Abstract
Humans lacking the CD3γ subunit of the pre-TCR and TCR complexes exhibit a mild αβ T lymphopenia, but have normal T cells. By contrast, CD3γ-deficient mice are almost devoid of mature αβ T cells due to an early block of intrathymic development at the CD4–CD8– double-negative (DN) stage. This suggests that in humans but not in mice, the highly related CD3δ chain replaces CD3γ during αβ T-cell development. To determine whether human CD3δ (hCD3δ) functions in a similar manner in the mouse in the absence of CD3γ, we introduced an hCD3δ transgene in mice that were deficient for both CD3δ and CD3γ, in which thymocyte development is completely arrested at the DN stage. Expression of hCD3δ efficiently supported pre-TCR–mediated progression from the DN to the CD4+CD8+ double-positive (DP) stage. However, αβTCR-mediated positive and negative thymocyte selection was less efficient than in wild-type mice, which correlated with a marked attenuation of TCR-mediated signaling. Of note, murine CD3γ-deficient TCR complexes that had incorporated hCD3δ displayed abnormalities in structural stability resembling those of T cells from CD3γ-deficient humans. Taken together, these data demonstrate that CD3δ and CD3γ play a different role in humans and mice in pre-TCR and TCR function during αβ T-cell development.
Introduction
The pre–T-cell receptor (TCR) and the αβTCR are multimeric protein complexes expressed on the surface of developing T cells at different stages of their ontogeny in the thymus. The pre-TCR complex consists of a disulfide-linked heterodimer of an invariant pre-Tα (pTα) and a variable TCRβ chain noncovalently associated with the CD3γ, δ, ϵ, and ζ polypeptides.1 The TCR contains an identical subunit composition but with a variable TCRα chain in lieu of pTα. Within the pre-TCR and TCR, the CD3 subunits are assembled as γϵ, δϵ, and ζζ dimers, with all but CD3ζζ being structurally related.2 Functionally the CD3 proteins play a role in the assembly, surface transport, and signal capacity of the pre-TCR and TCR complexes.3,4
During thymocyte development, the pre-TCR and αβTCR control discrete developmental checkpoints through which T-cell precursors have to pass to mature successfully.5,6 Signals initiated at the pre-TCR trigger progression from a CD44–CD25+ CD4–CD8– double-negative (DN) to a CD4+CD8+ double-positive (DP) differentiation stage, through CD44–CD25– DN intermediates. Thus, only cells with a productive TCRβ gene rearrangement undergo this transition (a checkpoint termed β selection). Subsequently, signals emanating from the TCR determine the positive (survival and differentiation) and negative (clonal deletion) selection of immature DP thymocytes, processes that shape the repertoire of mature CD4+CD8– and CD4–CD8+ single-positive (SP) T cells that exit from the thymus and populate the peripheral lymphoid organs. The position of the CD3 proteins at the initiation of the pre-TCR and TCR-mediated signaling cascade implies a critical role of these molecules in alternate signal transduction during thymic selection.
The overall structure and functioning of the pre-TCR and TCR complexes in mice and humans are quite similar. Despite this similarity, ablation of the highly related CD3δ and CD3γ subunits has markedly different effects in mice and humans. In mice made deficient for CD3δ, thymocyte development is completely blocked at the DP stage due to defective TCR expression and function,7,8 but pre-TCR function is normal. On the other hand, CD3δ-deficient humans exhibit an earlier developmental block at the pre-TCR–controlled checkpoint.9,10 Loss of CD3γ in the mouse results in a severe block at the DN-to-DP transition, indicating that pre-TCR signaling is defective.11 In particular, the majority of CD3γ-deficient thymocytes cannot pass the CD44–CD25+ DN checkpoint. In contrast, CD3γ-deficient humans show only slightly reduced numbers of peripheral T cells, which display decreased TCR surface expression, and functional defects that lead to immunodeficiency with a variable spectrum of clinical signs.12 Since CD3γ-deficient mice possess a different phenotype, a mouse model for this human disease is lacking to date.
To further dissect the roles of CD3γ and CD3δ in mouse and human T-cell development, we crossed mice lacking CD3γ and CD3δ, in which thymocyte development is completely arrested at the DN stage,13 with mice transgenic for a human CD3δ (hCD3δ) chain,14 resulting in the γδ– × hδTg mouse strain. These mice incorporated the hCD3δ protein in surface pre-TCR and TCR complexes, and displayed a nearly normal-sized population of TCR-expressing DP cells, indicative of normal pre-TCR function. In contrast, γδ– × hδTg TCR complexes displayed an impaired capability to signal for efficient positive as well as negative selection and differentiation of DP thymocytes into mature T cells. Of importance, the T-cell compartment of γδ– × hδTg mice, in contrast to the previously reported mice lacking CD3γ,11 resembled that of CD3γ-deficient humans. Therefore, this “humanized” mouse strain might be an adequate model system for the human CD3γ immunodeficiency.
Materials and methods
Mice
CD3γ-deficient mice expressing a human CD3δ chain (hereafter referred to as γδ– × hδTg mice) were generated by breeding CD3γδ doubly-deficient mice13 with a strain of transgenic mice, termed Tgδ4, which carries a low copy number of a human CD3δ transgene.14 Double mutant mice were identified by Southern blot and polymerase chain reaction (PCR). CD3γδ doubly-deficient mice expressing a mouse CD3δ transgene (γδ– × mδTg) will be described in detail elsewhere. In all experiments, C57BL/6 mice were used as wild-type (WT) controls. Mice were analyzed at 6 to 8 weeks of age. CD3γ-deficient and HY, OT-I, and AND TCR transgenic mice have been described elsewhere.11,15-17
Antibodies
For flow cytometry, the following biotinylated, FITC-, PE-, or PE-Cy5–conjugated antibodies were obtained from BD Biosciences Pharmingen (San Jose, CA): anti-CD3γϵ/δϵ (clone 145-2C11), anti-CD3γϵ (clone 17A2), anti-CD4 (clone RM4-5), anti-CD5 (clone 53-7.3), anti-CD8α (clone 53-6.7), anti-CD69 (clone H1.2F3), anti-TCRβ (clone H57-597), anti-TCRVα2 (clone B20.1), anti-TCRVβ3 (clone KJ25), and anti-TCRVβ8 (clone MR5-2). Streptavidin-PE-Cy5 was used as second-step reagent. Immunoblottings were performed using the following antibodies: anti–phosphotyrosine 4G10 (Upstate Biotechnology, Lake Placid, NY), anti–ZAP-70 (Zap4; a gift from S. Ley), anti–Cbl-1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-LAT (Upstate Biotechnology), anti-CD3ζ antibody 448,18 anti–phospho-MAPK (ERK, JNK, and p38), and anti–total MAPK (New England Biolabs, Beverly, MA).
Flow cytometry
Cells were stained with specific fluorochrome-conjugated or biotinylated antibodies and analyzed by 2- or 3-color flow cytometry in a FACSCalibur flow cytometer using the CellQuest software (Becton Dickinson, San Jose, CA).
TCR stimulation, immunoprecipitation, and immunoblotting
Thymocytes were resuspended in PBS and incubated for 10 minutes at 4°C in the presence or absence of biotin-conjugated anti-CD3 (145-2C11) and anti-CD4 (RM4-5) antibodies. After removal of unbound antibody, the cells were incubated at 37°C for varying periods of time with 100 μg/mL streptavidin. After stimulation, cells were lysed in a buffer containing 1% Brij-96, 20 mM Tris-HCL (pH 7.8), 150 mM NaCl, and a cocktail of protease and phosphatase inhibitors. For immunoprecipitations, lysates were incubated overnight with specific antibodies preadsorbed to protein G–sepharose beads. The beads were washed 4 times in lysis buffer and resuspended in SDS sample buffer. Total cell lysates or immunoprecipitates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon-P (Millipore) membranes, and immunoblotted with specific antibodies. Bound antibodies were detected using an enhanced chemiluminescence system (Amersham).
Protein analysis
For analysis of the TCR organization under nondenaturing conditions, blue native–polyacrylamide gel electrophoresis (BN-PAGE)19,20 was performed. Briefly, the TCR complex from digitonin lysates of pervanadate-stimulated thymocytes was immunoprecipitated with antiphosphotyrosine-coupled protein G–sepharose and eluted with phenylphosphate. After separation of the samples by BN-PAGE,21,22 the proteins were transferred to nitrocellulose membranes and detected by immunoblotting with anti-CD3ζ antiserum 448. Ferritin was used as the marker protein in its 24-mer, 48-mer, and 72-mer forms (440 kDa, 880 kDa, and 1320 kDa, respectively). For antibody-shift assays, the antibody amounts indicated were added to the samples and incubated for 30 minutes on ice before BN-PAGE loading, as described.22
TCR-induced thymocyte death assays
Mice were injected with either vehicle alone (PBS) or anti-TCRβ antibody (H57-597). After 48 hours, thymi were removed for analysis of thymocyte numbers and CD4/CD8 expression. For in vitro thymocyte death assay, cells were cultured in plastic wells precoated with the indicated amount of anti-TCRβ antibody. After 48 hours, thymocytes were harvested and cell death was assessed by propidium iodide (PI) staining followed by flow cytometry. Specific cell death (%) denotes the percentage of dead (PI positive) cells in antibody-coated wells, normalized to control wells without antibody as follows:
Calcium mobilization
Calcium mobilization was measured by flow cytometry in Fluo-3-AM–loaded thymocytes upon addition of biotin-conjugated antibodies to CD3 and CD4 followed by cross-linking with streptavidin.
Results
Restored DN-to-DP and impaired DP-to-SP transition in the thymus of CD3γδ doubly-deficient mice expressing a human CD3δ transgene
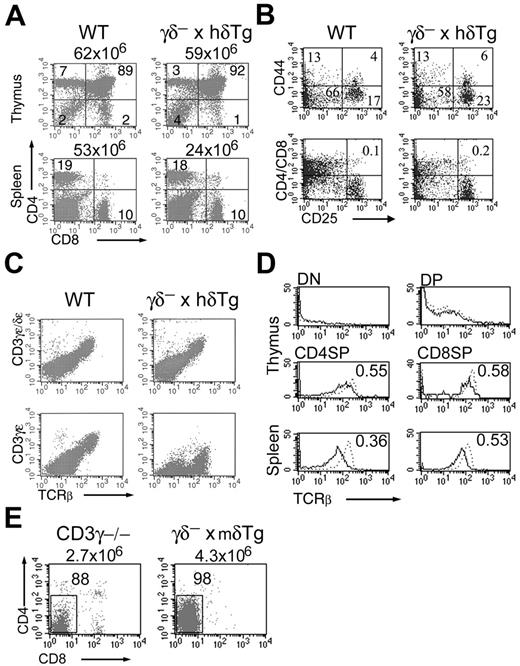
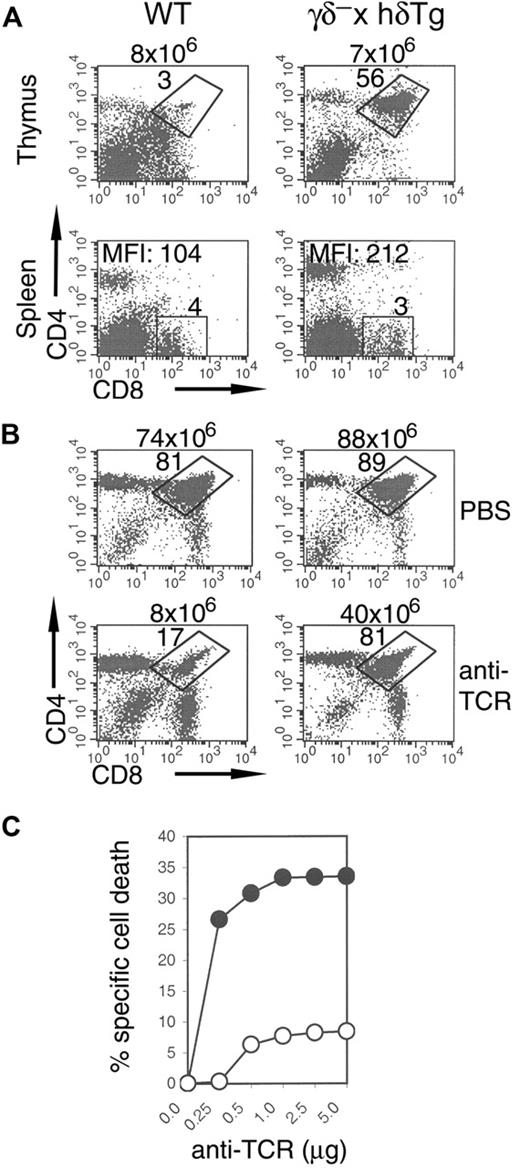
γδ– × hδTg mice were generated by crossing mice lacking both CD3γ and CD3δ, in which thymocyte development is completely arrested at the CD44–CD25+ DN stage, with mice carrying a low copy number of a complete human CD3δ transgene controlled by its own regulatory elements. The size of the thymi and total thymocyte number of these γδ– × hδTg mice were relatively normal compared with control WT mice. Total thymocyte numbers (n = 10) were as follows: WT, 88 ± 31 × 106; γδ– × hδTg, 74 ± 17 × 106. Total splenocyte numbers (n = 9) were as follows: WT, 48 ± 15 × 106; γδ– × hδTg, 25 ± 6 × 106. Further, all thymocyte subsets defined by expression of CD4 and CD8 coreceptors were present in γδ– × hδTg mice (Figure 1A).
More detailed analysis of the DN population revealed that progression beyond the CD44–CD25+ DN stage, severely impaired in CD3γ-deficient and CD3γδ doubly-deficient mice,11,13 proceeds efficiently in the thymi of γδ– × hδTg mice (Figure 1B upper panel). In contrast to DP cells in CD3γ-deficient mice,11 numbers of DP thymocytes were comparable between γδ– × hδTg and WT mice (Figure 1A). Further, γδ– × hδTg DP cells lacked surface CD25 (Figure 1B lower panel). Together, these data indicate that expression of a human CD3δ transgene is able to restore pre-TCR function in the absence of CD3γ in the mouse, resulting in the generation of DP thymocytes that were phenotypically normal. However, the numbers of CD4+ SP and CD8+ SP thymocytes in γδ– × hδTg mice were reduced on average to 36% and 42%, respectively, of those in WT mice (Figure 1A). These mature thymocytes were able to migrate to and accumulate in peripheral lymphoid organs, as shown by the presence of CD4+ and CD8+ SP cells in the spleens of γδ– × hδTg mice, in numbers averaging 50% of those in WT mice (Figure 1A).
Intrathymic T-cell development in γδ– × hδTg mice. (A) Thymocytes and splenocytes from WT and γδ– × hδTg mice were surface stained with anti-CD4 and anti-CD8 antibodies and analyzed by flow cytometry. Percentages of cells in quadrants and total cell numbers are shown within and above plots, respectively. (B) Dot plots show CD44/CD25 subsets in DN-gated cells (top panel), and CD25 expression in total thymocytes (bottom panel). TCR expression in total thymocytes (C) and in thymic and splenic CD4/CD8 subsets (D) of WT (dotted line) and γδ– × hδTg (solid line) mice. Numbers within histograms indicate mean fluorescence intensity (MFI) ratios of γδ– × hδTg versus WT cells. (E) Thymic CD4/CD8 subpopulations in CD3γ-deficient and γδ– × mδTg mice. Numbers within and above plots represent percentages of cells in the gated region and total cell numbers, respectively.
Intrathymic T-cell development in γδ– × hδTg mice. (A) Thymocytes and splenocytes from WT and γδ– × hδTg mice were surface stained with anti-CD4 and anti-CD8 antibodies and analyzed by flow cytometry. Percentages of cells in quadrants and total cell numbers are shown within and above plots, respectively. (B) Dot plots show CD44/CD25 subsets in DN-gated cells (top panel), and CD25 expression in total thymocytes (bottom panel). TCR expression in total thymocytes (C) and in thymic and splenic CD4/CD8 subsets (D) of WT (dotted line) and γδ– × hδTg (solid line) mice. Numbers within histograms indicate mean fluorescence intensity (MFI) ratios of γδ– × hδTg versus WT cells. (E) Thymic CD4/CD8 subpopulations in CD3γ-deficient and γδ– × mδTg mice. Numbers within and above plots represent percentages of cells in the gated region and total cell numbers, respectively.
The pattern of αβTCR expression on the surface of mutant thymocytes was indistinguishable from that of control WT thymocytes (Figure 1C upper panel), despite the specific absence of γϵ dimers in the TCRs of these cells (Figure 1C lower panel). Furthermore, the up-regulation of γδ– × hδTg TCR expression was normal during intrathymic development in the absence of CD3γ (Figure 1D). Yet, TCR surface expression in thymic and splenic SP cells from γδ– × hδTg mice was reduced in comparison with their WT counterparts (Figure 1D), although the extent of decrease was less than that reported for CD3γ-deficient mice23 or humans.24 Also, the impairment of TCR expression in peripheral mature T cells from γδ– × hδTg mice was more prominent in CD4+ SP than in CD8+ SP cells (Figure 1D). This was in contrast with either CD3γ-deficient mice or humans, where TCR expression is more markedly diminished in the CD8+ lineage.23,24 Collectively, these data confirm previous findings indicating that CD3γ is not essential for TCR assembly and surface expression in mouse T cells.11 Further, these results demonstrate that a human CD3δ transgene can effectively support the DN-to-DP and, albeit with lesser efficiency, the DP-to-SP transition in mice lacking both CD3δ and CD3γ. Rescue of intrathymic T-cell development does not appear to be related to simply transgene overexpression, since a mouse CD3δ transgene was unable to restore thymocyte cellularity and maturation in CD3γδ doubly-deficient mice (Figure 1E). Accordingly, thymic development in this γδ– × mδTg strain resembled that of CD3γ-deficient mice (Figure 1E), with most of the thymocytes arrested at the DN stage.
Human CD3δ substitutes both mouse CD3γ and CD3δ in the γδ– × hδTg TCR
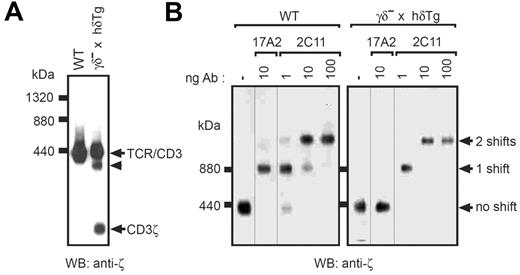
To determine the native structure of the TCR in γδ– × hδTg T cells, and to characterize the impact of the CD3γ-deficiency and incorporation of a human CD3δ on the composition and stability of the TCR complex, we performed blue native (BN)–PAGE analysis of the TCR expressed by WT and γδ– × hδTg thymocytes (Figure 2A). The digitonin-extracted native TCR/CD3 complex isolated from WT thymocytes was visualized with anti-CD3ζ as a single band with mobility just below the 440-kDa ferritin marker. It had the stoichiometry of αβγϵδϵζζ as reported before.22,25 In contrast, anti-CD3ζ detected 3 bands in the γδ– × hδTg complexes. The largest 2 bands had a size similar to the WT TCR complex. The highest mobility band consisted of free CD3ζ as a band with similar mobility appeared upon SDS treatment of the WT TCR (data not shown). SDS is a harsh detergent that disrupts all TCR complexes.
To characterize the subunit composition and stoichiometry of the TCR in γδ– × hδTg T cells, we used a novel technique combining antibody-mediated gel-shift and BN-PAGE, termed native antibody-based mobility-shift (NAMOS) assay22 (Figure 2B). Digitonin lysates from WT and γδ– × hδTg splenocytes were used to purify the TCR complex. Subsequently, the purified receptor was incubated with different amounts of the anti-CD3ϵ antibody 2C11 and then subjected to BN-PAGE. The WT TCR was shifted to 2 new bands (Figure 2B left). Since the complete (αβδϵγϵζζ) TCR complex has 2 binding sites for 2C11 (γϵ and δϵ), and since each antibody molecule bound to the complex produces a discrete change in electrophoretic mobility, the band appearing at the lowest amount of antibody tested is consistent with one antibody molecule bound to one TCR complex. The highest antibody amount generates a band likely corresponding to 2 antibody molecules associated with a single TCR complex, with an intermediate amount generating a mixture of the products observed at the highest and lowest amounts of shifting antibody (Figure 2B left). These data show that the WT TCR complex has 2 binding sites for 2C11 and, therefore, possesses 2 CD3 dimers (γϵ and δϵ).
Native structure and composition of the TCR complex expressed in γδ– × hδTg T cells. (A) The TCR complex was immunoprecipitated with antiphosphotyrosine antibodies from digitonin lysates of pervanadate-stimulated WT and γδ– × hδTg thymocytes, and subsequently eluted with phenylphosphate. After separation by BN-PAGE, proteins were transferred to a nitrocellulose membrane and blotted with an anti-CD3ζ antibody. The positions of the complete TCR complex (αβγϵδϵζζ in WT and αβ(hδϵ)2ζζ in γδ– × hδTg thymocytes), free CD3ζ, and a partial TCR complex (arrowhead) are indicated. (B) Phenylphosphate-eluted TCR complexes from WT and γδ– × hδTg splenocytes were incubated with the indicated amounts of the antibodies 17A2 (anti-CD3γϵ) or 2C11 (anti-CD3γϵ/δϵ), and subsequently resolved by BN-PAGE. The Western blot membrane was developed with anti-CD3ζ antibodies. The number of antibody-mediated shifts of the TCR complex is indicated to the right.
Native structure and composition of the TCR complex expressed in γδ– × hδTg T cells. (A) The TCR complex was immunoprecipitated with antiphosphotyrosine antibodies from digitonin lysates of pervanadate-stimulated WT and γδ– × hδTg thymocytes, and subsequently eluted with phenylphosphate. After separation by BN-PAGE, proteins were transferred to a nitrocellulose membrane and blotted with an anti-CD3ζ antibody. The positions of the complete TCR complex (αβγϵδϵζζ in WT and αβ(hδϵ)2ζζ in γδ– × hδTg thymocytes), free CD3ζ, and a partial TCR complex (arrowhead) are indicated. (B) Phenylphosphate-eluted TCR complexes from WT and γδ– × hδTg splenocytes were incubated with the indicated amounts of the antibodies 17A2 (anti-CD3γϵ) or 2C11 (anti-CD3γϵ/δϵ), and subsequently resolved by BN-PAGE. The Western blot membrane was developed with anti-CD3ζ antibodies. The number of antibody-mediated shifts of the TCR complex is indicated to the right.
The 2C11 antibody also produced 2 shifted bands when the γδ– × hδTg TCR complex was analyzed (Figure 2B right). This indicated that the mutant receptor contained 2 binding sites for 2C11 and corresponds therefore to a αβ(hδϵ)2ζζ complex. The shift pattern of the band migrating immediately below the predominant TCR complex (Figure 2A arrowhead) was consistent with a partial TCR complex containing only one hδϵ dimer (αβhδϵζζ) (data not shown). As expected, the TCR of γδ– × hδTg T cells was not shifted by the anti-CD3γϵ antibody 17A2 (Figure 2B right), whereas its WT counterpart was (Figure 2B left). Collectively, these data indicate that the majority of TCRs in γδ– × hδTg T cells incorporates 2 hδϵ dimers per complex, thus keeping with the typical architecture of the WT TCR.22 Further, a minor fraction of partial TCR containing only one CD3 dimer (hδϵ) within the complex was detected. To date, it is unclear whether these complexes existed on the cell surface or were generated upon detergent lysis. Both cases could indicate that the chimeric TCR is less stable than the WT TCR.
Inefficient positive selection of thymocytes in γδ– × hδTg mice
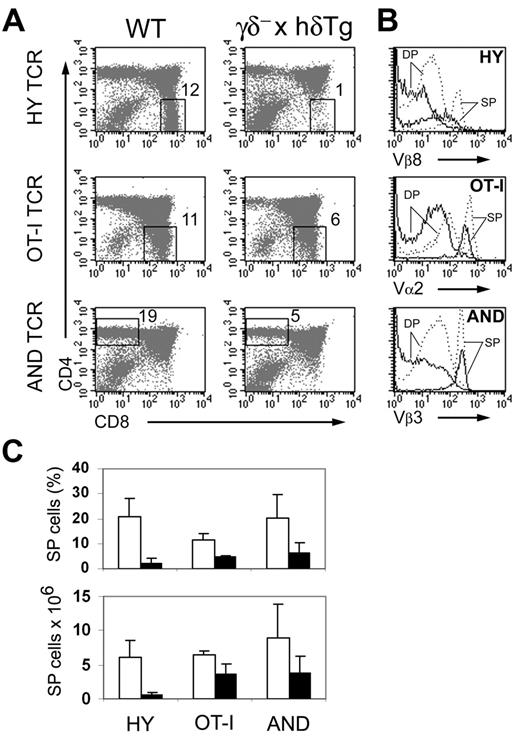
The decrease in the number of mature CD4+ and CD8+ SP thymocytes observed in γδ– × hδTg mice suggested an impairment of positive selection. To test this, 3 transgenic TCRs were independently introduced onto the γδ– × hδTg background: HY, OT-I, and AND. The HY TCR recognizes a male-specific peptide in the context of MHC class I H-2b. Thymocytes expressing the HY TCR are positively selected as CD8+ SP cells in female H-2b mice.15 Consequently, 20.7% ± 7.6% of total thymocytes are CD8+ SP (Figure 3A upper panel and 3C). In contrast, only 2.3% ± 1.7% CD8+ SP cells were present in female HY γδ– × hδTg mice (Figure 3A upper panel and 3C). Numbers of CD8+ SP thymocytes were also reduced by 90% in the HY TCR mutant mice (Figure 3C). Positive selection of another MHC class I–restricted TCR (OT-I,16 specific for an ovalbumin peptide in the context of H-2b) was also affected in γδ– × hδTg mice, albeit to a lesser degree (Figure 3A middle panel). In this case, the number of CD8+ SP cells was reduced by only 45% in OT-I transgenic γδ– × hδTg compared with WT mice (Figure 3C).
We next used mice expressing the pigeon cytochrome-C–specific TCR AND17 crossed with γδ– × hδTg mice to assess whether positive selection of class II–restricted CD4+ SP thymocytes was also affected. In AND γδ– × hδTg mice, the percentage and number of CD4+ SP cells were reduced to 32% and 43%, respectively, of those in WT mice (Figure 3A lower panel and 3C). This result demonstrated that the efficiency of positive selection of CD4+ T cells was also decreased in γδ– × hδTg mice. Thus, positive selection of both class I– and class II–restricted T cells appeared to proceed less efficiently in γδ– × hδTg mice. Yet, surface up-regulation of CD526 (data not shown) and the TCR (Figure 3B) along the DP-to-SP transition was comparable in WT and γδ– × hδTg TCR transgenics, suggesting that positive selection is not qualitatively affected in the mutant mice.
Impaired positive selection in γδ– × hδTg mice. (A) Dot plots show CD4 and CD8 expression in thymocytes from WT and γδ– × hδTg mice expressing transgenic HY (females), OT-I, or AND TCR. Numbers within dot plots indicate the percentage of CD8+ or CD4+ SP cells in the gated region. (B) Histograms depict transgenic TCR expression, assessed with anti-TCRVα or anti-TCRVβ antibodies, in DP and positively selected CD8+ (HY and OT-I) or CD4+ (AND) SP cells from WT (dotted line) and γδ– × hδTg (solid line) mice. (C) Percentages (top panel) and absolute numbers (bottom panel) of CD8+ (HY and OT-I) or CD4+ (AND) SP thymocytes in TCR transgenic WT (□) and γδ– × hδTg (▪) mice. Five to 8 mice per group were analyzed. Error bars indicate the mean plus or minus standard deviation.
Impaired positive selection in γδ– × hδTg mice. (A) Dot plots show CD4 and CD8 expression in thymocytes from WT and γδ– × hδTg mice expressing transgenic HY (females), OT-I, or AND TCR. Numbers within dot plots indicate the percentage of CD8+ or CD4+ SP cells in the gated region. (B) Histograms depict transgenic TCR expression, assessed with anti-TCRVα or anti-TCRVβ antibodies, in DP and positively selected CD8+ (HY and OT-I) or CD4+ (AND) SP cells from WT (dotted line) and γδ– × hδTg (solid line) mice. (C) Percentages (top panel) and absolute numbers (bottom panel) of CD8+ (HY and OT-I) or CD4+ (AND) SP thymocytes in TCR transgenic WT (□) and γδ– × hδTg (▪) mice. Five to 8 mice per group were analyzed. Error bars indicate the mean plus or minus standard deviation.
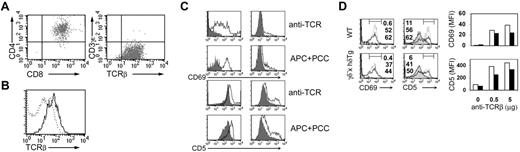
It could be argued that the defects in positive selection observed in γδ– × hδTg mice result from a decrease in surface TCR expression on the DP population (Figure 3B), which is the one subjected to this selection process. To address this issue further, we performed functional assays of TCR signaling using the AND.DP.γ–/– cell line. This cell line was established from a thymic tumor spontaneously arising in a mouse from our γδ– × hδTg colony bearing the AND TCR. It expresses surface CD4 and CD8, and TCR, but not CD3γ (Figure 4A). Expression of the transgenic hCD3δ was detected by PCR (data not shown). Of interest, surface TCR expression in AND.DP.γ–/– was even higher than that of DP cells from AND TCR transgenics (Figure 4B). Despite this high TCR expression, both CD69 and CD5 upregulation in response to TCR ligation with antibody or peptide-MHC,27,28 an event that correlates with positive selection,29 was clearly impaired in the DP thymocyte line when compared with its counterparts from WT AND TCR transgenics (Figure 4C). To exclude that this defect was entirely due to the transformed state of the cell line, we analyzed activation marker up-regulation in primary DP thymocytes from γδ– × hδTg mice, in which TCR surface expression is comparable with that of WT DP cells (Figure 1D). TCR-mediated up-regulation of CD69 and CD5 expression was also reduced in primary γδ– × hδTg DP thymocytes compared with their WT counterparts (Figure 4D), although to a lesser extent than in the thymocyte line. Together, these data suggest that TCR complexes incorporating a human CD3δ chain and lacking CD3γ have an impaired signaling capability, and that this defect is not related to the TCR density on the thymocyte surface.
Impaired negative selection and TCR-induced thymocyte death in γδ– × hδTg mice
To study negative selection in γδ– × hδTg mice, we analyzed male HY TCR transgenics. In male (but not in female) H-2b mice, thymocytes expressing the HY TCR are negatively selected as a result of extensive apoptotic cell death at the DP stage upon recognition of male-specific peptide and MHC.15 Indeed, only 3% DP cells were detected in WT HY TCR mice (Figure 5A top panel). In contrast, HY γδ– × hδTg mice exhibited a partial but clear defect in negative selection, demonstrated by the persistent DP population that constituted 56% of the thymocytes (Figure 5A top panel). Moreover, splenic CD8+ SP T cells that escaped intrathymic clonal deletion in γδ– × hδTg mice were CD8high, in contrast to WT mice where they expressed characteristically low levels of surface CD830 (Figure 5A bottom panel). Furthermore, thymocyte death induced by anti-TCR antibody in vivo31 (Figure 5B), and in vitro (Figure 5C), was markedly reduced in γδ– × hδTg mice. Collectively, these data indicate that effective coupling of the TCR to death-inducing pathways in thymocytes, and resulting negative selection, is impaired in γδ– × hδTg mice.
Reduced strength of TCR-initiated signals in γδ– × hδTg thymocytes
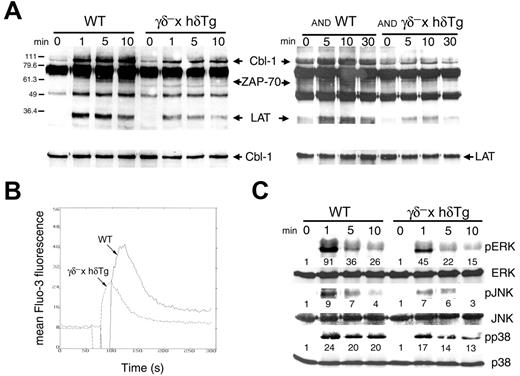
To determine whether thymocytes from γδ– × hδTg mice exhibited a TCR signaling defect, we analyzed several intracellular events associated with TCR signal transduction. Early biochemical events elicited upon TCR engagement,32 namely tyrosine phosphorylation of src- and syk-kinase substrates including ZAP-70, Cbl-1, and LAT, were markedly attenuated but not completely abrogated in γδ– × hδTg thymocytes, and also in AND TCR transgenic γδ– × hδTg thymocytes compared with their WT counterparts (Figure 6A). Although a reduced level of tyrosine phosphorylation of key signal-transduction molecules was found in anti-TCR–activated thymocytes (Figure 6A), it would appear though that no single pathway was affected specifically. TCR-induced calcium mobilization32 was also decreased in γδ– × hδTg thymocytes compared with their WT counterparts (Figure 6B).
TCR-dependent transient activation of mitogen-activated protein kinases (MAPKs) ERK, JNK, and p38 has been shown to affect thymocyte selection.33 We therefore examined the phosphorylation levels of these enzymes as an indication of their activation state. Upon in vitro TCR ligation with antibody, phosphorylation of the ERK, JNK, and p38 kinases was diminished in γδ– × hδTg thymocytes compared with control WT cells (Figure 6C). Thus, γδ– × hδTg mice display a general and quantitative defect in the TCR-induced activation of all MAPK pathways in the thymus. Taken together, these data demonstrate that proximal as well as distal signaling events coupled to TCR stimulation in thymocytes are markedly attenuated in γδ– × hδTg mice.
TCR expression and signaling in a DP thymocyte line expressing hCD3δ and lacking CD3γ. (A) The AND.DP.γ–/– line was surface stained with antibodies to CD4, CD8, TCRβ, and CD3γϵ and analyzed by flow cytometry. (B) The histogram shows surface TCR (TCRβ) expression in the AND.DP.γ–/– line (solid line) compared with DP thymocytes from AND TCR transgenic mice (dotted line). (C) CD69 and CD5 up-regulation in AND TCR transgenic DP thymocytes (left panels) or the AND.DP.γ–/– line (right panels) after 48-hour stimulation with 1 μg plastic-bound anti-TCRβ antibody or with DCEK cells preloaded with 2 μM pigeon cytochrome C (PCC) peptide 88-100. Shaded curves represent marker expression in unstimulated cells. (D) CD69 and CD5 up-regulation (left panels) in WT and γδ– × hδTg DP thymocytes stimulated for 48 hours with 0.5 μg (solid line) or 5 μg (dotted line) anti-TCRβ antibody, or in unstimulated cells (shaded curves), as determined by 3-color flow cytometry. Numbers within histograms represent the percentage of cells in the marked region for unstimulated (upper value) or anti-TCR–stimulated (0.5 μg, middle value; 5 μg, lower value) DP thymocytes. MFI values of CD69 and CD5 expression in WT (□) and γδ– × hδTg (▪) DP thymocytes stimulated with the indicated amount of anti-TCRβ antibody are plotted at the right.
TCR expression and signaling in a DP thymocyte line expressing hCD3δ and lacking CD3γ. (A) The AND.DP.γ–/– line was surface stained with antibodies to CD4, CD8, TCRβ, and CD3γϵ and analyzed by flow cytometry. (B) The histogram shows surface TCR (TCRβ) expression in the AND.DP.γ–/– line (solid line) compared with DP thymocytes from AND TCR transgenic mice (dotted line). (C) CD69 and CD5 up-regulation in AND TCR transgenic DP thymocytes (left panels) or the AND.DP.γ–/– line (right panels) after 48-hour stimulation with 1 μg plastic-bound anti-TCRβ antibody or with DCEK cells preloaded with 2 μM pigeon cytochrome C (PCC) peptide 88-100. Shaded curves represent marker expression in unstimulated cells. (D) CD69 and CD5 up-regulation (left panels) in WT and γδ– × hδTg DP thymocytes stimulated for 48 hours with 0.5 μg (solid line) or 5 μg (dotted line) anti-TCRβ antibody, or in unstimulated cells (shaded curves), as determined by 3-color flow cytometry. Numbers within histograms represent the percentage of cells in the marked region for unstimulated (upper value) or anti-TCR–stimulated (0.5 μg, middle value; 5 μg, lower value) DP thymocytes. MFI values of CD69 and CD5 expression in WT (□) and γδ– × hδTg (▪) DP thymocytes stimulated with the indicated amount of anti-TCRβ antibody are plotted at the right.
Discussion
In mice lacking CD3γ, due to targeted gene disruption, the DN-to-DP transition is severely impaired,11 most likely as a consequence of defective pre-TCR function. Thus, mature T cells are practically absent in the peripheral lymphoid organs of CD3γ-deficient mice. In contrast, T lymphocyte numbers are grossly normal or only slightly diminished in CD3γ-deficient humans,12 suggesting that in humans, but not in mice, the highly related CD3δ chain is capable of functionally replacing CD3γ during thymopoiesis. Here, we show that human CD3δ could restore mouse T-cell development in the absence of CD3γ by expressing a hCD3δ transgene in CD3γδ doubly-deficient mice.
In the γδ– × hδTg mouse strain, the pre-TCR–mediated DN-to-DP transition was efficiently restored by hCD3δ expression. It is rather intriguing that human but not mouse11 CD3δ can replace CD3γ for pre-TCR–mediated thymocyte development. In the absence of CD3γ, the amount of CD3δ could be limiting with regard to pre-TCR expression. In this scenario, enhanced expression of transgenic (relative to endogenous) CD3δ would overcome that limitation, allowing the expression of CD3γ-lacking pre-TCR complexes. This appears unlikely as the relative amount of intracellular CD3ϵ-containing dimers in DN thymocytes was comparable in WT, CD3γ-deficient, and γδ– × hδTg mice (data not shown). Also, CD3δϵ dimers present on the surface of CD3γ-deficient DN thymocytes are sufficient to mediate developmental progression when engaged with anti-CD3 antibodies,11 or to permit the expression of a transgenic αβTCR and rescue of the DN-to-DP transition.34 Finally, early thymocyte development was not restored in CD3γδ doubly-deficient mice expressing a mouse CD3δ transgene. In line with this, a human CD3 transgene that encodes full-length CD3δ and a truncated but functional form of CD3ϵ restored the defective pre-TCR function in CD3γ- and CD3γδ-deficient mice.35 Therefore, in the absence of CD3γ, human but not mouse CD3δ appears to endow the pre-TCR complex with specific properties essential for pre-TCR function. Whatever these properties might be, they appear to be independent of the signal-transducing ITAM motif, as early thymocyte development proceeded unaffected in mice expressing a CD3γ chain lacking the ITAM motif.34 Accordingly, the differential capability of human versus mouse CD3δ to functionally replace CD3γ within the pre-TCR must map in the extracellular and/or transmembrane domain, as it has previously been shown for CD3δ regarding TCR function and thymocyte selection.8 In the extracellular region, there are several amino acid positions where human but not mouse CD3δ matches mouse CD3γ. Whether these residues contribute specific structural and/or functional features to the pre-TCR complex deserves further investigation.
Impaired negative selection and antibody-induced thymocyte death in γδ– × hδTg mice. Dot plots (A) of CD4 and CD8 expression in thymocytes and splenocytes from WT and γδ– × hδTg HY TCR male mice, or (B) of thymocytes removed from WT (left) and γδ– × hδTg (right) mice 48 hours after intraperitoneal injection with either vehicle alone (PBS) or 200 μg anti-TCRβ antibody. Percentages of gated cells and total thymocyte numbers are shown within and above plots, respectively. MFI denotes mean fluorescence intensity of CD8 staining in the gated region. (C) In vitro anti-TCR antibody–induced death of WT (•) and γδ– × hδTg (○) thymocytes cultured for 48 hours in plastic wells precoated with the indicated amount of anti-TCRβ antibody. Cell death was assessed by propidium iodide staining and flow cytometry.
Impaired negative selection and antibody-induced thymocyte death in γδ– × hδTg mice. Dot plots (A) of CD4 and CD8 expression in thymocytes and splenocytes from WT and γδ– × hδTg HY TCR male mice, or (B) of thymocytes removed from WT (left) and γδ– × hδTg (right) mice 48 hours after intraperitoneal injection with either vehicle alone (PBS) or 200 μg anti-TCRβ antibody. Percentages of gated cells and total thymocyte numbers are shown within and above plots, respectively. MFI denotes mean fluorescence intensity of CD8 staining in the gated region. (C) In vitro anti-TCR antibody–induced death of WT (•) and γδ– × hδTg (○) thymocytes cultured for 48 hours in plastic wells precoated with the indicated amount of anti-TCRβ antibody. Cell death was assessed by propidium iodide staining and flow cytometry.
The reduced number of mature CD4+ and CD8+ SP thymocytes in γδ– × hδTg mice suggested a defect in TCR-mediated thymocyte selection. This was confirmed by using 3 different transgenic αβTCRs expressed in the γδ– × hδTg background. Positive selection of both these class I– or class II–restricted TCR transgenic T cells was markedly reduced in the mutant mouse, but without any sign of alteration in lineage commitment. Negative selection was similarly impaired in γδ– × hδTg mice. Further, these defects were not likely related to decreased TCR surface expression in mutant DP cells, as pointed out by our studies with the AND.DP.γ–/– thymocyte line, which presented defects in TCR signaling despite expressing high levels of receptor on the cell surface. Collectively, our results demonstrate that positive and negative selection proceeded less efficiently in γδ– × hδTg mice, probably because signals from CD3γ-lacking TCR incorporating human CD3δ are weaker. The latter is supported by the observation that signaling events proximal to TCR stimulation were markedly attenuated, but not totally abrogated, in thymocytes from γδ– × hδTg mice. More distal TCR-triggered events were also quantitatively but not qualitatively affected. A recent study further supports this notion by showing that the strength of signaling by CD4 and CD8 coreceptor tails determines the number but not the lineage direction of positively selected thymocytes,36 a phenotype similar to that observed in γδ– × hδTg mice. Our results are also consistent with the impairment in TCR-mediated tyrosine phosphorylation and calcium mobilization found in T cells from CD3γ-deficient patients.37,38 Similarly to mature T cells in CD3γ-deficient humans,24,37 but in contrast to CD3γ-null mutant mice,23 splenocytes from γδ– × hδTg mice showed normal up-regulation of CD69 and CD25 in response to anti-TCR antibody stimulation (data not shown). Further, like human T cells lacking CD3γ,37 γδ– × hδTg splenocytes exhibited defective phorbol ester–stimulated but only slightly reduced anti-TCR antibody-induced TCR down-regulation (data not shown). Taken together, these results indicate that TCR signaling is grossly similar in γδ– × hδTg mice and CD3γ-deficient humans.
Attenuated TCR-mediated signaling in γδ– × hδTg thymocytes. (A) Phosphotyrosine immunoblots of total cell lysates from WT and γδ– × hδTg thymocytes (left), or AND TCR transgenic WT and γδ– × hδTg thymocytes (right), unstimulated (time 0) or stimulated with anti-CD3 and anti-CD4 antibodies for the times indicated. The identities of some of the induced phosphoproteins were determined by immunoblotting with specific antibodies, as shown for Cbl-1 and LAT in the lower panels. (B) Calcium mobilization and (C) phosphorylated (active) MAPK (pERK, pJNK, and pp38) induction in thymocytes stimulated with cross-linked anti-CD3 and anti-CD4 antibodies. Fold activation of each MAPK relative to unstimulated (time 0) cells is indicated. Protein loading was assessed with antibodies to total ERK, JNK, and p38.
Attenuated TCR-mediated signaling in γδ– × hδTg thymocytes. (A) Phosphotyrosine immunoblots of total cell lysates from WT and γδ– × hδTg thymocytes (left), or AND TCR transgenic WT and γδ– × hδTg thymocytes (right), unstimulated (time 0) or stimulated with anti-CD3 and anti-CD4 antibodies for the times indicated. The identities of some of the induced phosphoproteins were determined by immunoblotting with specific antibodies, as shown for Cbl-1 and LAT in the lower panels. (B) Calcium mobilization and (C) phosphorylated (active) MAPK (pERK, pJNK, and pp38) induction in thymocytes stimulated with cross-linked anti-CD3 and anti-CD4 antibodies. Fold activation of each MAPK relative to unstimulated (time 0) cells is indicated. Protein loading was assessed with antibodies to total ERK, JNK, and p38.
The αβTCR complex expressed by thymocytes from γδ– × hδTg mice retained epitopes recognized by antibodies to murine TCRβ (H57-597) and CD3γϵ/δϵ dimers (145-2C11). Further, the mutant TCR complex displayed the same stoichiometry as that of the WT TCR. Together, these data indicate that the γδ– × hδTg TCR topologically resembles CD3γ-sufficient TCR in some aspects. Of importance, abnormal glycosylation of hCD3δ (data not shown) was observed for the γδ– × hδTg TCR. This might explain its slightly reduced electrophoretic mobility under native conditions compared with the WT TCR, and its inability to trigger late T-cell activation in response to the lectin concanavalin A (data not shown). In addition, a weak association of CD3 chains, including hCD3δ/CD3ϵ and CD3ζ, to the TCRαβ heterodimer was observed for the γδ– × hδTg TCR complex. These alterations in glycosylation and stability have also been reported for T cells from human CD3γ-deficient individuals,39 and they could be partly underlying the impaired signaling capability of the TCR in γδ– × hδTg mice.40,41 Whether CD3γ provides specific functions to the TCR that are essential for thymocyte selection34 remains an open question, awaiting the analysis of more CD3γ-deficient TCR transgenic mice.
CD3γ and CD3δ appeared for the first time in mammals as a result of the duplication of an ancestral gene found in birds with properties of both CD3 chains.42 The evolution of CD3γ and CD3δ as discrete subunits in mammals suggests that they might have some nonredundant but essential functions within the pre-TCR and TCR complexes. The present study further demonstrates that CD3δ and CD3γ play different roles in pre-TCR and TCR function in mice and humans.
The gross phenotype of γδ– × hδTg mice, in contrast to previously reported CD3γ-deficient mice,11 resembled that of humans who lack expression of the CD3γ protein.43 Thus, this humanized CD3γ-deficient mouse strain may constitute a valuable tool44 to analyze the consequences of the CD3γ deficiency in a more detailed way than is now possible in human patients, and for the design and preclinical evaluation of therapeutic strategies to correct human disorders associated with CD3 deficiencies.45
Prepublished online as Blood First Edition Paper, August 3, 2006; DOI 10.1182/blood-2006-03-010850.
Supported by grants BMC2002-01431 from the Spanish Ministry of Science and Education (MEC) (E.F.-M.), GR/SAL/0168/2004 from Community of Madrid (E.F.-M.), and Areces Foundation (to the Centro de Biología Molecular Severo Ochoa [CBMSO]). E.F.-M. is supported by the “Ramón y Cajal” Program of MEC. M.P. was supported by a BEFI fellowship from the Fondo de Investigaciones Sanitarias (FIS). W.W.A.S. was supported by the Deutsche Forschungsgemeinschaft through the Emmy Noether program (SCHA 976/1) and through SFB620.
The authors declare no competing financial interests.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal