCAAGAAGTGTTGATGAACATTTGGA-3′ for IL-15; forward 5′-TTTAGAATTTTTAATAGATCCATTG-3′ and reverse 5′-TTACACTTCTTGAAACTCTCTCTC-3′ for SCF; forward 5′-AACTACCAGAAAGGTATACCTGTTG-3′ and reverse 5′-TCAATCAGAAGGGAAGACAAGTTT-3′ for caspase-8; and forward 5′-GAGCTGCCCGATGGCCAGGTGATGACC-3′ and reverse 5′-TTAGAAGCATTTGCGGTGGACGATGGA-3′ for β-actin. Amplification was performed for 25 cycles for actin, 30 cycles for IL-15 and caspase-8, and 45 cycles for SCF.
Cytokine/chemokine protein detection
To determine the effect of CCL18 on cytokine production by monocytes, 3-day-old monocytes were stimulated with 40 nM CCL18 for 20 hours in serum-free conditions. Protein cytokine arrays that detected 23 or 40 cytokines/chemokines simultaneously (CytokineArray I and Inflammation III; RayBiotech, Norcross, GA) were used, in accordance with the manufacturer's protocol, to detect changes in cytokine expression in these monocytic supernatants. Concentrations of IL-6 and MIP-1α were quantified with commercial ELISA kits (BioLegend, San Diego, CA; R&D Systems).
Results
CCL18 stimulates hematopoiesis in human LTBMCs
To determine the effect of exposure to CCL18 on bone marrow hematopoiesis, myeloid LTBMCs were established from human bone marrow. Numbers of nonadherent cells and hematopoietic progenitors were significantly higher in CCL18-treated LTB-MCs during culture than in untreated LTBMCs or in heat-inactivated CCL18-treated LTBMCs (Figure 1A-B), and the effect of CCL18 on hematopoietic activity in LTBMCs showed nearly full activity at 10 nM (Figure 1C). Commercial CCL18 gave similar results. CCL18 stimulated the formation of foci of active hematopoiesis (cobblestone areas) within the adherent layers of LTBMCs (Figure 1D). In cultures treated with heat-inactivated CCL18, the size of cobblestone areas was not different from that of controls, suggesting that CCL18 stimulated hematopoiesis in LTBMCs.
To determine whether CCL18 directly stimulated the proliferation of committed progenitors, it was added in methylcellulose cultures of progenitor cells supplemented with lineage-specific cytokines. In the presence of CCL18, the number of colonies was moderately decreased (Figure 1E), consistent with previous findings.12 No effect on the size of colonies was seen. In cytokine-free methylcellulose cultures, no effect of CCL18 on colony formation was observed. One plausible interpretation of these results is that the stimulatory effect of CCL18 on hematopoiesis in human LTBMCs was indirect and mediated by accessory cells composing the hematopoietic microenvironmental niche.
Effect of CCL18 on LTBMCs. LTBMCs (n = 3 per group) were established from human bone marrow cells. Results of 1 of 3 representative experiments are shown. (A) Half the culture medium was harvested from LTBMCs after each feeding, and the number of nonadherent cells was counted and expressed as mean ± SD. (B) Nonadherent cells harvested from LTBMCs were plated at a concentration of 1 × 104 cells/mL in the presence of cytokine cocktail. After 7 days of culture, the number of colonies was counted, recalculated for the total number of nonadherent cells recovered from LTB-MCs, and expressed as mean ± SD of the number of CFCs. (C) Dose response to CCL18. Colony formation of cells harvested from 5-week-old LTBMCs was determined as described in panel B. The minimal dose showing a statistically significant increase in colony formation was 10 nM (Student t test). (D) Adherent layers from LTBMCs were photographed after 3 weeks of culture (Leica 10×/0.22 numerical aperture [NA] objective). Areas of hematopoiesis are evident in the adherent layers of LTBMCs and increased in the presence of CCL18. (E) CCL18 was added directly to methylcellulose cultures of progenitor cells supplemented with lineage-specific cytokines. In the presence of cytokines, CCL18 decreased colony formation; in their absence, it showed no effect on colony formation.
Effect of CCL18 on LTBMCs. LTBMCs (n = 3 per group) were established from human bone marrow cells. Results of 1 of 3 representative experiments are shown. (A) Half the culture medium was harvested from LTBMCs after each feeding, and the number of nonadherent cells was counted and expressed as mean ± SD. (B) Nonadherent cells harvested from LTBMCs were plated at a concentration of 1 × 104 cells/mL in the presence of cytokine cocktail. After 7 days of culture, the number of colonies was counted, recalculated for the total number of nonadherent cells recovered from LTB-MCs, and expressed as mean ± SD of the number of CFCs. (C) Dose response to CCL18. Colony formation of cells harvested from 5-week-old LTBMCs was determined as described in panel B. The minimal dose showing a statistically significant increase in colony formation was 10 nM (Student t test). (D) Adherent layers from LTBMCs were photographed after 3 weeks of culture (Leica 10×/0.22 numerical aperture [NA] objective). Areas of hematopoiesis are evident in the adherent layers of LTBMCs and increased in the presence of CCL18. (E) CCL18 was added directly to methylcellulose cultures of progenitor cells supplemented with lineage-specific cytokines. In the presence of cytokines, CCL18 decreased colony formation; in their absence, it showed no effect on colony formation.
CCL18 facilitates the formation of the adherent cell layer in LTBMCs
To determine the role of adherent cells in the stimulation of hematopoiesis by CCL18, the formation of the adherent layer was monitored in LTBMCs before cobblestone areas developed. After 1 week, the stromal fibroblast-like compartment of the adherent layer was better developed in CCL18-treated cultures than in controls (Figure 2A). Cultures treated with heat-inactivated CCL18 behaved like control cultures, indicating that the effect of CCL18 was specific. In addition, we observed large macrophage-like cells in CCL18-treated cultures, whereas in heat-inactivated and control cultures macrophages remained small (Figure 2B), suggesting that CCL18 provides better conditions for the adhesion and survival of monocytes in vitro.
We had previously observed that monocytes cultured for 3 to 4 days respond to CCL18.11 Therefore, these cells were used to determine whether CCL18 had an effect on cell adhesion, a prerequisite for monocyte survival and maturation.22 When monocyte adhesion was quantified, it was found that CCL18 increased adhesion to fibronectin and vitronectin (Figure 2C). Furthermore, when monocytes were cultured for 3 days and this was followed by incubation in the presence of CCL18 for 20 hours, annexin V staining indicated increased survival in CCL18-stimulated cultures (Figure 2D). If CCL18-stimulated cells were kept in continuous culture, by day 6 they developed into cells with macrophage morphology (Figure 2E).
Because cultured monocytes responded to CCL18 and because monocytes play an important role in structuring the hematopoietic niche, the question arose whether CCL18 regulates the supportive function of monocytes/macrophages in hematopoiesis.
CCL18 up-regulates the expression of monocytic cytokines
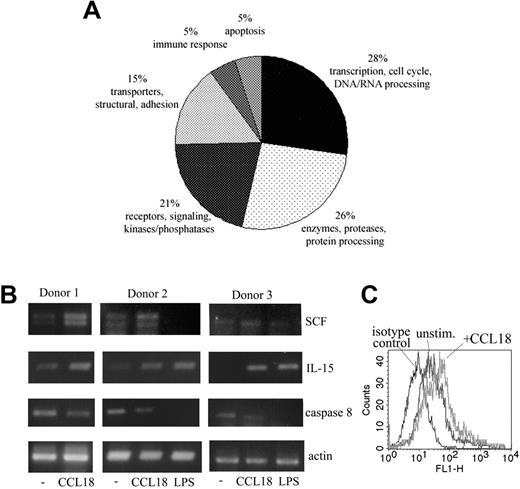
Given that factors such as cytokines and growth factors produced by monocytes are important components of the bone marrow regulatory network (for a review, see Cavaillon23 ), we sought to determine whether CCL18 influenced the expression profile of cell-surface molecules or cytokines involved in the regulation of HSPC behavior. We performed gene expression profiling using Stanford gene arrays. More than 200 genes were differentially expressed (greater than 2-fold difference) in the CCL18-treated monocytes compared with control samples (PBS). A replicate from separately treated cultures gave almost completely concordant data. Figure 3A illustrates the distribution of differentially expressed genes classified into functional classes (transcription, signaling, enzyme and protein processing, adhesion and structural, apoptosis, and immune response). Several proapoptotic proteins, including caspase-8 and programmed cell death 11, were down-regulated in CCL18-treated cells, in agreement with the antiapoptotic effect of CCL18 seen in Figure 2D. Interestingly, CCL18-treated cells expressed higher levels of IL-15 and SCF, which are both well documented as factors that support hematopoiesis.24-28
Effect of CCL18 on adherent cells in LTBMCs. Adherent layers from LTBMC were taken after 1 week of culture (Leica 10×/0.22 NA objective). Stromal fibroblast-like cells (A) and macrophage-like cells (B) are shown. (C) Effect of CCL18 on monocyte adhesion to fibronectin and vitronectin. Adhesion was determined as described in “Cell isolation and culture.” Results (mean ± SD) of 1 of 3 representative experiments are shown. Adhesion seen in the presence of 100 nM CCL18 was statistically different from that seen in unstimulated cells for both adhesion molecules (Student t test; *P < .01). (D) Effect of CCL18 on monocyte survival. Monocytes were isolated from peripheral blood (n = 4) or bone marrow (n = 2), cultured for 3 days, and stimulated with 40 nM CCL18 for 20 hours. Apoptosis was determined with an annexin V–FITC/propidium iodide staining kit in which viable cells remained unstained in the lower left quadrant of a FACS dot plot. Results of 1 representative experiment are shown. Cell viability ranged from 15% to 26% without stimulation and 36% to 44% in CCL18-stimulated cultures. (E) Effect of CCL18 on macrophage maturation. Monocytes were stimulated with 40 nM CCL18 or buffer control on day 2, cultured for 4 more days, and photographed (Leica 10×/0.22 NA objective).
Effect of CCL18 on adherent cells in LTBMCs. Adherent layers from LTBMC were taken after 1 week of culture (Leica 10×/0.22 NA objective). Stromal fibroblast-like cells (A) and macrophage-like cells (B) are shown. (C) Effect of CCL18 on monocyte adhesion to fibronectin and vitronectin. Adhesion was determined as described in “Cell isolation and culture.” Results (mean ± SD) of 1 of 3 representative experiments are shown. Adhesion seen in the presence of 100 nM CCL18 was statistically different from that seen in unstimulated cells for both adhesion molecules (Student t test; *P < .01). (D) Effect of CCL18 on monocyte survival. Monocytes were isolated from peripheral blood (n = 4) or bone marrow (n = 2), cultured for 3 days, and stimulated with 40 nM CCL18 for 20 hours. Apoptosis was determined with an annexin V–FITC/propidium iodide staining kit in which viable cells remained unstained in the lower left quadrant of a FACS dot plot. Results of 1 representative experiment are shown. Cell viability ranged from 15% to 26% without stimulation and 36% to 44% in CCL18-stimulated cultures. (E) Effect of CCL18 on macrophage maturation. Monocytes were stimulated with 40 nM CCL18 or buffer control on day 2, cultured for 4 more days, and photographed (Leica 10×/0.22 NA objective).
Differential gene expression in cultured monocytes/macrophages stimulated with CCL18. (A) Monocytes cultured for 3 days were stimulated with 40 nM CCL18 (or PBS control) for 3.5 hours. Differential gene expression was detected using cDNA arrays from the Stanford functional genomics facility, as described in “Gene array analysis and RT-PCR.” The 100 genes of known function with the greatest differential expression were plotted on a pie chart divided into protein classes. Results of the gene array analysis are provided as supplemental material (Table S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). (B) To verify gene array results, semiquantitative RT-PCR was performed with primers for SCF, IL-15, caspase-8, and actin as a household gene. Samples were prepared as described in panel A. In donors 2 and 3, LPS (100 ng/mL) was used as a known stimulus for monocytes. (C) Detection of IL-15 on cultured monocytes by FACS. Surface expression of IL-15 was detected by FACS in monocytes stimulated with CCL18 for 4 hours. Mean fluorescence channel increased from 63 in unstimulated cells to 118 in CCL18-stimulated cells. Results of 1 of 3 representative experiments are shown.
Differential gene expression in cultured monocytes/macrophages stimulated with CCL18. (A) Monocytes cultured for 3 days were stimulated with 40 nM CCL18 (or PBS control) for 3.5 hours. Differential gene expression was detected using cDNA arrays from the Stanford functional genomics facility, as described in “Gene array analysis and RT-PCR.” The 100 genes of known function with the greatest differential expression were plotted on a pie chart divided into protein classes. Results of the gene array analysis are provided as supplemental material (Table S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). (B) To verify gene array results, semiquantitative RT-PCR was performed with primers for SCF, IL-15, caspase-8, and actin as a household gene. Samples were prepared as described in panel A. In donors 2 and 3, LPS (100 ng/mL) was used as a known stimulus for monocytes. (C) Detection of IL-15 on cultured monocytes by FACS. Surface expression of IL-15 was detected by FACS in monocytes stimulated with CCL18 for 4 hours. Mean fluorescence channel increased from 63 in unstimulated cells to 118 in CCL18-stimulated cells. Results of 1 of 3 representative experiments are shown.
Results for IL-15, SCF, and caspase-8 were verified by semiquantitative RT-PCR. Increases of IL-15 and SCF were consistently seen in all donors tested (Figure 3B), and caspase-8 was down-regulated on all occasions. LPS used as a positive control in these experiments showed a different pattern of gene induction. It showed an enhancing effect on IL-15 similar to that of CCL18, and it inhibited caspase-8, but, in contrast to CCL18, down-regulated SCF expression (Figure 3B). Furthermore, our CCL18 preparation contained less than 20 pg/mL LPS (final concentration). At this concentration, LPS exerted no effect on the expression of tested genes (results not shown). Therefore, the observed effects could not have resulted from LPS contamination. Increased protein expression of IL-15 could be shown by FACS analysis of cultured monocytes stimulated with CCL18 for 4 hours (Figure 3C).
CCL18-stimulated monocytes/macrophages produce cytokines that support hematopoiesis
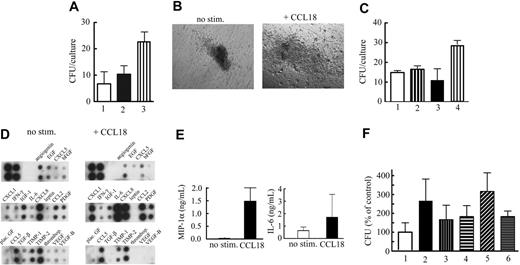
Monocytes are potent producers of cytokines, and we sought to determine whether supernatants of CCL18-stimulated cultured monocytes played a supportive role in hematopoiesis. Monocytes obtained from bone marrow or from peripheral blood were cultured for 3 days and treated with CCL18 for 20 hours, and conditioned media were collected and tested for colony-promoting activity with the methylcellulose colony-formation assay. Conditioned media from bone marrow macrophage or peripheral blood monocyte cultures stimulated with CCL18 increased colony-promoting activity compared with controls (Figure 4A). The number and size of colonies were increased (Figure 4B) but could not be accounted for by a direct effect of CCL18 on colony formation because the increases were not observed when CCL18 was added directly to the methylcellulose cultures (Figure 4C). Supernatants from CCL18-treated cultured monocytes were stimulatory; hence, the next question was which cytokines/growth factors were mediating the colony-promoting activity of CCL18-conditioned medium. Because the production of growth factors and cytokines by monocytes can be influenced at the transcriptional, translational, and protein storage/secretion levels,29,30 supernatants of CCL18-stimulated cells and their unstimulated controls were tested for the presence of several cytokines/chemokines. Conditioned media obtained from cultured monocytes—purified from whole blood or bone marrow—stimulated with CCL18 consistently contained increased levels of IL-6, IL-8/CXCL8, MCP-1/CCL2, and MIP-1α/CCL3 (Figure 4D-E). Other factors, such as interferon-γ and RANTES, were not affected or even down-regulated in the presence of CCL18. Down-regulated factors included angiogenin, TIMP-1, TIMP-2, TGF-β1, bFGF, and VEGF.
Effect of soluble factors produced by CCL18-stimulated monocyte/macrophages. After 3 days in culture, monocytes/macrophages (5 × 106 cells/condition) were incubated in serum-free RPMI 1640 for 20 hours in the presence of buffer control or 40 nM CCL18. (A) Effect of monocyte-conditioned medium on colony formation. CFUs were determined as described in “CFU assay.” The following additions were made: medium (RPMI 1640) only (bar 1); 1:10 dilution of conditioned medium obtained from monocytes prepared, as described, in the absence of stimulus (control CM; bar 2); 1:10 dilution of conditioned medium from monocytes treated with 40 nM CCL18 (CCL18 CM; bar 3). Mean ± SD of 3 experiments. (B) Image of colony cultured in the presence of CCL18-stimulated conditioned media or control-conditioned media (Leica 10×/0.22 NA objective). (C) Lack of direct effect of CCL18 on CFUs: medium only (bar 1); 40 nM CCL18 added to CFU assay (bar 2); 1:10 dilution of monocyte-conditioned medium (bar 3); 1:10 dilution of conditioned medium of CCL18-stimulated monocytes (bar 4). Mean ± SD from 1 experiment. (D) Antibody arrays were incubated with supernatants from unstimulated or CCL18-stimulated cultures (n = 6 from 6 different donors) using 2 different arrays with partially overlapping specificity. Results of 1 representative experiment are shown. Duplicate spots are represented in the following sequence, as indicated in the figure: positive control, angiogenin, EGF, ENA78/CXCL5, basic fibroblast growth factor, gro-α/CXCL1, interferon-γ, insulin-like growth factor 1, IL-6, IL-8, leptin, MCP-1/CCL2, platelet-derived growth factor, placental growth factor, RANTES/CCL5, transforming growth factor-β, tissue inhibitor of metalloproteinases-1, tissue inhibitor of metalloproteinases-2, thrombopoietin, VEGF, VEGF-B. CCL18 consistently inducedan increase in IL-6, IL-8, MCP-1, MIP-1α (present in an alternate blot), and gro-α, though the baseline of IL-6 production ranged from barely detectable to the highest signal in the unstimulated blot. Similar results were seen with monocytes purified from bone marrow and cultured for 3 days (n = 2; results not shown). (E) ELISA for CCL3/MIP-1α and IL-6 (n = 6) using supernatants prepared in the same way. MIP-1α concentrations were significantly different (Student t test; P < .01), and IL-6 concentrations were always increased, but no significant difference was reached because of the large variability in different donors. (F) Effect of antibody inhibition of SCF on colony formation by CM from CCL18-stimulated cultured monocytes: control CM (bar 1); CCL18 (40 nM) stimulated–CM (bar 2); control CM + control IgG (10 μg/mL; bar 3); control CM + anti-SCF (10 μg/mL; bar 4); CCL18-stimulated CM + control IgG (bar 5); CCL18-stimulated CM + anti-SCF (bar 6). Mean ± SD of 3 experiments performed in triplicate. A statistical difference was observed between CCL18-stimulated CM + control IgG and CCL-stimulated CM + anti-SCF (unpaired Student t test; n = 9; P < .05).
Effect of soluble factors produced by CCL18-stimulated monocyte/macrophages. After 3 days in culture, monocytes/macrophages (5 × 106 cells/condition) were incubated in serum-free RPMI 1640 for 20 hours in the presence of buffer control or 40 nM CCL18. (A) Effect of monocyte-conditioned medium on colony formation. CFUs were determined as described in “CFU assay.” The following additions were made: medium (RPMI 1640) only (bar 1); 1:10 dilution of conditioned medium obtained from monocytes prepared, as described, in the absence of stimulus (control CM; bar 2); 1:10 dilution of conditioned medium from monocytes treated with 40 nM CCL18 (CCL18 CM; bar 3). Mean ± SD of 3 experiments. (B) Image of colony cultured in the presence of CCL18-stimulated conditioned media or control-conditioned media (Leica 10×/0.22 NA objective). (C) Lack of direct effect of CCL18 on CFUs: medium only (bar 1); 40 nM CCL18 added to CFU assay (bar 2); 1:10 dilution of monocyte-conditioned medium (bar 3); 1:10 dilution of conditioned medium of CCL18-stimulated monocytes (bar 4). Mean ± SD from 1 experiment. (D) Antibody arrays were incubated with supernatants from unstimulated or CCL18-stimulated cultures (n = 6 from 6 different donors) using 2 different arrays with partially overlapping specificity. Results of 1 representative experiment are shown. Duplicate spots are represented in the following sequence, as indicated in the figure: positive control, angiogenin, EGF, ENA78/CXCL5, basic fibroblast growth factor, gro-α/CXCL1, interferon-γ, insulin-like growth factor 1, IL-6, IL-8, leptin, MCP-1/CCL2, platelet-derived growth factor, placental growth factor, RANTES/CCL5, transforming growth factor-β, tissue inhibitor of metalloproteinases-1, tissue inhibitor of metalloproteinases-2, thrombopoietin, VEGF, VEGF-B. CCL18 consistently inducedan increase in IL-6, IL-8, MCP-1, MIP-1α (present in an alternate blot), and gro-α, though the baseline of IL-6 production ranged from barely detectable to the highest signal in the unstimulated blot. Similar results were seen with monocytes purified from bone marrow and cultured for 3 days (n = 2; results not shown). (E) ELISA for CCL3/MIP-1α and IL-6 (n = 6) using supernatants prepared in the same way. MIP-1α concentrations were significantly different (Student t test; P < .01), and IL-6 concentrations were always increased, but no significant difference was reached because of the large variability in different donors. (F) Effect of antibody inhibition of SCF on colony formation by CM from CCL18-stimulated cultured monocytes: control CM (bar 1); CCL18 (40 nM) stimulated–CM (bar 2); control CM + control IgG (10 μg/mL; bar 3); control CM + anti-SCF (10 μg/mL; bar 4); CCL18-stimulated CM + control IgG (bar 5); CCL18-stimulated CM + anti-SCF (bar 6). Mean ± SD of 3 experiments performed in triplicate. A statistical difference was observed between CCL18-stimulated CM + control IgG and CCL-stimulated CM + anti-SCF (unpaired Student t test; n = 9; P < .05).
Because a negative effect of MIP-1α on proliferation of HSPCs has been reported,31 we performed ELISA to quantify the increase of MIP-1α after CCL18 stimulation. Although CCL18 caused a significant (P < .01) increase in MIP-1α (Figure 4E), the absolute concentration of MIP-1α (1.5 ng/mL) after the addition of CCL18, which was used at a 1:10 dilution in the CFU assay, was clearly lower than concentrations of MIP-1α (50-100 ng/mL), which have been shown to influence colony formation. Therefore, it was unlikely that the CCL18-induced increase in MIP-1α contributed to the effects of CCL18 on hematopoiesis.
Factors that show increased expression in CCL18-stimulated cells by gene or protein array and that are known to exhibit colony-promoting activity included IL-6,32 IL-15, and SCF. Given that IL-15 remains cell associated,33 the effect of inhibition of the 2 soluble factors, IL-6 and SCF, was determined. Function-blocking antibody against IL-6 showed variable effects, ranging from none to almost complete inhibition (results not shown). This was not unexpected, however, because IL-6 was the cytokine that showed the greatest variability among donors in CCL18-dependent up-regulation (Figure 4E). In contrast, antibody against SCF greatly reduced the colony-promoting activity of conditioned media of CCL18-stimulated mature monocytes on all occasions (Figure 4F).
Discussion
Here we present evidence that CCL18 participates in the regulatory network that controls HSPCs in the bone marrow. Although our evidence emphasizes the role of monocytes in this process, the contribution of additional cells, such as stromal cells, cannot be excluded.
To determine whether CCL18 is involved in the regulation of HSPCs, human LTBMCs were chosen as an in vitro model of hematopoiesis. CCL18 has not been found in the murine genome, and the human gene has supposedly evolved from duplication of the MIP-1α gene34,35 and thus far has been detected only in humans and monkeys.5 We found that the total number of mature hematopoietic cells and their progenitors produced in CCL18-treated LTBMCs was increased compared with control (nontreated or heat-inactivated CCL18). However, when it was added to methyl-cellulose cultures supplemented with lineage-specific cytokines at optimal concentrations, CCL18 slightly decreased the number of colonies, consistent with previous findings13 in which purified stem/progenitor cells had been used. These results suggest that hematopoietic stem/progenitor cells have receptors for CCL18 but that the observed effects of CCL18 on human hematopoiesis are mediated indirectly by accessory cells that are involved in the regulation of HSPCs.
It is well known that the behavior of hematopoietic stem cells is regulated by multiple signals provided by the hematopoietic microenvironmental niche, which responds to physiological and pathophysiological stimuli.36 The cellular compartment of the bone marrow niche consists of cells of hematopoietic (monocytes, lymphocytes, osteoclasts) and mesenchymal (stromal cells, osteoblasts, adipocytes) origin (for a review, see Bianco and Gehron Robey37 ). This complex scenario made it difficult to choose which cell type mediated the response to CCL18. Furthermore, several cell types in the hematopoietic niche respond to CCL18, including lymphocytes,1,2 maturing monocytes,11 endothelial cells (A.W., S.K.K., and I.U.S., unpublished observations, July 2006), and stromal cells.12 In fibroblasts, CCL18 has been shown to induce increased collagen formation and proliferation,12 consistent with our observation of increased growth of cells of the stromal cell layer in LTBMCs stimulated with CCL18 (Figure 2A). This is an area that deserves further investigation, especially because the extracellular matrix plays an important role in hematopoiesis.
Because of the prominent role of monocyte/macrophages in the bone marrow and their activation-induced up-regulation of cytokines/chemokines, growth factors, and adhesion molecules, these cells were the primary focus of our investigation. We have previously demonstrated that cultured, but not freshly isolated, monocytes respond to CCL18.11 Therefore, we hypothesized that CCL18-induced changes in the function of monocytes may mediate the hematopoiesis-supportive effects of CCL18. Indeed, bone marrow–and peripheral blood–derived cultured monocytes reacted to CCL18 treatment by producing hematopoiesis-supportive factors. The presence of these factors in the conditioned media from CCL18-treated monocytes was confirmed by HSPC culture in methylcellulose media. Under these conditions, the number and sizes of colonies were increased in the presence of CCL18-induced conditioned media (Figure 4A-B). By gene and protein array, we observed that CCL18 stimulation of cultured monocytes up-regulated the concentration of several cytokines/chemokines involved in the regulation of hematopoiesis, including SCF, IL-6, IL-15, IL-8, and MIP-1α (Figures 3C-D, 4D-E).
Concentrations of several chemokines, including IL-8, MIP-1α, and MCP-1, were increased in CCL18-stimulated monocyte/macrophage supernatants. Although none of these stimulate hematopoiesis, IL-8 and MIP-1α contribute to the mobilization of cells, including progenitors from the bone marrow.38,39 However, the concentrations of these chemokines appeared well below the concentrations that had been demonstrated to show a negative effect on hematopoiesis.31
IL-15 was one of the most highly (6-fold) up-regulated genes in the gene array. It is a pleiotropic cytokine that can have a direct effect on hematopoiesis.24,33 It also has indirect effects on hematopoiesis because it up-regulates the expression of hyaluronic acid, which plays an important role in structuring and regulating the hematopoietic niche.40,41 These direct or indirect mechanisms could indeed have occurred in the CCL18-stimulated LTBMCs, but they cannot explain the supportive role of the CCL18-conditioned monocyte supernatants given that IL-15 remains bound to the monocyte33 and could not be detected by ELISA in our supernatants. Monocytic IL-15 has been shown to up-regulate IL-6 and IL-842 ; therefore, it may be responsible, at least in part, for the increased concentrations of these cytokines observed in CCL18-activated monocyte supernatants. Finally, IL-15 has been shown to inhibit apoptosis in various leukocyte populations43-45 and in fibroblasts,46 and it might well have contributed to the protection from apoptosis seen in CCL18-stimulated monocytes.
Finally, SCF and IL-6 have direct effects on hematopoiesis. IL-6 is known to stimulate the proliferation of myeloid and lymphoid progenitors and of multipotent stem cells before commitment.32 Neutralizing anti–IL-6 antibody showed variable effects depending on the level of induction of IL-6 in a specific donor, which showed great variability. SCF supports the growth of human multipotential progenitor cells and promotes the effects of other colony-stimulating factors.26-28 Blocking antibody against SCF largely inhibited the colony-promoting activity in conditioned media derived from CCL18-stimulated monocytes, but it was probably not the only factor involved in this process.
The antiapoptotic effect of CCL18 on cultured monocytes is of potential physiological relevance. Monocytes circulate in the blood-stream for 24 to 48 hours, but only 10% of them differentiate into macrophages; the rest succumb to apoptosis. Apoptosis of these cells is prevented in the presence of growth factors/cytokines such as M-CSF47 ; however, because these monocytic growth factors lead to increased macrophage numbers, they contribute to disease with macrophage involvement.48 CCL18-stimulated monocytes matured into spheroid macrophages resembling IL-4–stimulated, so-called alternatively activated macrophages49 (Figure 2E). Although it is unusual for a chemokine to cause increased cell survival, CCL18 is not unique in this respect. Precedence for this behavior has been described for SDF-150 and MCP-1.51 Generally, apoptosis is regulated by the activation of a proteolytic cascade. In monocytes, however, it has been shown that factors that promote survival lead to the down-regulation of caspase-8 on the mRNA level,52 as was observed in the presence of CCL18 (Figure 3B).
Although gene arrays showed little up-regulation of adhesion molecules, increased adhesion was observed in 3-day-old monocytes plated on fibronectin or vitronectin (Figure 2C). Because adhesiveness is usually mediated by the increased avidity of adhesion molecules such as integrins rather than by transcriptional up-regulation, this observation will be pursued through cell biology methods, among them blocking antibodies.
Overall, we have described a new regulatory function for CCL18 and have demonstrated that CCL18 stimulates the hematopoiesis-supportive function of the HSPC microenvironment regulating gene expression and cytokine release in monocytes.
Authorship
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 3, 2006; DOI 10.1182/blood-2006-04-014399.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by National Institutes of Health grants HL55657 and TRDRP 13T-0083 (I.U.S.) and 1R21DK067084-01 and K18 HL081096 (S.K.K.).

![Figure 1. Effect of CCL18 on LTBMCs. LTBMCs (n = 3 per group) were established from human bone marrow cells. Results of 1 of 3 representative experiments are shown. (A) Half the culture medium was harvested from LTBMCs after each feeding, and the number of nonadherent cells was counted and expressed as mean ± SD. (B) Nonadherent cells harvested from LTBMCs were plated at a concentration of 1 × 104 cells/mL in the presence of cytokine cocktail. After 7 days of culture, the number of colonies was counted, recalculated for the total number of nonadherent cells recovered from LTB-MCs, and expressed as mean ± SD of the number of CFCs. (C) Dose response to CCL18. Colony formation of cells harvested from 5-week-old LTBMCs was determined as described in panel B. The minimal dose showing a statistically significant increase in colony formation was 10 nM (Student t test). (D) Adherent layers from LTBMCs were photographed after 3 weeks of culture (Leica 10×/0.22 numerical aperture [NA] objective). Areas of hematopoiesis are evident in the adherent layers of LTBMCs and increased in the presence of CCL18. (E) CCL18 was added directly to methylcellulose cultures of progenitor cells supplemented with lineage-specific cytokines. In the presence of cytokines, CCL18 decreased colony formation; in their absence, it showed no effect on colony formation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/12/10.1182_blood-2006-04-014399/4/m_zh80230604420001.jpeg?Expires=1769180083&Signature=RrreLkwFLhGEYh4Lqoj0COsYxkwbWUz-2cJxkijzasaWL7rnwhIyUPB3OoJ3KiVvFxB14wKLFFOQEY~e7~rRuZRBCZ9X8XC5oV5hw~l1jFYg-5z0r5roVZuizxlr-HU1P8U1EzS0sq88zC1EuGdJGLTeWzdY~hMMQTrzQxSWmx7wfJlUyYh1yZjTU5~H3Uf9OR-1KUIrE-VCrNbpCRWHNg~KglaLgBe1m42eOXZ9aZgiboNb4q2HjFEQvx~iZ1L94kOOUq4SyoqqNEQlQ22M58I-BE~R2C8~tq~syWOFi9I7Xdf8DI2OddUHE-vclFK7yBtJFX2AqLVVWRpH9FZSig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal