Abstract
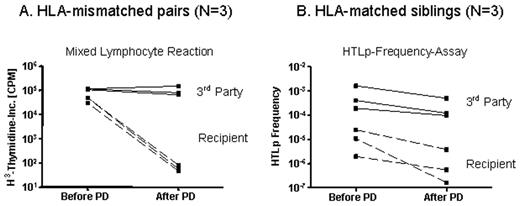
Selective depletion (SD) is a strategy to eliminate host-reactive donor lymphocytes from blood stem cell allografts to prevent GVHD and maintain GVL-effects. As an alternative to CD25-immunotoxin-based SD, we investigated a photodepletion (PD) process, whereby allo-activated donor cells are labeled with a photosensitizing rhodamine-based dye, 4,5-dibromorhodamide 123 (TH9402), and exposed to visible light, which preferentially eliminates allo-activated dye-retaining cells. Stimulator cells were prepared from recipient-derived leukapheresis mononuclear cells (MNCs) and cultured using anti-CD3 and 100 IU IL-2/ml. Responder cells (leukapheresis MNCs) from 3 random HLA-mismatched volunteers and 3 HLA-matched sibling donors were cocultured 1:1 with irradiated stimulators for 3 days. Cultured cells were incubated with 7.5 μM TH9402, followed by dye-extrusion and exposure to 5 Joule light energy in the PD light source (Celmed Bioscience Inc., Canada) at 5x106 cells/ml in FEP plastic bags. Flow cytometry was performed before and after PD and included surface phenotyping for CD25 and intracellular staining for forkhead protein3 (foxp3). Depletion efficacy was studied by mixed lymphocyte reactions (MLR) in mismatched pairs and by helper-T-lymphocyte precursor (HTLp) frequency assay in matched pairs. All six clinical-scale experiments provided sufficient reduction of allo-reactivity and retention of third party responses as measured against a pool of 5 donors. In mismatched pairs mean reduction of allo-reactivity was 703-fold (± 141) when compared to unmanipulated donors. Third-party responses were maintained, with a mean reduction of only 1.3 ± 0.15-fold (Figure A). In matched pairs alloreactivity was reduced below the “GVHD-threshold” of 1/100.000 whilst third party responses remained above 1/10.000 precursors (Figure B). Effective depletion was observed despite that fact that a small fraction of CD25+ T cells remained after PD. These were mainly CD4+CD25+ T cells (1.5 ± 1.1 % of CD4+) and very residual CD8+CD25+ T cells (0.3 ± 0.2 % of CD8+), and represented predominantly CD4+foxp3+ regulatory T cells (Tregs) which persisted after PD (1.4 ± 1.7 % of CD4+). This establishes a clinical scale PD process capable of highly efficient removal of alloreactive lymphocytes from mismatched and matched MLRs while maintaining desirable third party responses. As PD targets activation-based changes in MDR-1 that result in an altered dye efflux, the mechanism of action is distinct from surface-marker-based allodepletion (e.g. CD69, CD25). Thus, PD may overcome instability of activation-based surface marker expression resulting in more consistent and effective depletion. PD may further mitigate the risk of GVHD by conserving Tregs. This approach will now be tested in a clinical SD trial.
Disclosures: Christian Scotto is employed by Celmed Bioscience Inc., Canada.; Christian Scotto holds start-up stocks with Celmed Bioscience Inc, Canada.; Part of this work was performed under a collaborative research and development agreement (CRADA) between the NHLBI/NIH and Celmed Biosciences Inc.). The work of Stephan Mielke was supported in part by a grant of the Dr. Mildred-Scheel-Stiftung for Cancer Help, Germany.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal