Abstract
The receptor tyrosine kinase FLT3 is a promising molecular therapeutic target in acute myeloid leukemia (AML). Activating mutations of FLT3 are present in approximately one-third of patients, while many nonmutants show evidence of FLT3 activation, which appears to play a significant role in leukemogenesis. We studied the effects of lestaurtinib (CEP701) and PKC412, 2 small molecule inhibitors of FLT3, on 65 diagnostic AML blast samples. Both agents induced concentration-dependent cytotoxicity in most cases, although responses to PKC412 required higher drug concentrations. Cytotoxic responses were highly heterogeneous and were only weakly associated with FLT3 mutation status and FLT3 expression. Importantly, lestaurtinib induced cytotoxicity in a synergistic fashion with cytarabine, particularly in FLT3 mutant samples. Both lestaurtinib and PKC412 caused inhibition of FLT3 phosphorylation in all samples. Translation of FLT3 inhibition into cytotoxicity was influenced by the degree of residual FLT3 phosphorylation remaining and correlated with deactivation of STAT5 and MAP kinase. FLT3 mutant and wild-type cases both varied considerably in their dependence on FLT3 signaling for survival. These findings support the continued clinical assessment of FLT3 inhibitors in combination with cytotoxic chemotherapy: Entry to future clinical trials should include FLT3 wild-type patients and should remain unrestricted by FLT3 expression level.
Introduction
Despite gradual improvements in combination chemotherapy regimens and supportive care, most acute myeloid leukemia (AML) patients continue to die from their disease. Only 35% to 45% of patients below the age of 60 years will be long-term survivors, and this figure falls to less than 15% in older patients.1 Advances in the understanding of molecular pathogenetic mechanisms of hematologic malignancy have fueled a drive for the development of targeted therapeutic approaches that are able to improve disease remission rates without increasing treatment-related toxicity. Dys-regulated receptor tyrosine kinase activity has been implicated in many neoplastic processes,2,3 and the remarkable success of the tyrosine kinase inhibitor imatinib mesylate in chronic myeloid leukemia has stimulated a search for similar small molecule inhibitors effective in AML.4
The important role played by FMS-like tyrosine kinase 3 (FLT3) in the survival and proliferation of AML blasts, and its mutation and overexpression in large numbers of AML patients, makes FLT3 an attractive therapeutic target.5-9 The FLT3 receptor is expressed at high levels in most cases of AML.10-12 Constitutive activation of FLT3 in AML cells occurs through either endogenous coexpression of FLT3 ligand (FL)13,14 or through the presence of activating mutations that include internal tandem duplication (ITD) mutations within the juxtamembrane region15 and single amino acid point mutations within the tyrosine kinase domain of the receptor (TKD-PMs).16,17 Activating mutations of FLT3 are among the most common molecular lesions so far described in AML, with a combined prevalence of 31%.7 The presence of a FLT3 ITD mutation is now recognized as an independent predictor of poor clinical outcome.18-20 Activation of FLT3, either through FL binding or mutation, appears to play a significant role in leukemogenesis, causing activation of downstream targets including proteins in the STAT, MAP kinase, and AKT pathways, which are involved in regulation of proliferation, transcription, and apoptosis.21-23
A number of FLT3-selective small molecule tyrosine kinase inhibitors have been developed by the biotechnology industry in recent years.5 Lestaurtinib (CEP701) and PKC412 are indolocarbazole alkaloid compounds that are synthetically derived from compounds of microbial origin. Both are orally bioavailable, relatively selective inhibitors of FLT3 kinase.
Lestaurtinib inhibits phosphorylation of ITD and wild-type (WT) FLT3 with an IC50 of 3 nM.24 It is cytotoxic to human AML cell lines expressing both mutant and WT FLT3 at doses similar to those required to inhibit phosphorylation of the FLT3 receptor, and it prolongs survival in a mouse model of FLT3 ITD leukemia.24 In vitro cytotoxicity assays using primary pediatric AML cells showed preferential killing of samples with ITD mutations.25 Cell line evidence suggests that lestaurtinib acts synergistically with standard cytotoxic agents if used simultaneously with or immediately following chemotherapy.26
PKC412 was developed originally as an inhibitor of protein kinase C.27 It is also a potent FLT3 inhibitor (IC50 10 nM) and inhibits PDGF-Rβ with an IC50 of 80 nM and KIT at doses above 500 nM.28 PKC412 inhibits FLT3 phosphorylation in Ba/F3 cells transected with WT and mutant FLT3, inhibiting cell proliferation and inducing apoptosis.28 It also causes dose-dependent cytotoxicity in primary acute lymphocytic leukemia (ALL) blasts expressing high levels of FLT3.29 Orally administered PKC412 prolongs survival in mice with activated FLT3-induced myeloproliferative syndrome.28,30
Lestaurtinib, PKC412, and other candidate FLT3 inhibitors have so far shown similar but limited monotherapeutic clinical activity in phase 1 and 2 trials in adults with relapsed/refractory FLT3 mutant AML.31-34 Transient peripheral blood and bone marrow responses were seen in both FLT3 mutant and WT patients when lestaurtinib was used as first-line treatment for older patients not considered fit for intensive chemotherapy.35
Several important questions regarding the role and potential target populations of these drugs highly pertinent to the planning of future clinical trials remain unanswered. It has not been fully established whether FLT3 inhibitors are likely to benefit FLT3/WT patients or whether baseline FLT3 expression level is likely to influence drug response. The fundamental relationship between FLT3 inhibition, its consequences on downstream signaling, and the onset of cytotoxicity remains poorly understood. We studied the in vitro cytotoxic effects of lestaurtinib and PKC412, used alone and in combination with chemotherapy, on primary adult AML blasts to allow the correlation of patient response with FLT3 mutation status and expression. We also assessed the effects of FLT3 inhibition on proliferative and antiapoptotic signaling to enable greater understanding of the interpatient variations in signaling patterns that appear to influence the onset of cytotoxicity.
Materials and methods
Primary cells
Bone marrow and venous blood samples were collected in preservative-free heparin or EDTA from newly diagnosed AML patients entering the United Kingdom Medical Research Council (UK MRC) AML14 and 15 trials and the National Cancer Research Institute (NCRI) CEP701 trial35 at hospitals throughout the United Kingdom. Samples were enriched by Ficoll-Histopaque density gradient centrifugation (Sigma-Aldrich, Gillingham, United Kingdom) and cryopreserved at -80°C in 10% dimethyl sulfoxide (DMSO) until required for use. Minimum pretreatment cell viability (assessed by trypan blue exclusion) of more than 90% was required for drug culture experiments. Cells were cultured at 37°C with 5% CO2 in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, l-glutamine, and penicillin/streptomycin. Human samples involved in this study were donated following written informed consent using documentation approved by the Medical Research Ethics Committee and the institutional review board of the University Hospital of Wales Cardiff.
Reagents
Lestaurtinib (Cephalon, West Chester, PA) was stored at -20°C as a 4 mM stock solution in DMSO and diluted with RPMI culture medium immediately prior to use. PKC412 (Novartis Pharma, Basel, Switzerland) was stored at -20°C as a 10 mM stock solution in DMSO. Prior to use an initial 10-fold dilution was made with 50% DMSO/50% distilled water, with the resulting solution being diluted with RPMI culture medium. Cytarabine was dissolved in distilled water: a 10-mM stock solution was prepared by dilution with RPMI culture medium and stored at -20°C. Anti-FLT3 S-18 and anti-STAT5 C17 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antiphosphotyrosine 4G10 antibody and protein A-agarose were purchased from Upstate Biotechnology (Lake Placid, NY). Anti-MAP kinase (p44/42), anti-AKT, antiphospho-STAT5 (Tyr694), antiphospho-MAP kinase (p44/42), and antiphospho-AKT (Ser473) antibodies were purchased from Cell Signaling Technology (Beverly, MA).
FLT3 mutation analysis
Messenger RNA was extracted from Ficoll-Histopaque-enriched mononuclear cells (MNCs) using Trizol and Eppendorf Phase Lock Gel tubes (Fischer Scientific, Loughborough, United Kingdom). FLT3 ITD reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using the forward primer 5′-GCAAATTAGGTATGAAAGCCAGC-3′ and the reverse primer 5′-CTTTCAGCATTTTGACGGCAACC-3′. FLT3 TKD-PMs were detected using ALM forward primer 5′-CCGCCAGGAACGTGTTTG-3′ and ALM reverse primer 5′-CACAGTAATATTCCATATGACCAGATATC-3′ followed by EcoRV or HinfI digestion. PCR products were analyzed, and the ratio between mutant and WT FLT3 alleles calculated using DNA500 chips on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA).
RT-PCR quantification of FLT3 mRNA expression
Expression level of the FLT3 transcript was quantified by a real-time fluorescence detection method, relative to expression of the stably expressed ribosomal housekeeping gene S14, using a LightCycler System (Roche, Mannheim, Germany). The sense and antisense primer sequences were the same as those used for FLT3 ITD mutation analysis. FLT3 cDNA was amplified in glass capillaries using the LightCycler FastStart Master SYBR Green I mix (Roche) containing FastStart Taq DNA polymerase and the double-stranded DNA-specific fluorescent dye SYBR Green I, with fluorescence being quantified over time. A second run was performed for each patient sample to quantify amplification of the housekeeping gene S14. Relative gene expression levels were calculated using LightCycler Relative Quantification Software (Roche).
Cytotoxicity assay
Cytotoxicity was assessed using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium (MTS) assay. Stock solutions of lestaurtinib and PKC412 were diluted with culture medium to twice the highest concentration to be assayed and serially diluted into triplicate wells of 96-well plates. Control DMSO solutions containing equivalent concentrations of DMSO to those found in the drug solutions were prepared and similarly serially diluted. Forty-five-microliter aliquots of MNCs resuspended in culture medium at 5 × 106/mL were added to each well (final, 225 000 cells per well in 90 μL volume). After incubation for 72 hours at 37°C 5% CO2, MTS assay was performed according to the manufacturer's instructions (Promega, Madison, WI). Cell viability at each drug concentration was expressed as a percentage of viability of untreated cells cultured in an equivalent concentration of DMSO.
The cytotoxic response to combinations of 2 drugs (lestaurtinib/cytarabine [AraC], PKC412/AraC, and lestaurtinib/PKC412) was also quantified. Each drug, when used in combination, was assessed over the same range of concentrations that had been used in the single-drug assay. For each drug combination, a stock medium containing the 2 agents at a fixed ratio was prepared and serially diluted, along with appropriate DMSO control solutions. MTS assay was performed after 72-hour incubation.
Immunoprecipitation and immunoblotting
Aliquots of 1 × 107 to 2 × 107 MNCs were treated with a range of concentrations of lestaurtinib, PKC412, or DMSO control for 1 hour at 37°C 5% CO2 and then washed 3 times in ice-cold PBS and lysed in detergent buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 1% Igepal (Sigma), 10% glycerol, 10 mM EDTA, 20 mM sodium fluoride, and 3 mM sodium orthovanadate with MiniComplete protease inhibitor cocktail tablet (Roche) for 30 minutes at 4°C followed by centrifugation at 23 500g. A total of 500 μg of clarified protein lysate was incubated overnight at 4°C with anti-FLT3 antibody prior to the addition of protein A-agarose beads for 2 hours. For analysis of downstream signaling proteins, 20 μg of whole cell lysate was used. Following electrophoresis using NuPage precast 4% to 12% Bis-Tris gels (Invitrogen, Paisley, United Kingdom) and transfer to PVDF membranes (Invitrogen), immunoblotting was performed with antiphosphotyrosine, antiphospho-STAT5, antiphospho-AKT, or antiphospho-MAPK antibody, followed by stripping and reblotting, respectively, with anti-FLT3, anti-STAT5, anti-AKT, or anti-MAPK antibody. Blots were visualized by chemiluminescence (ECL Advance; Amersham Biosciences, Piscataway, NJ) with the resultant images being analyzed using a UVIDoc Acquisition Unit and UVISoft Image Analysis Software (UVITec, Cambridge, United Kingdom). FLT3 phosphorylation at each drug concentration was expressed as a percentage of FLT3 phosphorylation in untreated control cells after correction for any variation in total FLT3 concentration.
Statistical analysis
Statistical significance of the difference in mean MTS cytotoxicity responses and FLT3 dephosphorylation IC50 values by group was determined by Student t test. Drug combination data were analyzed using the median effect method of Chou and Talalay.36 The software package Calcusyn 1.2 (Biosoft, Cambridge, United Kingdom) was used to perform linear regression analysis of dose-response data from each experiment and calculate a combination index (CI) for each individual patient for each drug combination. According to this method, convention generally defines CI values between 0.9 and 1.1 as implying additivity; with 0.3 to 0.9 implying synergism; less than 0.3, strong synergism; 1.1 to 3.3, antagonism; and more than 3.3, strong antagonism. During analysis, mutual nonexclusivity with independent modes of action was assumed for combinations of FLT3 inhibitor with AraC, while mutual exclusivity with similar modes of action was assumed for the combination of lestaurtinib and PKC412. The V-squared (V2) test37 was used to determine the statistical significance of postulated synergistic population effects.
Results
In vitro cytotoxic effects of FLT3 inhibitors on primary AML mononuclear cells
MTS cytotoxicity assay was performed in triplicate with lestaurtinib and PKC412 using cryopreserved primary AML MNCs obtained from 96 patients. Results were considered evaluable if there was adequate viability, equating to a mean optical density of at least 0.300, in the untreated control wells following the 72-hour drug incubation period. By this criterion, assays were successful in 65 cases (68%). Blasts accounted for 41% to 100% (median, 85%) of MNCs prior to drug incubation in these samples. All patient samples were screened for FLT3 activating mutations, with investigators blinded to the FLT3 mutation status of each case until after the cytotoxicity assay had been performed. Of the 65 evaluable patients, 21 (32%) harbored FLT3 ITD mutations, ranging in size from 15 to 136 base pairs and accounting for 0.4% to 86% of total FLT3 RNA. Six further cases (9%) showed FLT3 TKD-PMs, with the remaining 38 cases expressing only WT FLT3. Clinical parameters are summarized in Table 1: FLT3 ITD mutant cases showed a trend toward a higher presenting white blood cell (WBC) count and were exclusively of intermediate-risk karyotype.
Clinical parameters of 65 MTS cytotoxicity assay-evaluable patients grouped according to FLT3 mutation status
Characteristic . | FLT3 ITD . | FLT3 TKD-PM . | FLT3 WT . | p* . |
|---|---|---|---|---|
| No. | 21 | 6 | 38 | |
| Sex, no. (%) | 5 (24) | 3 (50) | 18 (47) | .082 |
| Male | 15 (71) | 3 (50) | 19 (50) | |
| Female | 1 (5) | 0 (0) | 1 (3) | |
| Median age, y (range) | 63 (16-87) | 65 (17-74) | 61 (17-87) | .811 |
| Median presenting WBC count, × 109/L (range) | 79.5 (3-230) | 42.4 (29-258) | 25.8 (1-312) | .073 |
| Cytogenetics, no. (%) | ||||
| Favorable | 0 (0) | 1 (17) | 6 (16) | .065 |
| Intermediate | 13 (62) | 4 (67) | 17 (45) | .011 |
| Adverse | 0 (0) | 0 (0) | 4 (11) | .174 |
| Not available | 8 (38) | 1 (17) | 11 (29) | — |
| FAB classification, no. (%) | ||||
| M0 | 0 (0) | 1 (17) | 2 (5) | .315 |
| M1 | 4 (19) | 2 (33) | 8 (21) | .957 |
| M2 | 4 (19) | 0 (0) | 10 (26) | .701 |
| M4 | 7 (33) | 1 (17) | 11 (29) | .488 |
| M5 | 2 (10) | 1 (17) | 3 (8) | .714 |
| RAEB | 0 (0) | 0 (0) | 1 (3) | .482 |
| Not available | 4 (19) | 1 (17) | 3 (8) | — |
Characteristic . | FLT3 ITD . | FLT3 TKD-PM . | FLT3 WT . | p* . |
|---|---|---|---|---|
| No. | 21 | 6 | 38 | |
| Sex, no. (%) | 5 (24) | 3 (50) | 18 (47) | .082 |
| Male | 15 (71) | 3 (50) | 19 (50) | |
| Female | 1 (5) | 0 (0) | 1 (3) | |
| Median age, y (range) | 63 (16-87) | 65 (17-74) | 61 (17-87) | .811 |
| Median presenting WBC count, × 109/L (range) | 79.5 (3-230) | 42.4 (29-258) | 25.8 (1-312) | .073 |
| Cytogenetics, no. (%) | ||||
| Favorable | 0 (0) | 1 (17) | 6 (16) | .065 |
| Intermediate | 13 (62) | 4 (67) | 17 (45) | .011 |
| Adverse | 0 (0) | 0 (0) | 4 (11) | .174 |
| Not available | 8 (38) | 1 (17) | 11 (29) | — |
| FAB classification, no. (%) | ||||
| M0 | 0 (0) | 1 (17) | 2 (5) | .315 |
| M1 | 4 (19) | 2 (33) | 8 (21) | .957 |
| M2 | 4 (19) | 0 (0) | 10 (26) | .701 |
| M4 | 7 (33) | 1 (17) | 11 (29) | .488 |
| M5 | 2 (10) | 1 (17) | 3 (8) | .714 |
| RAEB | 0 (0) | 0 (0) | 1 (3) | .482 |
| Not available | 4 (19) | 1 (17) | 3 (8) | — |
RAEB indicates refractory anemia with excess blasts
P values refer to statistical comparisons made between FLT3/WT and FLT3/ITD groups. Statistical significance of differences in FAB type, cytogenetic risk group, and sex was determined by the χ2 independence test. Statistical significance of differences in age and presenting WBC count was determined by the Student t test
Figure 1A shows dose-response curves of the mean MTS responses to lestaurtinib and PKC412 treatment for all 65 evaluable samples, normalized to untreated controls and grouped according to FLT3 mutation status. At most concentrations studied, lestaurtinib was significantly more cytotoxic to primary AML blasts with FLT3 ITD mutations than to those that expressed only WT FLT3. There was also a trend toward greater drug sensitivity in blasts harboring FLT3 TKD-PMs. There was no evidence of a differential level of cytotoxic response to PKC412 according to FLT3 mutation status. For both drugs, cytotoxic responses of MNCs from different patients were highly heterogeneous, even after accounting for FLT3 mutation status. This variation in cytotoxic response was also independent of blast percentage in the MNC fraction, presenting WBC count, cytogenetic risk group, and French-American-British (FAB) classification. For each individual patient, Calcusyn software (Biosoft) was used to calculate a 50% inhibitory concentration (IC50) for cytotoxic responses to both lestaurtinib and PKC412. The heterogeneity of IC50 obtained, with marked overlap between FLT3 mutant and WT cases, is illustrated in Figure 1B.
Several studies have suggested that FLT3 ITD mutant cases with a high mutant-wild-type FLT3 allelic ratio have a distinctly worse clinical prognosis.18,38,39 In the current study, lestaurtinib proved more cytotoxic to primary AML blasts with high FLT3 ITD mutant-wild-type allelic ratios than to blasts with ITD mutations that accounted for only a low percentage of total FLT3, with this differential response being particularly evident at low concentrations of lestaurtinib of similar magnitude to those known to inhibit FLT3 receptor phosphorylation24 (Figure 1C). There was no evidence of differential cytotoxicity according to FLT3 ITD length.
In vitro cytotoxic response of primary AML blasts to single-agent lestaurtinib and PKC412. (A) MTS assay dose-response curves showing mean cytotoxic response of 65 patient samples, grouped according to FLT3 mutation status, to lestaurtinib and PKC412, normalized to untreated controls. Error bars represent standard error of the mean (SEM). P values refer to statistical comparison of the FLT3 ITD and WT groups at each dose using the Student t test (only significant P values [P < .05] shown). (B) Dot plots showing the range of individual patient in vitro MTS IC50 values for lestaurtinib and PKC412. The median and quartiles for each mutation group are indicated. P values refer to intergroup statistical analysis of log-transformed data by the Student t test, with significant differences in lestaurtinib IC50 distribution being seen between WT and ITD cases and between WT cases and all FLT3 mutants grouped together. No significant group differences were seen with PKC412. (C) MTS assay dose-response curves showing mean cytotoxic response of 21 ITD samples to lestaurtinib and PKC412, grouped according to percentage FLT3 ITD RNA.
In vitro cytotoxic response of primary AML blasts to single-agent lestaurtinib and PKC412. (A) MTS assay dose-response curves showing mean cytotoxic response of 65 patient samples, grouped according to FLT3 mutation status, to lestaurtinib and PKC412, normalized to untreated controls. Error bars represent standard error of the mean (SEM). P values refer to statistical comparison of the FLT3 ITD and WT groups at each dose using the Student t test (only significant P values [P < .05] shown). (B) Dot plots showing the range of individual patient in vitro MTS IC50 values for lestaurtinib and PKC412. The median and quartiles for each mutation group are indicated. P values refer to intergroup statistical analysis of log-transformed data by the Student t test, with significant differences in lestaurtinib IC50 distribution being seen between WT and ITD cases and between WT cases and all FLT3 mutants grouped together. No significant group differences were seen with PKC412. (C) MTS assay dose-response curves showing mean cytotoxic response of 21 ITD samples to lestaurtinib and PKC412, grouped according to percentage FLT3 ITD RNA.
In vitro cytotoxic effects of FLT3 inhibitors in combination with chemotherapy
Of the 65 patient samples that were successfully studied in single-drug cytotoxicity assays, sufficient material was available to perform MTS assessment of the combinations of lestaurtinib and AraC in 55 cases, PKC412 and AraC in 58 cases, and lestaurtinib and PKC412 in a further 38 cases. The drugs were applied simultaneously in fixed ratios over the same range of concentrations that were used in the single-drug experiments.
Cytotoxic dose-response data were analyzed according to the median effect principle of Chou and Talalay.36 Figure 2 illustrates the range of CI values obtained for each of the 3 drug combinations across the sample population. Again, primary blasts from different patients displayed a high degree of heterogeneity of drug response. The median CI values obtained for lestaurtinib/AraC, PKC412/AraC,and lestaurtinib/PKC412 were 0.73, 0.78 and 0.96, respectively, suggesting an overall minor degree of synergy when the FLT3 inhibitors were combined with AraC and an approximately additive effect when the 2 FLT3 inhibitors were used in tandem. To determine statistical significance, the distribution of the study population relative to a CI cutoff value of 1 was compared with an equally sized hypothetical “additive” population with a median CI of 1 using the V2 test. Statistical significance was just reached with lestaurtinib/AraC (P = .043) but not with PKC412/AraC (P = .061). When cases were stratified according to FLT3 mutation status, a significant overall synergistic effect was seen with lestaurtinib/AraC in FLT3 mutant (P = .008) but not WT cases (P = .619). This significance was lost when FLT3 mutant cases were subdivided into ITD (P = .052) and TKD-PM (P = .056) subpopulations. There was no statistical evidence to support synergy in any of the FLT3 mutational subpopulations treated with the PKC412/AraC combination.
In vitro cytotoxic effects of lestaurtinib and PKC412 in combination with cytarabine. (A) Dot plot showing the range of individual patient combination indices (CIs) obtained following analysis of MTS in vitro cytotoxicity assay fixed-ratio drug combination data using the method of Chou and Talalay. Median and quartiles are shown for each drug combination. P values refer to the significance of a postulated synergistic population effect for each drug combination and were obtained using the V2 test to assess the distribution of the study population relative to an equally sized hypothetical population with a median CI of 1. (B) Dot plots showing the range of individual patient CIs obtained following in vitro treatment with lestaurtinib/AraC and PKC412/AraC. Patients are grouped according to FLT3 mutation status, and P values again refer to V2 test used to assess for significance of synergistic effects.
In vitro cytotoxic effects of lestaurtinib and PKC412 in combination with cytarabine. (A) Dot plot showing the range of individual patient combination indices (CIs) obtained following analysis of MTS in vitro cytotoxicity assay fixed-ratio drug combination data using the method of Chou and Talalay. Median and quartiles are shown for each drug combination. P values refer to the significance of a postulated synergistic population effect for each drug combination and were obtained using the V2 test to assess the distribution of the study population relative to an equally sized hypothetical population with a median CI of 1. (B) Dot plots showing the range of individual patient CIs obtained following in vitro treatment with lestaurtinib/AraC and PKC412/AraC. Patients are grouped according to FLT3 mutation status, and P values again refer to V2 test used to assess for significance of synergistic effects.
Variations in FLT3 expression level have relatively little impact on in vitro sensitivity to the cytotoxic effects of FLT3 inhibitors. (A) Scatter plot showing FLT3 mRNA expression levels of cryopreserved MNCs from 64 patients measured by a real-time PCR method relative to expression of the housekeeping gene S14. Cases are grouped according to FLT3 mutation status. Analysis by the Student t test showed no significant differences in FLT3 RNA expression between mutation groups. (B) Mean MTS-derived cytotoxic dose responses of cells from 43 patients to lestaurtinib and PKC412. Cases are grouped by FLT3 mRNA expression level relative to the population median. Error bars represent the SEM. P values refer to a comparison of high and low FLT3 expression groups at each dose using the Student t test (only significant P values [P < .05] shown).
Variations in FLT3 expression level have relatively little impact on in vitro sensitivity to the cytotoxic effects of FLT3 inhibitors. (A) Scatter plot showing FLT3 mRNA expression levels of cryopreserved MNCs from 64 patients measured by a real-time PCR method relative to expression of the housekeeping gene S14. Cases are grouped according to FLT3 mutation status. Analysis by the Student t test showed no significant differences in FLT3 RNA expression between mutation groups. (B) Mean MTS-derived cytotoxic dose responses of cells from 43 patients to lestaurtinib and PKC412. Cases are grouped by FLT3 mRNA expression level relative to the population median. Error bars represent the SEM. P values refer to a comparison of high and low FLT3 expression groups at each dose using the Student t test (only significant P values [P < .05] shown).
Variations in FLT3 expression level have little impact on in vitro sensitivity to the cytotoxic effects of FLT3 inhibitors
FLT3 RNA expression was quantified by real-time PCR relative to expression of the housekeeping gene S14 in 64 patients, including 17 with FLT3 ITD mutations, 5 with FLT3 TKD-PMs, and 42 patients who expressed only WT FLT3. FLT3/S14 expression ratios ranged from 0.005 to 14.026 (median, 1.495), a 2750-fold difference in FLT3 RNA expression from lowest to highest. In agreement with previously reported data,40,41 no significant difference (or trend) in FLT3 RNA expression was seen between patients that harbored FLT3 activating mutations and WT cases (Figure 3A).
The in vitro effects of lestaurtinib and PKC412 were assessed by MTS assay using MNCs from 43 of these 64 patients, including 24 WT cases. Mean in vitro cytotoxic dose responses, stratified according to FLT3 RNA expression level, are shown in Figure 3B. Patients were divided into high and low FLT3 expression groups according to whether FLT3 RNA expression fell above or below the population median. There was a trend toward increased sensitivity to the cytotoxic effects of both agents in cells from patients with high FLT3 RNA expression levels, but this difference between “high and low expressers” only reached statistical significance at isolated drug concentrations. The trend toward greater cytotoxic response in cases with high FLT3 expression was also evident when WT cases were assessed in isolation.
The in vitro effects of lestaurtinib and PKC412 on FLT3 phosphorylation in primary AML mononuclear cells
To assess the in vitro effects of FLT3 inhibitors on FLT3 receptor phosphorylation, MNCs from 12 patients were exposed for 1 hour to a range of concentrations of lestaurtinib and PKC412. The cases included both FLT3 inhibitor-responsive and -resistant patients as identified by MTS assay, including 6 with ITD mutations, 1 with D835Y point mutant, and 5 FLT3/WT cases (Table 2). FLT3 was isolated by immunoprecipitation, which was followed by sequential immunoblotting with antiphosphotyrosine and anti-FLT3 antibodies.
FLT3 mutation data and presentation clinical parameters for patient samples studied in Western blotting experiments
. | . | . | . | . | . | . | MTS IC50, nM . | . | |
|---|---|---|---|---|---|---|---|---|---|
| No. . | Sex . | Age, y . | FAB type . | Presenting WBC count, × 109/L . | Mutation type* . | % mutant RNA† . | Lestaurtinib . | PKC412 . | |
| M-1 | F | 74 | M1 | 140.7 | ITD 33 bp | 34 | 44 | 647 | |
| M-2 | F | 40 | M1 | 112.8 | ITD 40 bp | 84 | 173 | ++ | |
| M-3 | F | 56 | M4 | 73.6 | ITD 25 bp | 7 | ++ | ++ | |
| M-4 | F | 66 | M2 | 52.5 | ITD 33 bp | 18 | 178 | ++ | |
| M-5 | F | 39 | M4 | 230.2 | ITD 60 bp | 30 | 95 | 320 | |
| M-6 | M | 45 | M4 | 162.0 | ITD 24 bp | 62 | 14 | 704 | |
| M-7 | M | 71 | M1 | 50.5 | D835Y | 45 | 24 | 32 | |
| W-1 | M | 18 | M1 | 29.0 | WT | — | ++ | ++ | |
| W-2 | M | 17 | M1 | 75.0 | WT | — | ++ | ++ | |
| W-3 | NA | 42 | M5 | 150.0 | WT | — | 42 | 312 | |
| W-4 | F | 44 | n/a | 190.0 | WT | — | ++ | ++ | |
| W-5 | F | 41 | n/a | n/a | WT | — | 69 | ++ | |
. | . | . | . | . | . | . | MTS IC50, nM . | . | |
|---|---|---|---|---|---|---|---|---|---|
| No. . | Sex . | Age, y . | FAB type . | Presenting WBC count, × 109/L . | Mutation type* . | % mutant RNA† . | Lestaurtinib . | PKC412 . | |
| M-1 | F | 74 | M1 | 140.7 | ITD 33 bp | 34 | 44 | 647 | |
| M-2 | F | 40 | M1 | 112.8 | ITD 40 bp | 84 | 173 | ++ | |
| M-3 | F | 56 | M4 | 73.6 | ITD 25 bp | 7 | ++ | ++ | |
| M-4 | F | 66 | M2 | 52.5 | ITD 33 bp | 18 | 178 | ++ | |
| M-5 | F | 39 | M4 | 230.2 | ITD 60 bp | 30 | 95 | 320 | |
| M-6 | M | 45 | M4 | 162.0 | ITD 24 bp | 62 | 14 | 704 | |
| M-7 | M | 71 | M1 | 50.5 | D835Y | 45 | 24 | 32 | |
| W-1 | M | 18 | M1 | 29.0 | WT | — | ++ | ++ | |
| W-2 | M | 17 | M1 | 75.0 | WT | — | ++ | ++ | |
| W-3 | NA | 42 | M5 | 150.0 | WT | — | 42 | 312 | |
| W-4 | F | 44 | n/a | 190.0 | WT | — | ++ | ++ | |
| W-5 | F | 41 | n/a | n/a | WT | — | 69 | ++ | |
++ indicates IC50 not reached at 200 nM lestaurtinib or 1000 nM PKC412; n/a, not available; and —, not applicable
FLT3 ITD with size in number of base pairs or TKD point mutation
Mutant FLT3 RNA expression as a percent of total FLT3 RNA expression
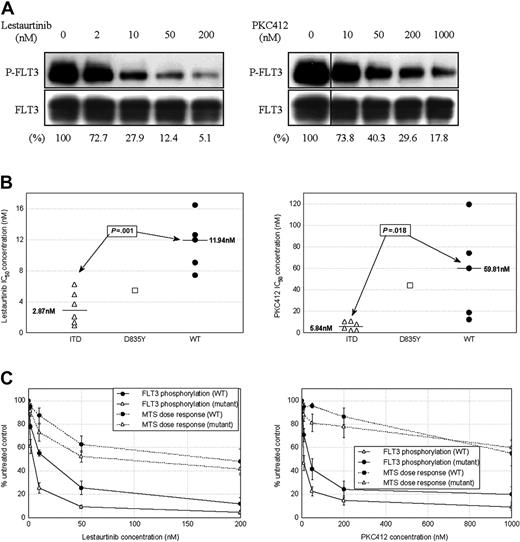
FLT3 protein was found to be present and phosphorylated in all 12 patient samples, with wide variation in levels of both total and phosphorylated FLT3 in untreated cells. There was very little observed overall difference in levels of baseline FLT3 phosphorylation between WT and mutant cases. Treatment with lestaurtinib and PKC412 caused dose-dependent dephosphorylation of FLT3 in all samples (Figure 4A). There was a significant degree of intercase variation in terms of both dose response and levels of residual FLT3 phosphorylation remaining following treatment. Several WT cases displayed more than 20% residual FLT3 phosphorylation at maximal drug concentrations. IC50 concentrations for inhibition of FLT3 phosphorylation were significantly lower in ITD mutant than WT cases: The median IC50 for FLT3 inhibition in response to lestaurtinib was 2.9 nM in mutant and 11.9 nM in WT cases (P = .001), while for PKC412 the respective values were 5.8 nM and 59.8 nM (P = .018) (Figure 4B).
To assess the relationship between FLT3 inhibition and cytotoxicity, 72-hour MTS assays were performed using cells from each of the 12 cases exposed to a similar range of concentrations of both agents. Cytotoxic effects tended to occur at higher drug concentrations than those required to simply inhibit FLT3. Although cytotoxic dose responses paralleled the inhibition of FLT3 phosphorylation, providing supportive evidence that the cytotoxic effects of the drugs are mediated primarily through the direct inhibition of FLT3 (Figure 4C), cytotoxic responses again showed much greater heterogeneity, with marked overlap occurring between the cytotoxic responses of FLT3 mutant and WT cases.
Almost complete dephosphorylation of FLT3 appears necessary for the achievement of a cytotoxic response to lestaurtinib and PKC412
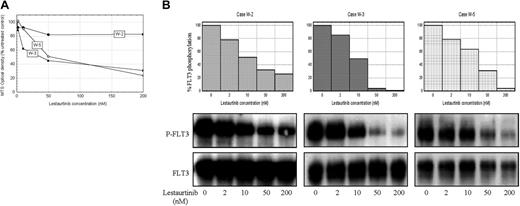
There appears to be a direct relationship between the degree of FLT3 inhibition achieved and the induction of cytotoxicity. Cases in which more than 15% residual FLT3 activation persisted at maximal drug concentrations displayed resistance to the cytotoxic effects of both agents, as illustrated in Figures 5 and 6. Significant in vitro cytotoxic responses to lestaurtinib were achieved in FLT3/WT cases W-3 and W-5, in which FLT3 was almost fully dephosphorylated by the drug (Figure 5). In contrast, cells from FLT3/WT case W-2 displayed more than 15% residual FLT3 phosphorylation at maximal doses of lestaurtinib and were largely resistant to the cytotoxic effects of this agent. In the same 3 WT cases, a cytotoxic response to PKC412 was seen only in cells from W-3, where almost complete inhibition of FLT3 phosphorylation occurred (Figure 6). Cases W-2 and W-5 both displayed more than 15% residual FLT3 activation at maximal drug concentrations and were thus resistant to the cytotoxic effects of PKC412.
Inhibition of signaling downstream of FLT3 appears integral to achieving a cytotoxic response to lestaurtinib and PKC412
In 11 of the 12 cases that were studied in FLT3 phosphorylation assays, sufficient whole cell lysate material was available to assess the effects of FLT3 inhibitors on signaling proteins downstream of FLT3. A wide variation in protein expression was seen among both FLT3 mutant and WT cases, with WT cases showing greater MAP kinase activation at baseline in comparison with ITD samples, while relatively low levels of AKT-signaling were seen irrespective of mutation status. Figure 7 illustrates the changing signaling profiles seen in MNCs from 5 typical cases, including 2 ITD mutants, 1 D835Y point mutant, and 2 WT cases, following 1-hour in vitro exposure to lestaurtinib and PKC412.
The in vitro effects of lestaurtinib and PKC412 on FLT3 phosphorylation in primary AML mononuclear cells. (A) Western blot images showing in vitro inhibition of FLT3 phosphorylation by lestaurtinib and PKC412 in a representative patient sample (case M-6, with FLT3 ITD mutation). Percentages refer to percentage FLT3 phosphorylation relative to untreated control as determined by densitometric analysis of blot images. (B) Dot plots showing the range of individual patient IC50 values for in vitro inhibition of FLT3 phosphorylation by lestaurtinib and PKC412. The 12 cases are grouped according to FLT3 mutation status, with the median values indicated. P values refer to comparison of ITD and WT groups using the Student t test. (C) Mean FLT3-inhibitory and MTS-derived cytotoxic dose responses of cells from 12 patients to lestaurtinib and PKC412. Cases are divided according to the presence or absence of a FLT3 activating mutation. Error bars refer to the SEM.
The in vitro effects of lestaurtinib and PKC412 on FLT3 phosphorylation in primary AML mononuclear cells. (A) Western blot images showing in vitro inhibition of FLT3 phosphorylation by lestaurtinib and PKC412 in a representative patient sample (case M-6, with FLT3 ITD mutation). Percentages refer to percentage FLT3 phosphorylation relative to untreated control as determined by densitometric analysis of blot images. (B) Dot plots showing the range of individual patient IC50 values for in vitro inhibition of FLT3 phosphorylation by lestaurtinib and PKC412. The 12 cases are grouped according to FLT3 mutation status, with the median values indicated. P values refer to comparison of ITD and WT groups using the Student t test. (C) Mean FLT3-inhibitory and MTS-derived cytotoxic dose responses of cells from 12 patients to lestaurtinib and PKC412. Cases are divided according to the presence or absence of a FLT3 activating mutation. Error bars refer to the SEM.
Almost complete dephosphorylation of FLT3 appears necessary for the achievement of a cytotoxic response to lestaurtinib. (A) MTS assay-derived cytotoxic dose responses of MNCs from 3 FLT3/WT cases exposed to lestaurtinib for 72 hours. (B) Comparative phospho-FLT3 and total FLT3 immunoblots with densitometric dose-response histograms from the same 3 cases following 1-hour exposure to lestaurtinib.
Almost complete dephosphorylation of FLT3 appears necessary for the achievement of a cytotoxic response to lestaurtinib. (A) MTS assay-derived cytotoxic dose responses of MNCs from 3 FLT3/WT cases exposed to lestaurtinib for 72 hours. (B) Comparative phospho-FLT3 and total FLT3 immunoblots with densitometric dose-response histograms from the same 3 cases following 1-hour exposure to lestaurtinib.
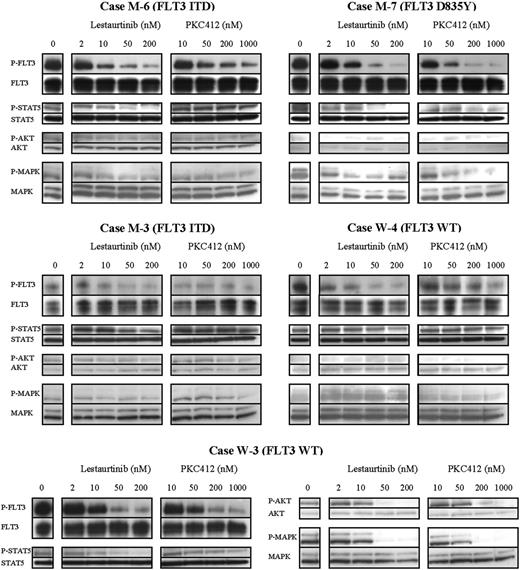
In case M-6, an ITD mutant, FLT3 was almost fully inhibited by lestaurtinib, with a corresponding reduction in STAT5 activation. At maximal doses of PKC412, however, enough FLT3 activation persisted (17.6% of baseline) to maintain STAT5 signaling. MTS assay confirmed cytotoxicity with lestaurtinib but resistance to PKC412. In contrast, case M-7, a D835Y mutant, showed a marked cytotoxic response to both FLT3 inhibitors. In M-7, FLT3 was almost completely inhibited by both agents, with correlating downstream reductions of STAT5 and, to a lesser degree, MAP kinase activation. In these and other FLT3 mutant cases, cytotoxicity correlated with inhibition of signaling through STAT5.
In some cases that harbor FLT3 activating mutations, FLT3 may not provide the predominant prosurvival signal. In case M-3, an ITD mutant, relatively low levels of FLT3 activation were seen in untreated cells. Despite inhibition of this activity by lestaurtinib and PKC412, there was minimal corresponding reduction in STAT5 and MAP kinase activation, suggesting FLT3-independent activation of these pathways. No cytotoxic response occurred to either drug.
Conversely, blasts that express only WT FLT3 may be dependent on FLT3 signaling. W-3, a FLT3/WT patient, showed a high degree of sensitivity to the cytotoxic effects of both agents. Cells from W-3 demonstrated high levels of baseline FLT3 activation. Exposure to both lestaurtinib and PKC412 resulted in marked reduction of STAT5, AKT, and MAP kinase activation, suggesting that, in this case, survival signaling was FLT3 dependent. In contrast, W-4, another WT case, was resistant to the cytotoxic effects of both drugs. While W-4 also showed a high level of baseline FLT3 activation, which was inhibited by both lestaurtinib and PKC412, there was no corresponding reduction in STAT5 activity, suggesting that STAT5 activation was occurring via alternative pathways.
Almost complete dephosphorylation of FLT3 appears necessary for the achievement of a cytotoxic response to PKC412. (A) MTS assay-derived cytotoxic dose responses of MNCs from 3 FLT3/WT cases exposed to PKC412 for 72 hours. (B) Comparative phospho-FLT3 and total FLT3 immunoblots with densitometric dose-response histograms from the same 3 cases following 1-hour exposure to PKC412.
Almost complete dephosphorylation of FLT3 appears necessary for the achievement of a cytotoxic response to PKC412. (A) MTS assay-derived cytotoxic dose responses of MNCs from 3 FLT3/WT cases exposed to PKC412 for 72 hours. (B) Comparative phospho-FLT3 and total FLT3 immunoblots with densitometric dose-response histograms from the same 3 cases following 1-hour exposure to PKC412.
Inhibition of signaling downstream of FLT3, especially of STAT5, appears critical to achieving a cytotoxic response to lestaurtinib and PKC412. Western blot images showing the in vitro effects of lestaurtinib and PKC412 on FLT3 phosphorylation and phosphorylation of the downstream signaling proteins STAT5, AKT, and MAP kinase in MNCs following 1-hour exposure to a range of concentrations of each drug. Three of the 5 cases (M-6, M-7, and W-3) were in vitro cytotoxic responders to lestaurtinib, while 2 of the 5 cases (M-7 and W-3) were responsive to PKC412. MTS assay-derived cytotoxic IC50 concentrations for case-by-case comparison are shown in Table 2.
Inhibition of signaling downstream of FLT3, especially of STAT5, appears critical to achieving a cytotoxic response to lestaurtinib and PKC412. Western blot images showing the in vitro effects of lestaurtinib and PKC412 on FLT3 phosphorylation and phosphorylation of the downstream signaling proteins STAT5, AKT, and MAP kinase in MNCs following 1-hour exposure to a range of concentrations of each drug. Three of the 5 cases (M-6, M-7, and W-3) were in vitro cytotoxic responders to lestaurtinib, while 2 of the 5 cases (M-7 and W-3) were responsive to PKC412. MTS assay-derived cytotoxic IC50 concentrations for case-by-case comparison are shown in Table 2.
Discussion
In this study, the largest to date of the in vitro effects of FLT3 inhibitors on primary AML patient material, lestaurtinib and PKC412 both induced cytotoxicity in blasts from most of the 65 cases studied. Responses were highly heterogeneous, and although samples from patients with activating mutations of FLT3 were significantly more sensitive to the cytotoxic effects of lestaurtinib than those from WT cases, there was considerable overlap in the range of responses seen between the 2 patient groups. Cytotoxic responses to PKC412 were more limited and tended to occur at higher drug concentrations.
A previous in vitro study using primary childhood ALL lymphoblasts demonstrated a direct relationship between FLT3 expression and sensitivity to lestaurtinib-induced cytotoxicity in that disorder.42 Quantification of FLT3 RNA expression in the current study provided little evidence to support a similar relationship with FLT3 inhibitor response in AML. Although trends favoring greater sensitivity to both agents in high expressers of FLT3 emerged, these data provide no clear basis for restriction of the clinical application of FLT3 inhibitors according to FLT3 expression level, a parameter that does not necessarily reflect the baseline degree of FLT3 activation.
Cell line evidence suggests that lestaurtinib and PKC412 act synergistically with standard cytotoxic agents if used simultaneously with or immediately following chemotherapy.26,43,44 Cytotoxicity assays using FLT3 inhibitors in combination with cytarabine in the current study again showed the response of primary samples to be more heterogeneous, although median effect analysis confirmed an overall population synergistic effect when lestaurtinib and cytarabine were combined, especially when used to treat blasts that harbored FLT3 activating mutations. All other patient groups and drug combinations showed at least additive effects. Primary sample size constraints unfortunately precluded investigation of the effects of time sequencing of drug combinations.
In Western blotting experiments, lestaurtinib and PKC412 caused in vitro inhibition of FLT3 phosphorylation in blasts from all cases studied. Higher concentrations of PKC412 were required to achieve comparable dephosphorylation of FLT3, and higher concentrations of both agents were required to dephosphorylate the WT FLT3 receptor. The translation of this FLT3 inhibition into the induction of a cytotoxic response appears to be a complex phenomenon influenced by factors including the degree of residual FLT3 phosphorylation present following drug treatment and the variable dependence of blasts from different patients on FLT3 signaling for cell survival and proliferation. In several, particularly WT, cases FLT3 was incompletely inhibited, with significant residual phosphorylation persisting at drug concentrations likely to far exceed those achievable in clinical practice. Cases that displayed more than 15% residual FLT3 phosphorylation after lestaurtinib or PKC412 treatment showed resistance to the cytotoxic effects of the respective compound (Figures 5, 6), broadly in agreement with previous data regarding lestaurtinib.31
In the primary samples studied, induction of cytotoxicity also correlated closely with deactivation of STAT5 and MAP kinase. In a number of cases including both FLT3 mutant and WT patients, minimal cytotoxic responses were seen when STAT5 and/or MAP kinase activation persisted despite complete inhibition of FLT3. Blasts from these drug-resistant cases may have been dependent on proliferation and survival pathways independent of FLT3 or, alternatively, FLT3 signaling may have been only one of several pathways that needed to be inhibited to achieve a cytotoxic response: the tandem use of additional molecularly targeted agents (for example, inhibitors of AKT/mTOR) could potentially be a method of overcoming such resistance.45,46 These experiments appear to confirm that, in some AML cases with FLT3 activating mutations, FLT3 may not provide the predominant prosurvival signal and that, conversely, FLT3/WT blasts may in some cases depend on FLT3 signaling for their survival, thus explaining the overlap seen between the in vitro cytotoxic responses of the 2 patient groups. “Degree of dependency on FLT3 signaling” will unfortunately not be an easily quantifiable laboratory parameter by which to restrict patient recruitment into future clinical trials. Indeed, on the basis of this work there is a strong rationale for the continued inclusion of FLT3/WT patients in such studies.
The reasons for the marked difference between the limited cytotoxic effects of PKC412 on primary material and the more marked responses observed both in vitro in leukemia cell lines and clinically in patients treated with this agent28,29,32 are not well understood. In the only previously published study of the in vitro cytotoxic effects of PKC412 on primary leukemic cells, only 1 of 4 primary ALL samples that expressed high levels of FLT3 reached an IC50 at PKC412 doses of up to 1 μM.29 The current study confirmed that PKC412-induced inhibition of FLT3 was generally comparable to that achieved by lestaurtinib, although cytotoxic responses to PKC412 were more limited. This discrepancy could, in part, be explained by a recent hypothesis that lestaurtinib inhibits other cellular targets upstream of STAT5 while PKC412 is more selective for FLT3.47 In the clinical setting, however, the greater in vitro potency of lestaurtinib may be offset by other factors, including the better protein binding profile of PKC412 and the accumulation of metabolites of PKC412 with direct inhibitory activity against the FLT3 kinase,47 which result in similar effective concentrations of the 2 agents. Phase 1 and 2 clinical trials of lestaurtinib and PKC412 monotherapy in similar AML patient groups have so far demonstrated very similar rates and degrees of clinical response.31,32
A clear causal relationship is now emerging between FLT3 inhibition and the achievement of a cytotoxic response in AML, although, as the data in this study demonstrate, inhibition of FLT3 is in itself frequently insufficient to induce cytotoxicity. Blasts from patients that harbor activating mutations of FLT3 and those from FLT3/WT patients show variable dependence on FLT3 signaling for survival, and patients within both of these groups may derive benefit from FLT3 inhibitor therapy. The multifactorial pathogenesis of AML means that any molecularly targeted agent is unlikely to be curative if used alone, and this is borne out by the results of clinical trials of FLT3 inhibitors in adult AML to date.31-35 Simultaneous inhibition of multiple targets is likely to be necessary in most patients, and this may in the future be achievable through combination molecular therapy. The current work adds to a growing body of evidence to support a synergistic relationship between FLT3 inhibitors and conventional cytotoxic agents.26,43,48,49 Both lestaurtinib and PKC412 are the subjects of ongoing clinical trials in combination with chemotherapeutic agents,50,51 and the results of these studies as well as future trials of FLT3 inhibitors in combination with other molecularly targeted agents are eagerly anticipated.
Prepublished online as Blood First Edition Paper, July 25, 2006; DOI 10.1182/blood-2006-04-015487.
S.K. designed the research, performed the in vitro cytotoxicity assays and immunoblotting experiments, analyzed the data, and wrote the manuscript; S.J.A. performed FLT3 mutation analysis; A.F.G. and V.W. performed RT-PCR FLT3 expression analysis; K.I.M. contributed to experimental design and data analysis; and A.K.B. established the research plan, supervised the project, and approved the data and final version of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank our colleagues at Cephalon and Novartis Pharma and, in particular, Peter Brown (Cephalon) and Pamela Cohen and Johannes Roesel (Novartis Pharma) for the provision of lestaurtinib and PKC412, for their technical advice in storing, handling, and administering the compounds, and for their thorough review of and contributions to the final manuscript. We also thank our colleagues at the Small Laboratory, Johns Hopkins Medical Institute, Baltimore, MD, especially Donald Small, Mark Levis, Patrick Brown, and Rosalyn Pham, for their invaluable advice on experimental methodology.

![Figure 1. In vitro cytotoxic response of primary AML blasts to single-agent lestaurtinib and PKC412. (A) MTS assay dose-response curves showing mean cytotoxic response of 65 patient samples, grouped according to FLT3 mutation status, to lestaurtinib and PKC412, normalized to untreated controls. Error bars represent standard error of the mean (SEM). P values refer to statistical comparison of the FLT3 ITD and WT groups at each dose using the Student t test (only significant P values [P < .05] shown). (B) Dot plots showing the range of individual patient in vitro MTS IC50 values for lestaurtinib and PKC412. The median and quartiles for each mutation group are indicated. P values refer to intergroup statistical analysis of log-transformed data by the Student t test, with significant differences in lestaurtinib IC50 distribution being seen between WT and ITD cases and between WT cases and all FLT3 mutants grouped together. No significant group differences were seen with PKC412. (C) MTS assay dose-response curves showing mean cytotoxic response of 21 ITD samples to lestaurtinib and PKC412, grouped according to percentage FLT3 ITD RNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/10/10.1182_blood-2006-04-015487/4/m_zh80220603590001.jpeg?Expires=1767728837&Signature=cJiEXpp-T3qBwxtSy4m-NJ8Wjsz0urwzG-6BgHu-D4WYY9rrqZJsiksWo1aO2wBvK8eGzzaKocOVqtwSoSDb-j0eqazfwu0u55bYz~ArlyWQ2QpDCRB3ATXaRpyIjKEi0cDFTQn4-wxsaO315KEGgL2grXODUddXmfwyyCq6lrYX7GcXSqjPxy0dL5~4X3nBdZsa5Xp63UQGAEf3PHtyCwdmzLnBx0wV7AVjskwmMo3OFXlKSUjkNHAOd69mp23uMHedreGt-VsFqcMQPMWOvcCQO6K3~iMkrLljyGu7GozlpTWftvHOqbpBllIG-tQyYMA8gocXUrTAMDPw4j9Uxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Variations in FLT3 expression level have relatively little impact on in vitro sensitivity to the cytotoxic effects of FLT3 inhibitors. (A) Scatter plot showing FLT3 mRNA expression levels of cryopreserved MNCs from 64 patients measured by a real-time PCR method relative to expression of the housekeeping gene S14. Cases are grouped according to FLT3 mutation status. Analysis by the Student t test showed no significant differences in FLT3 RNA expression between mutation groups. (B) Mean MTS-derived cytotoxic dose responses of cells from 43 patients to lestaurtinib and PKC412. Cases are grouped by FLT3 mRNA expression level relative to the population median. Error bars represent the SEM. P values refer to a comparison of high and low FLT3 expression groups at each dose using the Student t test (only significant P values [P < .05] shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/10/10.1182_blood-2006-04-015487/4/m_zh80220603590003.jpeg?Expires=1767728837&Signature=sJfpHgTxlGIW6F4S8RFNS7RYBRPVsJESP9yoi5eTWhp4aAl45MrwAV6Valv8sm1DWbnK6t2PsVg3RUarK5cC1wn3vOuTfRpXjaofyxiPJqOboMz5Ct-AH76ecgnsbwwHMXzQ~txpH43FsevnMtHog8ryfhn8heDncB3pUR2C5tZuHjMZIelNUqhA9ot5lMtTG6m5ggG5gSz7H7S36WULSvOlHfv-VBFtMI9BOqHrJYBLYpt6eYY5XIk6Q3~RozjLs6UlsvRuZOJp1N2Ku1W4dsBDj2iBx0Sv~onWCPswogjY4o1BMzrZC7NaF1qVStjj1zaitjmLgLR8Sq-NHCq3pQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal