Abstract
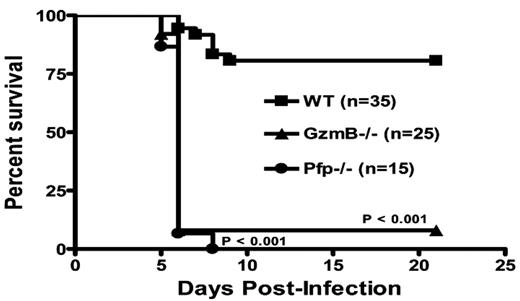
NK cells predominantly utilize the granule exocytosis pathway to kill virus-infected and malignant target cells. Current paradigms suggest that resting NK cells have pre-formed granules containing granzymes A, B, and perforin and are ready to kill targets immediately upon proper recognition by NK receptors. Here, we report that resting murine NK cells in the spleen exhibit poor cytotoxicity (5.4±1.6% target cell death, 20:1 E:T ratio and 4 hour incubation), compared with cytokine-activated (IL-15, 48 hours) splenic NK cells (59.7±10.6% target cell death), against the RMAS tumor cell line in vitro as measured by a flow-based killing assay. In addition, using intracellular flow cytometric analysis with monoclonal antibodies specific for granzymes A, B, and perforin, we find that resting murine NK cells express abundant granzyme A (86.2±1.9% positive), but little or no granzyme B (4.4±5.4% positive) or perforin (2.6±1.8% positive). Activation of murine NK cells with IL-15 induces robust expression of both perforin (59.1±2.0% positive) and granzyme B (91.5±7.9% positive), which correlates with increased cytotoxicity. Further, granzyme B cluster −/− (26±6.7% target cell death) and perforin −/− (5.7±1.3% target cell death) NK cells have poor cytotoxicity in vitro despite IL-15 activation. Poly I:C simulates RNA virus infection and activates NK cell cytotoxicity in vivo through TLR3 and cytokine cascades. NK cell granzyme B and perforin expression is induced in vivo 24 hours after poly I:C injection, correlating with increased in vitro NK killing of tumor targets. In wild type mice infected with murine cytomegalovirus (MCMV), NK cell expression of both perforin (83.5±4.9% positive) and granzyme B (89.3±2.1% positive) is upregulated in the spleen, peaking 2–4 days post-infection and returning to baseline by 8 days post-infection. In addition, MCMV titers are significantly elevated at day 3 post-infection in both granzyme B cluster −/− (P<0.01) and perforin −/− (P<0.01) mice, compared to wild type mice. Moreover, survival following MCMV infection was significantly lower in granzyme B cluster −/− and perforin −/− mice, compared with wild type mice (P<0.001, see survival curve). Thus, our findings show that murine NK cells require the activation of granzyme B and perforin to become potent cytotoxic effectors. We also demonstrate for the first time that granzyme B is critical for early host defense against MCMV. These findings explain the long-standing observation that murine NK cells require prior activation for potent natural killing of tumor targets in vitro. Further, this requirement for activation-dependent granzyme B and perforin expression in NK cells may influence outcomes in murine models of innate immune anti-tumor and anti-viral responses.
Disclosures: Timothy J. Ley holds the license for the anti-mouse granzyme A monoclonal antibody (clone 3G8.5) in conjunction with Santa Cruz Biotechnology, Inc.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal