Abstract
Here, we report that the MHC class I-related neonatal Fc receptor (FcRn) is expressed within azurophilic and specific granules of neutrophils and relocates to phagolysosomes on phagocytosis of IgG-opsonized bacteria. We found FcRn to enhance phagocytosis in a pH-dependent manner which was independent of IgG recycling. IgG-opsonized bacteria were inefficiently phagocytosed by neutrophils from β2M knock-out or FcRn α-chain knock-out mice, which both lack expression of FcRn. Similarly, low phagocytic activity was also observed with mutated IgG (H435A), which is incapable of binding to FcRn, while retaining normal binding to classical leukocyte Fcγ receptors. Finally, a TAT peptide representing intracellular endocytosis and transport motifs within FcRn strongly inhibited IgG-mediated phagocytosis. These findings support a novel concept in which FcRn fulfills a major role in IgG-mediated phagocytosis.
Introduction
Phagocytic cells express members of 3 classes of leukocyte IgG-Fc receptors, FcγRI, FcγRII, and FcγRIII. All share considerable structural and functional homology and recognize similar residues within the CH2 region of IgG.1 FcγR activates phagocytes on interaction with IgG-opsonized particles, involving immunoreceptor tyrosine-based activation motifs (ITAMs). This activation signal may possibly be down-regulated by the immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing FcγRIIb receptor on polymorphonuclear neutrophils (PMNs) and monocytes.2 No other signaling motifs have been implicated in FcγR-mediated phagocytosis. Crosslinking of ITAM-bearing receptors (eg, T-cell receptor, FcϵRI) does not initiate phagocytosis, but it generally triggers fusion and release of granule contents into sealed immunologic synapses between effector cells and targets. In phagocytes these granules contain various components, including enzyme complexes that initiate the respiratory burst and phagosome acidification, as well as antimicrobial peptides and enzymes that serve to kill invading pathogens.3,4
A distinct IgG receptor, the neonatal FcγR (FcRn), consisting of a unique α-chain and β2-microglobulin (β2M), is a major histocompatibility class I (MHC-I) homolog.5 FcRn is present in epithelial cells, placental syncytiotrophoblasts, as well as endothelial cells. In these cells, FcRn has been implicated in transport of IgG across mucosal cells,5,6 from mother to fetus,7 and regulation of IgG half-life,8-11 respectively. This receptor has been found in human monocytes,12 albeit that no function has been attributed to monocyte FcRn. FcRn does not bind IgG at physiologic pH (7.4). Only in the acidic environment of endocytic vacuoles (pH ≤ 6.5), where histidine residues in the Fc-tail of IgG become protonated, can FcRn bind IgG with high affinity. Both β2M and the FcRn α-chain participate in IgG binding within the CH2-CH3 interface.13
In this study we document expression of FcRn within PMNs. Furthermore, we observed FcRn translocation to nascent phagosomes, where FcRn facilitates IgG-mediated bacterial phagocytosis through signaling motifs found within the cytoplasmic tail. These results point to a novel role for FcRn in phagocyte biology.
Materials and methods
Recombinant antipneumococcal 6A/B antibodies
The generation and functional characterization in vitro and in vivo of human antipneumococcal serotype 6A/B GDob1 antibodies used in this study have been described in detail before in Saeland et al.14 The H435A IgG1 variant was generated by mutating the γ1 heavy chain with a Quickchange Site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's guidelines, using the CH3-specific primer gaggctctgcacaacGCctacacgcagaagagcc (mutated bases underlined and capitalized) and its complementary primer. After verification of the expected incorporation of the mutated bases by sequencing (ABI 373 Stretch automated sequencing machine; Applied Biosystems, Foster City, CA), resulting in the corresponding amino-acid change from a histidine to an alanine in position 435, the heavy chain was transfected together with the corresponding light chain, produced, and purified as described in Saeland et al14 and Vidarsson et al.15
Detection antibodies
Mouse IgG1 MAC-1 (CR3)-specific mAb was purchased from Becton Dickinson (San Jose, CA), and Cy3-labeled goat anti-mouse IgG F(ab′)2 fragments from Jackson (West Grove, PA). Protein G-isolated mouse IgG1 directed against the α-chain of FcRn (mAb 1G3) was obtained from ATCC (Manassas, VA).16 Mouse IgM mAb 22H4C11 was raised by immunizing balb/c mice with a peptide stretch composing the FcRn α-chain intracellular tail. Rabbit antiserum against human FcRn was a generous gift from Dr Neil Simister (Brandeis University, Waltham, MA). Mouse IgG1 was detected in fluorescence-activated cell scanner (FACS) experiments by means of FITC-labeled goat F(ab′)2 fragments of anti-mouse IgG (Protos, Burlingame, CA). Rabbit antiserum was detected with biotin-labeled swine anti-rabbit IgG F(ab′)2 fragments (DAKO, Glostrup, Denmark), followed by streptavidin-Alexa555 (Molecular Probes, Leiden, The Netherlands). Human recombinant IgG1 concentrations were quantified by a sandwich enzyme-linked immunoabsorbent assay (ELISA), capturing the recombinant antibodies by a rabbit anti-human-kappa antiserum, with alkaline phosphatase-conjugated rabbit anti-IgG (Fc specific) antisera for detection as described in Vidarsson et al15 and by antigen-specific ELISA as detailed in Saeland et al14 with either an Fc-specific HRP-labeled goat anti-human IgG or with FITC-labeled goat anti-kappa F(ab′)2 fragments (Southern Biotechnology Associates, Birmingham, AL) followed by an HRP-labeled sheep anti-FITC (Roche, Mannheim, Germany).
FcRn TAT peptides
Peptides consisting of the HIV-originating TAT sequence (YGRKKRRQRRRG) and part of the intracellular tail of FcRn containing the tryptophan motif and the dileucine motif (APWISLRGDDTGVLLPTP) were synthesized at The Netherlands Cancer Institute, Amsterdam, The Netherlands (final sequence, n-YGRKKRRQRRRGAPWISLRGDDTGVLLPTP-c). Biotinylated control peptides with identical amino acid composition, but scrambled signaling motifs, were generated likewise (n-YGRKKRRQRRRGAPLILDRGLSTGVWDPTP-c, rearranged amino acids underlined). Both peptides were N-terminally biotinylated through an aminohexanoic acid spacer.
Mice
Cells
Human PMNs were isolated as described in Vidarsson et al15 by Ficoll (Pharmacia, Almeda, CA)-Histopaque (Sigma, St Louis, MO) gradients, followed by hypotonic lysis of residual red blood cells in water (for 30 seconds at 4°C). Natural killer (NK) cells and monocytes were isolated from the mononuclear fraction with a MoFlo cell sorter (Cytomation, Fort Collins, CO) after staining with PE-labeled CD56 mAb (CLB, Amsterdam, The Netherlands) and biotinylated CD14 mAb (Sigma) and streptavidin-APC (Molecular Probes). Mouse PMNs were isolated from mice treated with PEG-G-CSF (Amgen, Thousand Oaks, CA) as described in van Spriel et al.18 All experiments involving mice and human subjects were approved by the ethics and scientific review boards of UMC Utrecht, The Netherlands.
FcRn mRNA expression
RNA was isolated from cell populations by means of RNAzol (Campro Scientific, Veenendaal, The Netherlands), and first-strand cDNA was generated with an oligo(T) primer and avian myeloblastosis virus reverse transcriptase (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions. FcRn-specific mRNA was quantified by subsequent real-time quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis (Taqman), with the forward CTCAGGGTGGAGCTGGAATC, reverse CTCCACGAAGGGAGATCCAA primer, and the probe ATCGTCATCGGTGTCTTGCTACTCACGG (passes an intron/exon boundary). Arbitrary values were obtained by calculating FcRn expression levels as a ratio of ABL (Abelson) gene expression.19
Immunoelectron microscopy
Human neutrophils were fixed for 24 hours in 2% paraformaldehyde in 0.1 M PHEM buffer (60 mM PIPES, 25 mM HEPES, 2 mM MgCl2,10mM EGTA, pH 6.9) and processed for ultrathin cryosectioning as described in Calafat et al.20 Briefly, 50-nm cryosections were cut at -120°C using diamond knives in an ultracryomicrotome (Leica Aktiengesellschaft, Vienna, Austria) and transferred with a mixture of sucrose and methylcellulose onto formvar-coated copper grids. The grids were placed on 35-mm Petri dishes containing 2% gelatin. Ten- and 15-nm protein-A-conjugated colloidal gold probes (EM Lab, Utrecht University, The Netherlands) were used for double immunolabeling using anti-FcRn rabbit sera, and either rabbit anti-human lactoferrin from Cappel Laboratories (Cochranville, PA) or rabbit anti-human myeloperoxidase from DAKO. After immunolabeling, the cryosections were embedded in a mixture of methylcellulose and uranyl acetate and examined with a Philips CM10 electron microscope (Eindhoven, The Netherlands). As controls, the primary antibody was replaced by a preimmune rabbit.
FcRn expression by confocal microscopy and FACS
For all confocal microscopy experiments, vital cells were allowed to attach to poly-l-lysine-coated superfrost (Sigma) microscopy slides for 30 minutes at 37°C in a humidified chamber. All phagocytosis experiments for confocal microscopy were carried out as described for FACS phagocytosis experiments (see “Phagocytosis experiments”) but on microscopy slides. Before all stainings, cells were fixed with paraformaldehyde (3.7%), washed with saponin buffer (PBS with 0.5% saponin and 1% bovine serum albumin; saponin was omitted for extracellular staining), and incubated for 60 minutes at room temperature with either mouse IgG1 MAC-1 (CR3) or rabbit anti-FcRn antiserum, followed by appropriate conjugates (see “Detection antibodies”). All detection antibodies and conjugates were diluted in saponin buffer to allow intracellular staining. After staining, the microscopy slides were treated with ProlongGold (Molecular Probes) according to the manufacturer's recommendations, and samples were visualized by confocal microscopy (Digital Eclipse C1; Nikon, Kanagawa, Japan). MAC-1 or FcRn staining was used to center the scanning (Z) plane in the cell center before acquisition of final images. A DXM1200 digital camera was used to capture images, which were magnified under a 100×/1.30 oil-immersion objective lens. EZ software version 1.0 (Nikon) was used to acquire digital images. Densitometry analyses were performed with ImageJ version 1.34S (http://rsb.info.nih.gov/ij/index.html). All FACS stainings were performed identically, but in 50-μL volumes in polypropylene tubes (Micronics, Lelystad, The Netherlands) and analyzed by flow cytometry (FACSCalibur; Becton Dickinson). Biotinylated TAT peptides were detected after incubations with 1 mg/mL TAT peptides for 10 minutes, using streptavidin-Alexa555 (Molecular Probes). Samples were washed twice with buffer or saponin buffer between all incubations depending on whether cell membrane or intracellular staining was performed (see above).
Phagocytosis experiments
Heat-killed Streptococcus pneumoniae, serogroup 6B, was FITC-labeled and stored at -20°C in PBS as described in Saeland et al.14 Phagocytosis experiments were carried out as in Vidarsson et al15 using freshly isolated human PMNs. All phagocytosis experiments were carried out at 37°C for 15 minutes by mixing IgG, 105 PMNs, and 5 × 106 FITC-labeled bacteria, except where indicated otherwise. PMN-adhered or ingested bacteria were evaluated by confocal microscopy and flow cytometry. Live gates were used on mouse PMNs (as distinguished by PE-labeled Rat IgG anti-Gr-1/Ly-6; Becton Dickinson). In experiments where ingested bacteria were quantified by FACS, Trypan Blue21 or ethidium bromide22 was used to quench extracellular FITC-labeled pneumococci, both of which resulted in similar and efficient quenching. TAT peptides and inhibitors, when used, were added together with IgG1 and pneumococci to isolated cell preparations without prior incubations. Phagocytic index (PI) was calculated as described in Vidarsson et al.15
IgG recycling
PMNs were allowed to ingest recombinant antipneumococcal IgG1 (10 μg/mL), either alone or in the presence of 6B pneumococci for 5 minutes at 37°C, followed by 2 washes with medium (RPMI 1640 medium with 2% FCS, adjusted to pH 2.5) to wash away extracellularly bound IgG (confirmed by FACS; data not shown). Cells were then incubated for an additional 30 minutes, and supernatants were harvested and analyzed for the presence of human anti-6B pneumococcal antibodies by antigen-specific ELISA.14
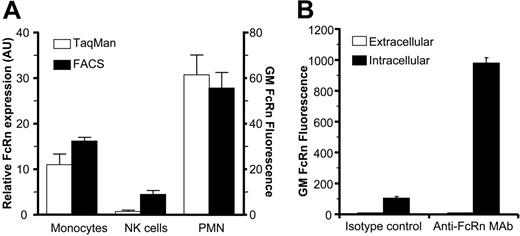
Expression of FcRn in human blood monocytes, NK cells, and PMNs. (A) FcRn mRNA and protein levels in freshly isolated human monocytes, NK cells, and PMNs as measured by real-time quantitative RT-PCR (AU defined as a ratio of ABL expression) and flow cytometry (geometric mean fluorescence). FcRn expression in cells was detected by mAb 22H4C11 in fixed and permeabilized cells. (B) FcRn is localized intracellularly in resting PMNs. Freshly isolated PMNs or paraformaldehyde-fixed and saponin-treated PMNs were stained with mouse anti-FcRn mAb 1G3 and measured by FACS to detect extracellular and intracellular FcRn levels, respectively. (A-B) Data are presented as means plus standard deviations. Data are representative of at least 5 experiments.
Expression of FcRn in human blood monocytes, NK cells, and PMNs. (A) FcRn mRNA and protein levels in freshly isolated human monocytes, NK cells, and PMNs as measured by real-time quantitative RT-PCR (AU defined as a ratio of ABL expression) and flow cytometry (geometric mean fluorescence). FcRn expression in cells was detected by mAb 22H4C11 in fixed and permeabilized cells. (B) FcRn is localized intracellularly in resting PMNs. Freshly isolated PMNs or paraformaldehyde-fixed and saponin-treated PMNs were stained with mouse anti-FcRn mAb 1G3 and measured by FACS to detect extracellular and intracellular FcRn levels, respectively. (A-B) Data are presented as means plus standard deviations. Data are representative of at least 5 experiments.
Statistical analyses
One-way ANOVA or 2-tailed Student t tests were used to compare differences in phagocytosis and binding indexes after testing for normal distribution using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). Significance was set at P less than .05.
Results
FcRn is expressed in PMNs
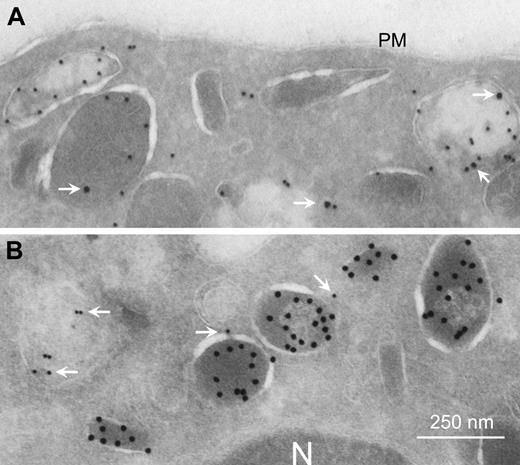
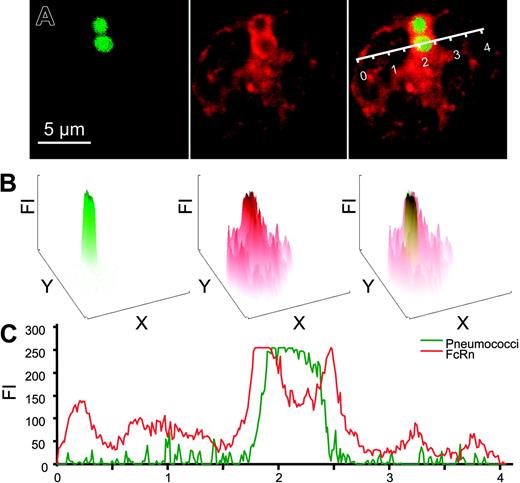
We observed FcRn to be highly expressed in freshly isolated human and mouse neutrophils (PMNs), as analyzed by real-time quantitative RT-PCR analyses and flow cytometry (Figure 1A). FcRn was exclusively localized intracellularly in resting PMNs (Figure 1B). By electron microscopy, FcRn was found in granular structures but not on the plasma membrane (Figure 2A). The most prominent staining was observed within myeloperoxidase-positive azurophil granules (33% of myeloperoxidase-positive granules also stained FcRn positive). A lower level was also detected in specific granules (18% of lactoferrin-positive granules; Figure 2B). FcRn could be detected transiently on the surface by FACS analysis on stimulation with the degranulation agent phorbol 12-myristate 13-acetate but not with N-formyl-methionyl-leucyl-phenylalanine (n = 3, data not shown). This is in agreement with previously published data on intracellular trafficking of monocyte FcRn.12 Furthermore, we observed that FcRn enveloped target pathogens following human IgG1-mediated phagocytosis (Figure 3A), suggesting that FcRn-containing granules fuse with developing phagosomes and/or phagolysosomes during or after internalization. On phagocytosis, the bulk of FcRn expression was observed around phagosomes (Figure 3B-C).
Localization of FcRn within PMNs. FcRn expression in human granulocytes was analyzed by costaining with (A) myeloperoxidase (10-nm gold particles) or (B) lactoferrin (15-nm gold particles) using transmission electron microscopy. FcRn is visualized as 15-nm (A) or 10-nm (B) particles (white arrows). N indicates nucleus; PM, plasma membrane.
Localization of FcRn within PMNs. FcRn expression in human granulocytes was analyzed by costaining with (A) myeloperoxidase (10-nm gold particles) or (B) lactoferrin (15-nm gold particles) using transmission electron microscopy. FcRn is visualized as 15-nm (A) or 10-nm (B) particles (white arrows). N indicates nucleus; PM, plasma membrane.
FcRn is involved in IgG-mediated phagocytosis
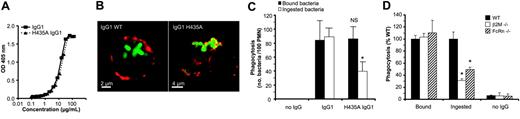
To assess the role of FcRn in PMN phagocytosis, we initially attempted to knock-down expression of FcRn in human PMNs and in various monocytic cell lines (U937, K562, 28SC) by different siRNA-based techniques and constructs. However, these attempts all resulted in severe loss of cell viability and eventual cell death (but not of scrambled controls). We therefore investigated the role of FcRn on human PMNs with recombinant WT and mutated IgG1 mAb and with PMNs from mice that lacked either the FcRn α-chain or the β2M subunit. We first compared the phagocytosis of S pneumoniae serotype 6B, using the 6B-specific recombinant human IgG1 mAb (GDob1),14 with a mutated derivative, where histidine 435 was replaced by alanine (H435A). This mutation abrogates specifically the IgG binding to FcRn, without affecting binding to leukocyte FcγR.23 The H435A mutation did not alter the antibody's antigen-binding capacity, as observed by ELISA (Figure 4A). Confocal microscopy revealed reduced PMN phagocytosis of pneumococci opsonized with H435A IgG1, relative to WT IgG1, with approximately 4-fold more PMNs with bacteria on the outside when bacteria were opsonized with H435A IgG1 (Figure 4B). After quenching of noningested bacteria and quantification of internalized bacteria by FACS, we also observed significantly less phagocytosis when using H435A IgG1-opsonized pneumococci (Figure 4C). IgG1 and H435A IgG1 induced similar binding of FITC-labeled bacteria to PMNs at 4°C (Figure 4C), confirming a normal interaction of H435A IgG1 with FcγRIIa and FcγRIIIb. No adherence to PMNs or phagocytosis of pneumococci was observed in the absence of IgG (Figure 4C) or in the presence of normal human serum without serotype-specific antibodies.14,24 Similar data were obtained with mouse PMNs when comparing IgG1 with H435A (data not shown).
Colocalization of FcRn and bacteria in PMNs. (A) Paraformaldehyde-fixed and saponin-treated PMNs after phagocytosis of Alexa488-labeled IgG-opsonized pneumococci (green) were analyzed through middle sections of cells by confocal microscopy. FcRn was visualized with a rabbit anti-FcRn antiserum (red). Bacteria, FcRn staining, and overlay are shown from left to right, respectively. (B) Fluorescence intensity (FI) of corresponding color channels shown in panel A were analyzed for whole cells showing enhanced localization of FcRn around phagosomes. In the rightmost panel, FI of FcR (red) and pneumococcal (green) are overlaid. (C) FI of pneumococci (green) and FcRn (red) across the cell along the line indicated (A, right; scale in arbitrary units). Experiments were repeated 3 times, yielding similar results.
Colocalization of FcRn and bacteria in PMNs. (A) Paraformaldehyde-fixed and saponin-treated PMNs after phagocytosis of Alexa488-labeled IgG-opsonized pneumococci (green) were analyzed through middle sections of cells by confocal microscopy. FcRn was visualized with a rabbit anti-FcRn antiserum (red). Bacteria, FcRn staining, and overlay are shown from left to right, respectively. (B) Fluorescence intensity (FI) of corresponding color channels shown in panel A were analyzed for whole cells showing enhanced localization of FcRn around phagosomes. In the rightmost panel, FI of FcR (red) and pneumococcal (green) are overlaid. (C) FI of pneumococci (green) and FcRn (red) across the cell along the line indicated (A, right; scale in arbitrary units). Experiments were repeated 3 times, yielding similar results.
FcRn facilitates PMN internalization of IgG-opsonized bacteria. (A) Antigen-binding capacity of a WT IgG1 mAb (GDob1) and its H435A IgG1 derivative to polysaccharide was indistinguishable by ELISA. IgG1 concentrations were quantified by anti-kappa capturing, and anti-Fc detection sandwich ELISA. Equal amounts of IgG were subsequently allowed to bind to pneumococcal polysaccharide 6B-coated plates, and bound IgG was detected with anti-kappa antisera. (B) Alexa488-labeled pneumococci (green) were ingested efficiently by human PMNs when incubated with WT IgG1 but not on incubation with the H435A IgG1 derivative. PMNs were visualized by red membrane staining using MAC-1 mAb (CR3/CD11b). (C) Binding (4°C) and phagocytosis (37°C) of IgG-opsonized FITC-labeled Streptococcus pneumoniae serogroup 6B measured by FACS on incubation with human PMNs in the absence (no IgG) or presence of 5 μg/mL human IgG1 (IgG1) or a mutated H435A IgG1 (H435A IgG1) variant. For evaluation of ingested bacteria (□), fluorescence of PMNs was measured after quenching of extracellular bacteria. Data are represented as phagocytic index (PI).15 (D) Ingestion of FITC-labeled pneumococci incubated at 37°C with PMNs from WT, β2M-, and FcRn-knockout mice in the presence of IgG1. Fluorescence of bound but not ingested bacteria were quenched as described in “Phagocytosis experiments.” NS indicates not significant; *P < .05 when compared with WT. Data are presented as means plus standard deviations from 2 (A) or 4 (D) experiments, respectively. Experiments (B-C) were performed 3 times, yielding similar results.
FcRn facilitates PMN internalization of IgG-opsonized bacteria. (A) Antigen-binding capacity of a WT IgG1 mAb (GDob1) and its H435A IgG1 derivative to polysaccharide was indistinguishable by ELISA. IgG1 concentrations were quantified by anti-kappa capturing, and anti-Fc detection sandwich ELISA. Equal amounts of IgG were subsequently allowed to bind to pneumococcal polysaccharide 6B-coated plates, and bound IgG was detected with anti-kappa antisera. (B) Alexa488-labeled pneumococci (green) were ingested efficiently by human PMNs when incubated with WT IgG1 but not on incubation with the H435A IgG1 derivative. PMNs were visualized by red membrane staining using MAC-1 mAb (CR3/CD11b). (C) Binding (4°C) and phagocytosis (37°C) of IgG-opsonized FITC-labeled Streptococcus pneumoniae serogroup 6B measured by FACS on incubation with human PMNs in the absence (no IgG) or presence of 5 μg/mL human IgG1 (IgG1) or a mutated H435A IgG1 (H435A IgG1) variant. For evaluation of ingested bacteria (□), fluorescence of PMNs was measured after quenching of extracellular bacteria. Data are represented as phagocytic index (PI).15 (D) Ingestion of FITC-labeled pneumococci incubated at 37°C with PMNs from WT, β2M-, and FcRn-knockout mice in the presence of IgG1. Fluorescence of bound but not ingested bacteria were quenched as described in “Phagocytosis experiments.” NS indicates not significant; *P < .05 when compared with WT. Data are presented as means plus standard deviations from 2 (A) or 4 (D) experiments, respectively. Experiments (B-C) were performed 3 times, yielding similar results.
Importantly, PMNs from both β2M knock-out mice (that lack FcRn expression9 ) and FcRn α-chain knock-out mice showed similar reduced levels of pneumococcal phagocytosis (Figure 4D).
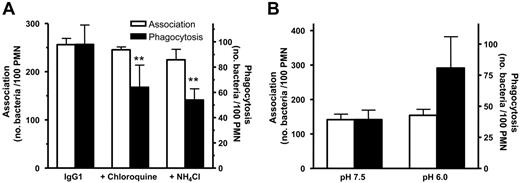
Next, we studied the influence of pH on phagocytosis using chloroquine as inhibitor of the ATP-dependent vacuolar proton pump (V-ATPase) and the weak base NH4Cl. Both agents induced a similar reduction in phagocytic activity of IgG1-opsonized pneumococci (Figure 5A). Conversely, by performing the phagocytosis assay in medium with pH 6.0, allowing for immediate IgG binding by FcRn, the phagocytosis rate was increased (Figure 5B).
FcRn-mediated IgG recycling
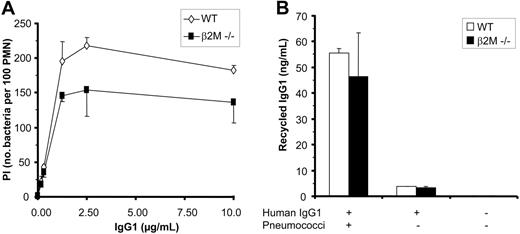
If FcRn-mediated IgG recycling was responsible for enhancing phagocytosis of IgG-opsonized bacteria, this effect would be expected to be more prominent at lower IgG concentrations, when IgG is a limiting factor. We noted that the lower level of phagocytosis observed in the absence of FcRn engagement was consistently more pronounced at saturating IgG concentrations and to be lower (or absent) at limiting IgG concentrations (Figure 6A). This was observed when IgG1 was compared with H453A IgG1 with human PMNs and when comparing knock-out with wild-type mouse PMNs. We next tested whether FcRn-dependent IgG recycling may underlie the lowered phagocytic activity of PMNs in the absence of FcRn engagement. However, both WT and β2M knock-out PMNs mediated similar low-level recycling of IgG, with less than 1% of IgG1 offered to the cells being recycled to culture medium following IgG1-mediated phagocytosis (Figure 6B). At this IgG1 concentration no significant phagocytosis was observed (Figure 6A). No IgG recycling was observed in the absence of bacteria (Figure 6B). In conclusion, our data do not support the hypothesis that FcRn mediates recycling of IgG after IgG-mediated phagocytosis.
Involvement of FcRn signaling motifs in phagocytosis
We next studied whether FcRn was actively involved with the enhanced internalization of IgG-opsonized bacteria. Peptides consisting of a TAT sequence and previously recognized internalization and transport motifs within the FcRn intracellular tail25-27 (Figure 7A) readily diffused over the plasma membrane (Figure 7B) and inhibited phagocytosis of both PMNs and monocytes in a concentration-dependent manner (Figure 7C). Control TAT peptides, with identical amino acid composition but with the key amino acids within the motifs shuffled, were detected intracellularly at levels similar to the wild-type peptide (Figure 7B) but had no significant effect on phagocytosis (Figure 7D).
Effect of pH on IgG1-mediated phagocytosis. (A) The V-ATPase inhibitor chloroquine and the weak base NH4Cl inhibit phagocytosis but have no effect on association of IgG1-osonized pneumococci to PMNs. (B) Phagocytosis is increased in medium of pH 6.0 but has no effect on association of IgG1-opsonized pneumococci. **P < .01 when compared with WT by ANOVA. Data represent means plus standard deviations and are representative of at least 3 individual experiments.
Effect of pH on IgG1-mediated phagocytosis. (A) The V-ATPase inhibitor chloroquine and the weak base NH4Cl inhibit phagocytosis but have no effect on association of IgG1-osonized pneumococci to PMNs. (B) Phagocytosis is increased in medium of pH 6.0 but has no effect on association of IgG1-opsonized pneumococci. **P < .01 when compared with WT by ANOVA. Data represent means plus standard deviations and are representative of at least 3 individual experiments.
Recycling of IgG is not responsible for enhanced phagocytosis mediated by FcRn. (A) Effect of IgG level on phagocytosis of pneumococci by wild-type and β2M-/- mouse PMNs. Note that phagocytosis by β2M-/- PMNs is more impaired at higher concentrations of human IgG1. (B) Recycling of human IgG1 by wild-type and β2M-/- mouse PMNs. Wild-type mouse PMNs and β2M-/- PMNs were allowed to take up human IgG1 antiserotype 6B mAb (10 μg/mL) for 5 minutes at 37°C in the presence of serotype 6B pneumococci. After extensive washing, cells were incubated for an additional 30 minutes in medium to allow for exocytosis/recycling of IgG, and supernatants were subsequently analyzed for the presence of human anti-6B pneumococcal antibodies by ELISA. Control samples in which mouse PMNs were incubated with IgG1 only (without bacteria) did not result in significant recycling. Experiments were repeated 4 times, with essentially identical results. Data represent means plus or minus standard deviation.
Recycling of IgG is not responsible for enhanced phagocytosis mediated by FcRn. (A) Effect of IgG level on phagocytosis of pneumococci by wild-type and β2M-/- mouse PMNs. Note that phagocytosis by β2M-/- PMNs is more impaired at higher concentrations of human IgG1. (B) Recycling of human IgG1 by wild-type and β2M-/- mouse PMNs. Wild-type mouse PMNs and β2M-/- PMNs were allowed to take up human IgG1 antiserotype 6B mAb (10 μg/mL) for 5 minutes at 37°C in the presence of serotype 6B pneumococci. After extensive washing, cells were incubated for an additional 30 minutes in medium to allow for exocytosis/recycling of IgG, and supernatants were subsequently analyzed for the presence of human anti-6B pneumococcal antibodies by ELISA. Control samples in which mouse PMNs were incubated with IgG1 only (without bacteria) did not result in significant recycling. Experiments were repeated 4 times, with essentially identical results. Data represent means plus or minus standard deviation.
Discussion
The central initiator of IgG-mediated phagocytosis on human PMNs, FcγRIIa (CD32),28 is expressed primarily on the cell surface and is quickly internalized together with IgG-coated particles on receptor engagement.1,4 Unlike the classical leukocyte FcγRs, which are the only IgG receptors described so far on PMNs, we found FcRn to be exclusively localized in intracellular compartments in freshly isolated PMNs. The intracellular localization to both azurophilic and specific granules corroborates previous studies showing the presence of β2M in specific granules and secretory vesicles in PMNs29 and with studies documenting FcRn expression within human macrophages12 and in human and porcine monocytes.12,30 Our data are also in agreement with previous work showing that degranulation mediates translocation of FcRn from intracellular compartments to the cell surface on monocytes.12
Effect of FcRn-TAT peptides on phagocytosis of IgG-opsonized pneumococci. (A) Sequence of the FcRn tail included in the TAT peptides. Signaling motifs of FcRn documented within the FcRn intracellular tail, the tryptophan motif, the 2 aspartic acids, and the dileucine motif are underlined (FcRn WT). Shuffled amino acids in the control peptide with otherwise identical composition are shown in bold (shuffled FcRn). (B) Both wild-type and the shuffled control peptide are translocated over the plasma membrane. Biotinylated TAT peptides were incubated with PMNs. Extracellular staining was done on fixed PMNs, and internalized TAT peptides were detected on fixed and permeabilized cells. (C) WT FcRn-TAT peptides inhibit internalization of IgG1-opsonized bacteria. (D) Control FcRnS peptides had no effect on phagocytosis of IgG1-opsonized pneumococci (experiments were carried out with 0.8 mg/mL TAT peptides). Experiments were repeated 3 times, yielding comparable results. Data in panels C and D represent means plus or minus standard deviations. *P < .05; **P < .001 when compared with WT.
Effect of FcRn-TAT peptides on phagocytosis of IgG-opsonized pneumococci. (A) Sequence of the FcRn tail included in the TAT peptides. Signaling motifs of FcRn documented within the FcRn intracellular tail, the tryptophan motif, the 2 aspartic acids, and the dileucine motif are underlined (FcRn WT). Shuffled amino acids in the control peptide with otherwise identical composition are shown in bold (shuffled FcRn). (B) Both wild-type and the shuffled control peptide are translocated over the plasma membrane. Biotinylated TAT peptides were incubated with PMNs. Extracellular staining was done on fixed PMNs, and internalized TAT peptides were detected on fixed and permeabilized cells. (C) WT FcRn-TAT peptides inhibit internalization of IgG1-opsonized bacteria. (D) Control FcRnS peptides had no effect on phagocytosis of IgG1-opsonized pneumococci (experiments were carried out with 0.8 mg/mL TAT peptides). Experiments were repeated 3 times, yielding comparable results. Data in panels C and D represent means plus or minus standard deviations. *P < .05; **P < .001 when compared with WT.
To investigate FcRn's involvement in IgG-mediated phagocytosis by PMNs, we performed a series of confocal microscopic and FACS analyses, in which we observed a prominent FcRn staining around phagolysosomes on internalization of IgG-opsonized bacteria (Figure 3A), indicating FcRn to be selectively concentrated to phagolysosomes on IgG-mediated phagocytosis (Figure 3B-C).
We observed phagocytosis of both human and mouse PMNs to be severely impaired under experimental conditions preventing FcRn engagement. For experiments with mouse PMNs, we used human IgG1 which has been documented to have high affinity at pH 6.0 to mouse and human FcRn31 and to mediate efficient FcγRIII-dependent phagocytosis of pneumococci by mouse PMNs.14 By blocking acidification of phagolysosomes, IgG1-mediated phagosytosis was inhibited. Conversely, phagocytosis was found to be enhanced on adjustment of the extracellular pH to pH 6.0 (Figure 5). PMNs from β2M knock-out mice, lacking functional FcRn expression,9-11 were unable to efficiently phagocytose IgG1-opsonized pneumococci. PMNs from FcRn α-chain knock-out mice8 showed a similar phenotype (Figure 4D). Likewise, lowered phagocytic activity was observed by human PMNs when allowed to internalize pneumococci opsonized with a mutated IgG derivative (H435A) incapable of binding FcRn (Figure 4B-C).23 These studies show that a functional pH-dependent interaction between FcRn and IgG-opsonized pneumococci is required for optimal phagocytosis.
It has been established in various models that FcRn binds IgG at low pH and recycles or transports IgG through cells.5,8 IgG recycling by FcRn might, therefore, theoretically underlie the observed differences in phagocytosis. However, the differences in phagocytosis with and without FcRn engagement were more extreme at saturating IgG concentrations (Figure 6A). Although low levels of IgG recycling were observed, these were not due to FcRn expression (Figure 6B), suggesting that other mechanisms underlie the observed IgG recycling.32 Importantly, the level of IgG recycling was too low to account for significant phagocytosis (Figure 6A-B). IgG recycling is therefore unlikely to explain the apparent role of FcRn in phagocytosis.
Furthermore, we assessed the possibility that FcRn contributed to phagocytosis by actively meditating signals or links to the cellular IgG-transport machinery. We constructed 2 peptides; one containing a recently described tryptophan-based endocytosis motif,25 a serine within this motif important for apical to basolateral transport,26 and a separate endocytosis signal consisting of a dileucine motif which apparently functions in the context of 2 aspartic acids,27 and another peptide with these key amino acids scrambled (Figure 7A). Both peptides were constructed in the context of the HIV-derived TAT peptide,33 which mediates translocation over eukaryotic cellular membranes. The wild-type peptide, but not the scrambled control peptide, inhibited IgG-mediated phagocytosis of PMNs and monocytes, providing evidence that the intracellular FcRn tail is important for phagocytosis.
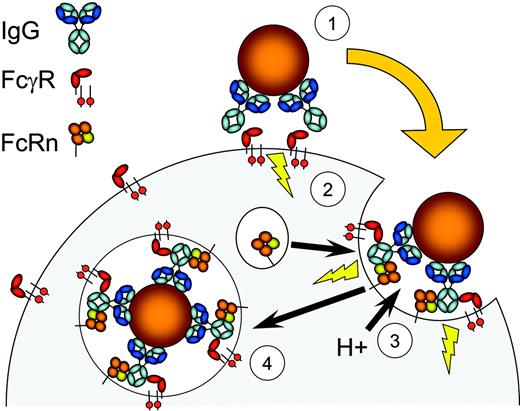
Our present data are consistent with the current paradigm within FcγR biology, where FcγR bind IgG-opsonized targets, resulting in degranulation and translocation of FcRn to nascent phagosomes. Subsequently, additional binding of FcRn to the IgG-opsonized targets may occur34 after phagosome acidification, which approaches pH 6.5 in around 8 minutes,35,36 and facilitate phagocytosis (summarized in Figure 8). How exactly FcRn mediates the observed effects in PMNs, monocytes, and macrophages is at present unclear. One attractive potential mechanism is that FcRn provides a direct link to molecular components of both endocytic and phagocytic machineries through association of the tryptophan motif with adaptor protein complex 2 (AP-2).25 AP-2 is known to associate with clathrin (and associated molecules), both of which have been implicated in IgG-mediated phagocytosis.37,38 The nature of the molecular components and signals involved in these processes warrants more detailed studies.
The MHC class I-like family of proteins arose early in vertebrate evolution,39 and its members have been implicated in a variety of biologic pathways, ranging from antigen presentation of peptides and phospholipids (MHC-I, CD1),40 coagulant and inflammatory responses (EPCR),41 fat (ZAG)42 and iron metabolism (HFE),43 pheromone perception,44 and IgG and albumin catabolism (FcRn).8,45 FcRn has been found in mammals and marsupials46 and is evolutionary highly conserved, with mouse and human FcRn overall sharing 65% identical amino acid sequences. This degree of conservation includes the intracellular tail, suggesting a strong selection pressure on FcRn-encoded functions.
In summary, we observed that FcRn envelops pneumococci-containing phagosomes in PMNs in the presence of pneumococcal-specific IgG and found phagocytosis to be impaired under conditions when FcRn-binding to IgG was abrogated. We evaluated this by introducing specific mutations in IgG and by knocking out either
β2M or FcRn. By interfering with select FcRn intracellular signaling motifs, a similar reduction in phagocytosis was observed. These findings implicate FcRn as directly involved in a cellular mechanism that has not been described for this molecule before, namely IgG-mediated phagocytosis. Our observations may explain why monocytes efficiently mediate phagocytosis through the ITAM-bearing FcγRIIIa (CD16), whereas NK cells (that do not express FcRn, Figure 1A) are incapable of phagocytosis on triggering of the same receptor.47 Furthermore, the present data may well provide a rationale for the relative inability of the phagocyte IgA receptor (FcαRI, CD89) to mediate phagocytosis by IgA (that does not bind FcRn), despite its potent capacity to trigger PMN degranulation.15,48
Illustration of a proposed role for FcRn in phagocytosis. IgG-opsonized bacteria engage leukocyte FcγR (1), which initiates the phagocytic process involving FcR-ITAM signaling motifs and downstream effectors (2). Activation leads to fusion of granules containing proton-pump components and FcRn with nascent phagosomes, which lowers the pH and promotes FcRn recognition of IgG (3). This process subsequently facilitates internalization of IgG-coated targets (4). In the absence of FcRn, efficient phagocytosis does not take place (see main text).
Illustration of a proposed role for FcRn in phagocytosis. IgG-opsonized bacteria engage leukocyte FcγR (1), which initiates the phagocytic process involving FcR-ITAM signaling motifs and downstream effectors (2). Activation leads to fusion of granules containing proton-pump components and FcRn with nascent phagosomes, which lowers the pH and promotes FcRn recognition of IgG (3). This process subsequently facilitates internalization of IgG-coated targets (4). In the absence of FcRn, efficient phagocytosis does not take place (see main text).
In conclusion, IgG-mediated phagocytosis, pathogen elimination, and the role of FcγR in phagocyte biology in particular need to be re-evaluated in light of these data.
Prepublished online as Blood First Edition Paper, July 18, 2006; DOI 10.1182/blood-2006-05-024539.
Supported by the Eijkman Graduate School for Immunology and Infectious Diseases (G.V.).
The authors declare no competing financial interests.
G.V. and A.M.S. contributed equally to this study.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Dr Derry Roopenian for supplying FcRn knock-out mice for this study; Drs Jeffrey Beekman, Eirikur Saeland, Nina van Sorge, Frank Miedema, Ellen van der Schoot, Frank A. Redegeld, and Maurice W. van der Heijden for helpful discussions; Marc Jansen, Marco Jansen, and Henriëtte Vilé for technical assistance; and Dr Dirk Roos for critically reviewing the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal