Abstract
Blastoid MCL has a very poor prognosis, with a median survival of 16–20 months after treatment with CHOP-like chemotherapy regimens. We recently described an intense regimen where R-HyperCVAD is alternated with R-M/A for 6–8 cycles, capable of achieving 87% complete remission (CR) rates and an overall 3-year FFS rate of 64% in a group of 97 patients with newly diagnosed aggressive MCL treated under an institutionally approved clinical trial from March 1999 to March 2001 (J Clin Oncol 23:7013–7023).
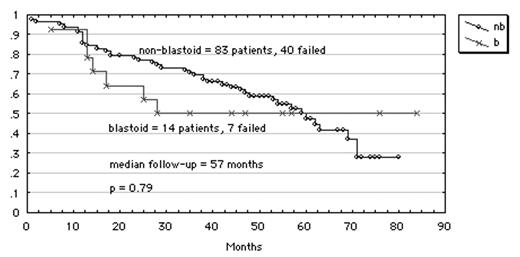
Fourteen of these patients presented with the blastoid cytologic variant and the following clinical features were compared with those without a blastoid cytology: age > 65 years, beta 2 microglobulin ≥ 3 gm/dL, elevated serum lactic dehydrogenase (LDH), and high international prognostic score (IPI). Only serum LDH was significantly different, being higher for those with blastoid presentation (p = 0.03). Rates of complete remission were lower for blastoid when compared to non-blastoid cytology (79% vs 89%, respectively) but not statistically significant (p = 0.72). With a median follow up of 57 months, there is a plateau in the FFS curve for blastoid MCL as shown below. At 57 months the median overall survival has not been reached. Contrary to what we had expected, the improved outcome in this group of patients with blastoid cytology suggests a potential for long term remission after intense, non-myeloablative chemotherapy.
Disclosures: Amgen, IDEC, Genentech partially support clinical studies that Dr. Rodriguez chairs; research funding provided by Novartis - Dr. Hagemeister; grant support from Genentech - Dr. Cabanillas.; Genentech, Biogen-IDEC, Ortho-Biotech, Eli Lilly, Celgene, Millennium - Dr. Hagemeister.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal