Abstract
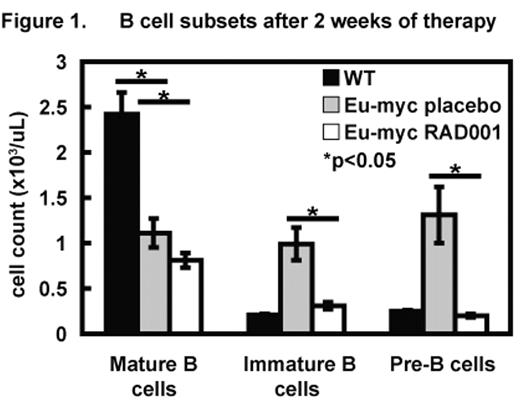
The c-Myc proto-oncogene encodes a bHLH-LZ transcription factor that regulates proliferation, differentiation and apoptosis. Deregulated expression of c-MYC is a frequent finding in a wide variety of human cancers, including B cell lymphoma. One emerging function of c-MYC is the regulation of ribosome biogenesis, protein synthesis and metabolism i.e. cell growth. mTOR, a key downstream signal transduction molecule in the PI3K/AKT growth regulatory pathway, is amenable to pharmacological inhibition by rapamycin analogues such as RAD001. We hypothesized that control of cell growth by c-MYC is important for its ability to regulate differentiation and act as an oncogene and that RAD001, by limiting cell growth, would attenuate the transforming properties of c-MYC. In Eμ-myc mice the c-myc transgene is under the control of the immunoglobulin heavy chain enhancer (Eμ). Constitutive expression of c-MYC results in a polyclonal expansion of B cell precursors followed by lymphoma development. In the current study ‘pre-lymphomatous’ Eμ-myc mice were randomized to receive RAD001 5mg/kg (n=20) or placebo (n=18) 6 days per week from 4 weeks of age. Peripheral blood B cells were analyzed by surface marker expression after 2, 4 and 8 weeks of therapy. Mice were monitored weekly for the development of lymphadenopathy. 2 weeks of treatment with RAD001 significantly reduced the numbers of B cells in the blood of Eμ-myc mice compared to placebo (1.37±0.13 ×103/μL in the RAD001 arm versus 3.41±0.64 ×103/μL in the placebo arm, p<0.05). In particular, there was preferential suppression of less mature circulating B cell precursors over mature B cells by RAD001 resulting in B cell developmental subset profiles more closely approaching those of wild-type mice (Figure 1). Treatment with RAD001 was associated with improved lymphoma-free survival; 13/14 lymphoma-free mice (92.9%) versus 6/11 (54.6%) in the placebo group in an interim analysis of mice that had received at least 60 days of therapy. These results indicate that RAD001 can firstly oppose the expansion of B cell precursors and secondly reduce the incidence of malignant transformation induced by deregulated expression of c-MYC in B lymphocytes. These findings have implications for the application of mTOR inhibitors in the treatment or prevention of malignancies associated with MYC.
Disclosures: Consultancy work for Novartis and Pfizer.; Collaborative research agreements with Merck, Pfizer and Novartis.; Honoraria from Novartis.; Advisory committees Novartis and Pfizer.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal