Abstract
Early viral infection is often associated with lymphopenia, a transient reduction of blood lymphocyte counts long before the onset of clinical symptoms. We have investigated lymphopenia in mice infected with vesicular stomatitis virus (VSV) or treated with the Toll-like receptor (TLR) agonists poly(I:C) and R-848. In all cases analyzed, lymphopenia was critically dependent on type I interferon receptor (IFNAR) signaling. With the use of bone marrow–chimeric mice, radioresistant cells, such as stroma and endothelium, could be excluded as type I interferon (IFN-α/β) targets for the induction of lymphopenia. Instead, adoptive transfer experiments and studies in conditionally gene-targeted mice with a B- or T-cell–specific IFNAR deletion demonstrated that IFN-α/β exerted a direct effect on lymphocytes that was necessary and largely sufficient to induce lymphopenia. Furthermore, after treatment with R-848, we found that other cytokines such as TNF-α also played a role in T-cell lymphopenia. Investigation of the molecular mechanism revealed that lymphopenia was mainly independent of G protein–coupled receptors (GPCRs) and chemokines. In an adhesion assay, B cells of poly(I:C)–treated mice showed moderately increased adhesion to ICAM-1 but not to VCAM-1. In conclusion, our data identify a new effect of direct IFN-α/β stimulation of lymphocytes that profoundly affects lymphocyte redistribution.
Introduction
The incubation period of many viral infections is characterized by a transient reduction of peripheral blood lymphocyte counts, a phenomenon called lymphopenia. In mice infected with virus or treated with TLR ligands, lymphopenia in blood and lymph was related to IFN-α/β.1,2 In line with this, lymphopenia is reported as a side effect of IFN-α/β therapy for multiple sclerosis and chronic hepatitis.3,4 Similarly, treatment with IFN-γ, IL-2, TNF-α,5 and IL-126 and chemical compounds including FTY7207 were reported to cause a reduction of leukocyte subsets in patients' blood.
Interferons are classified into 2 distinct families, IFN-α/β (or type I IFN) and IFN-γ (or type II IFN). IFN-α/β binds to the heterodimeric type I interferon receptor (IFNAR), composed of an α-chain, which is essential for signaling, and a β-chain. In addition to antiviral activity,8-10 IFN-α/β can have an impact on lymphocyte proliferation,11 apoptosis,12 and expression of cytokines and cytokine receptors,13-16 causing immune modulation in vivo.17-19
IFN-α/β exerts direct and indirect effects on lymphocytes. A critical role of IFN-α/β in CD8+ T-cell cross-priming has been shown.20 This could recently be identified as a direct effect on CD8+ T cells, promoting clonal expansion and memory T-cell formation by increased cell survival.21 Furthermore, IFN-α/β can directly stimulate naive T cells,22,23 promote plasma cell differentiation, and enhance B-cell responses.24-28 Many indirect effects of IFN-α/β on lymphocytes are presumably mediated by the activation of antigen-presenting cells,26,29-31 which in turn secrete various cytokines, such as IL-15, that can act on lymphocytes.16 Apart from activating B and T cells, IFN-α/β can influence lymphocyte homeostasis by suppressing hematopoiesis32 or enhancing the output of lymphocyte precursors.33
Lymphocytes continuously home to secondary lymphoid organs (SLOs) and recirculate through lymph and blood. Homing relies on a cascade of events guided by selectins, integrins, chemokine receptors, and sphingosine 1-phosphate receptor 1 (S1P1).34-36 The expression of homing receptors on lymphocytes and endothelium is stringently controlled and correlates with the cellular differentiation state.37-41 It is conceivable that IFN-α/β affects circulation and homing of lymphocytes because it can induce chemokines and modulate adhesion molecules in human T-cell lines42 and endothelial cells.43 Stimulation with the TLR7 ligand R-848 causes pronounced lymphopenia and enhances rolling and sticking of lymphocytes, concomitant with increased expression of several adhesion molecules on endothelia.44 Furthermore, treatment with FTY720 reduces S1P1 expression and leads to lymphopenia and lymphocyte sequestration in lymphoid organs.7,45,46 In essence, however, the mechanism of lymphopenia and the cellular targets of cytokines and other factors remain elusive.
In the present study, we have analyzed the role of IFN-α/β in lymphopenia induced by vesicular stomatitis virus (VSV) infection or by treatment with the Toll-like receptor (TLR) agonists poly(I:C) and R-848. We found that under all conditions tested, lymphopenia was caused by direct action of IFN-α/β on B and T cells. Using conditionally targeted mice with B- or T cell–specific IFNAR inactivation, we demonstrated that the direct effect of IFN-α/β on lymphocytes is necessary and largely sufficient to induce lymphopenia. Lymphopenic B and T cells are not preferentially recruited to one specific target organ, and induction of lymphopenia is mainly independent of G protein–coupled receptors (GPCRs) expressed on lymphocytes.
Materials and methods
Mice
C57BL/6 mice were purchased from Charles River. Mice expressing a conditional IFNAR (IFNARflox/flox), transgenic mice that express Cre specifically in B cells (CD19-Cre)47 or in T cells (CD4-Cre),48 IFNAR–/–9 mice, and TNF-α–/–49 mice were bred at the Paul-Ehrlich-Institut, and CCR7–/– mice50 were bred at the University of Zürich, Switzerland. All genetically modified mice were 10-fold backcrossed to the C57BL/6 background. BM chimeras were produced by lethally irradiating recipients (11 Gy) and injecting 5 to 10 × 106 BM cells intravenously the next day. Six weeks later, reconstitution was verified by fluorescence-activated cell sorter (FACS) analysis. Lymphocyte chimerism of mice usually exceeded 94%, whereas 5% of recipient-derived T cells was typically found in blood. Animal experiments were conducted under specific pathogen-free conditions, in compliance with German federal and state legislation on animal experiments or legislation on animal protection of the Kanton Zürich.
Viruses
VSV-Indiana (Mudd-Summers isolate), originally obtained from D. Kolakofsky (University of Geneva, Switzerland), was grown on BHK-21 cells. Virus was harvested from cell culture supernatants, and titers were determined by plaque formation on Vero cells.
Induction of lymphopenia
Mice were given intraperitoneal injections of 200 μg poly(I:C) (Sigma) or 25 to 50 μg R-848 (3M, St Paul, MN; InvivoGen, San Diego, CA) in 200 μL PBS, and blood lymphocyte counts were assessed 16 to 20 hours later. Alternatively, mice were treated subcutaneously with 2 × 105 IU IFN-α4 (kindly provided by D. Tough, GlaxoSmithKline).20 IFN-β (106 IU/mL) used for cell culture studies was purchased from R&D Systems (Minneapolis, MN).
Adoptive transfer of lymphocyte populations
Untouched splenic B cells were purified by MACS (Miltenyi Biotec, Bergisch Gladbach, Germany) and labeled either by 5-(6-) carboxytetramethylrhodamine succinimidyl ester (TAMRA; Molecular Probes, Eugene, OR) or by carboxyfluorescein diacetate succinimidyl ester (CSFE; Molecular Probes). For labeling, 107 cells were resuspended in 5 mL of 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered RPMI, and 10 μL of 5 mM TAMRA was added. Alternatively, cells were resuspended in 0.5 mL PBS with 0.1% BSA, and 0.6 μL of 0.25 or 5 mM CSFE was added. Cells (1-5 × 107 per labeling type) were adoptively transferred into recipients. Unless otherwise indicated, the transferred cells were counted 1 day after injection.
Histology
Effective GPCR blockade was confirmed by analysis of sections of fresh spleens and inguinal lymph nodes (LNs), embedded at room temperature in moviol, on a laser scan microscope (LSM 510 Meta; Carl Zeiss, Jena, Germany) connected to an inverted microscope (Axiovert 200M; Carl Zeiss). Images were taken with a Plan-Neofluar lens at 10 × magnification with 0.3 numerical aperture using LSM 5 image browser software (Carl Zeiss). Figures were composed in Photoshop (Adobe, San Jose, CA). For immunohistochemistry, cryosections of 6-mm thickness were treated at room temperature with the antibody 2.4G2 to block Fc receptors and were stained with MOMA-1–FITC and Thy1.2-APC or B220-APC.
Antibodies and flow cytometry
All antibodies were purchased from BD Biosciences (San Jose, CA), except for PE-labeled anti–mouse CD3ϵ, CD11a, and CD62L (Caltag, Burlingame, CA) and MOMA-1-FITC (Serotec, Raleigh, NC). Staining of CCR7 with the CCL19-Fc fusion protein was performed as described previously.41 Labeled cells were analyzed on FACScan or LSR II flow cytometers (Becton Dickinson) using CellQuest Pro, BD FACSDiva, and WinList 5.0 software (Verity Software House, Topham, ME).
Counting numbers of peripheral-blood lymphocytes
To determine absolute blood cell counts, peripheral blood was collected in heparinized microtainers (BD Biosciences). Then, 15 μL blood was mixed with 15 μL counting beads (approximately 1000 beads/μL; Caltag), and samples were stained for T cells (CD3ϵ+) and B cells (B220+). After treatment with FACS lysing solution (BD Biosciences), samples were subjected to FACS analysis with the acquisition of 5000 counting beads. Therefore, data shown correspond to approximately 5 μL blood. In some experiments (Figure 5C-D), 100-μL blood samples were taken, erythrolyzed, and analyzed. Based on the total lymphocyte counts, percentages of CFSEhi and CFSElo cells were calculated and depicted as percentages of pretreatment values. Pretreatment mean values were normalized to 100% for CCR7–/– and WT cells.
Adhesion assay
For adhesion assays,51 96-well plates were coated overnight at 4°C with ICAM-Fc or VCAM-Fc (R&D Systems) in TBS. After blocking with HBSS + 1% BSA for 60 to 90 minutes at 37°C and subsequent washing, 3 × 105 CD43–-sorted (Miltenyi Biotec) B cells in HBSS, 0.5% BSA, containing 2 mM Mg2+ and 2 mM Ca2+, were added. B-cell purity was typically greater than 97%. After incubation in triplicate for 30 minutes at 37°C, plates were gently washed, fixed with PBS + 10% glutaraldehyde, and stained for 45 minutes with 0.5% crystal violet in methanol. After washing with water and short drying, cells were lysed by the addition of 1% SDS. Plates were read at 570 nm. Uncoated wells containing 3 × 105 B cells were fixed and stained directly as positive controls (100%). Adhesion to wells coated with 4% BSA in TBS was taken as background control. Of note, nonspecific adhesion of B cells to plastic was typically higher than adhesion to BSA-coated wells.
Statistical analyses
Data are depicted as means plus or minus SD. B/T cell ratios were analyzed with the Wilcoxon rank-sum test, and differences were considered statistically significant for P less than .05. For adhesion assays, means of triplicates in each experimental condition were compared using paired t tests.
Results
IFNAR signaling is critically involved in reduction of peripheral-blood lymphocyte counts and CD69 up-regulation after VSV infection or treatment with TLR3 or TLR7 agonists
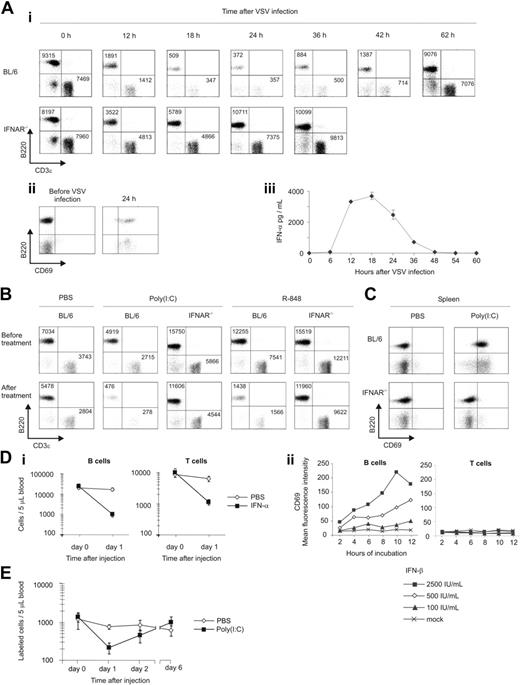
After intravenous infection with VSV, B- and T-cell counts were massively decreased in C57BL/6 (BL/6) mice. This lymphopenia was mediated by IFNAR signaling because B- and T-cell numbers in mice devoid of IFNAR (IFNAR–/–)9 were only partially reduced (Figure 1Ai). During lymphopenia, the few lymphocytes still found in blood showed a stimulated phenotype, as characterized by high expression of CD69 (Figure 1Aii). Before lymphopenia was manifested, we found an early and significant IFN-α production (Figure 1Aiii). Similar to VSV infection, IFN-α responses had an impact on lymphopenia induced by intraperitoneal treatment with the TLR3 ligand synthetic, double-stranded RNA (poly(I:C)) (Figure 1B). Given that TLR7 is involved in VSV-mediated IFN-α/β induction,52 mice were further given injections of the synthetic TLR7 agonist R-848, which also induced IFNAR-dependent lymphopenia (Figure 1B). Lymphocytes of poly(I:C)–treated BL/6 mice showed CD69 up-regulation (Figure 1C) that was absent in IFNAR–/– mice. Similar results were observed after injection with R-848 (E.K., unpublished data, August 2005 and May-June 2006). In conclusion, IFNAR signaling played a critical role in the induction of lymphopenia and the up-regulation of CD69 on lymphocytes.
IFN-α/β plays a critical role in the induction of lymphopenia. (Ai) BL/6 and IFNAR–/– mice were intravenously infected with 2 × 106 PFU VSV. Blood samples were taken at the indicated time points and stained for CD3ϵ and B220. For FACS analysis, data equivalent to approximately 5 μL blood were acquired. Representative results of 1 of 2 similar experiments are shown. (Aii) Twenty-four hours after VSV inoculation, blood samples were stained for CD69 and B220 and subjected to FACS analysis. Representative data of 1 of 4 animals tested are depicted. (Aiii) Serum samples of mice infected with 2 × 106 PFU VSV were taken at the indicated time points and analyzed for IFN-α by ELISA. Results are expressed as mean ± SD (n = 2). (B) Poly(I:C)– and R-848–induced lymphopenia are dependent on IFNAR signaling. BL/6 and IFNAR–/– mice were intraperitoneally treated with poly(I:C), R-848, or PBS, and blood lymphocyte counts were determined. Representative data from 1 of 2 similar experiments are shown. (C) Poly(I:C) induces IFNAR-dependent up-regulation of CD69 in spleen. Splenocytes of PBS or poly(I:C)–treated mice were stained for CD69 and B220 and subjected to FACS analysis. Representative data of 3 similar experiments are depicted. (Di) IFN-α treatment induces lymphopenia. BL/6 mice were treated subcutaneously with 2 × 105 IU IFN-α or PBS, and blood lymphocyte counts were determined. Results are expressed as mean ± SD for 4 mice per group. (Dii) CD69 expression on B and T cells in splenocyte cultures stimulated with graded concentrations of IFN-β was determined by FACS analysis. (E) Poly(I:C)–induced lymphopenia is reversible. Syngeneic CFSE-labeled splenocytes were adoptively transferred to WT recipients. Mice were given injections of poly(I:C) or PBS, and labeled cells in blood were counted. Results are expressed as mean ± SD (n = 3) and are representative of 2 similar experiments.
IFN-α/β plays a critical role in the induction of lymphopenia. (Ai) BL/6 and IFNAR–/– mice were intravenously infected with 2 × 106 PFU VSV. Blood samples were taken at the indicated time points and stained for CD3ϵ and B220. For FACS analysis, data equivalent to approximately 5 μL blood were acquired. Representative results of 1 of 2 similar experiments are shown. (Aii) Twenty-four hours after VSV inoculation, blood samples were stained for CD69 and B220 and subjected to FACS analysis. Representative data of 1 of 4 animals tested are depicted. (Aiii) Serum samples of mice infected with 2 × 106 PFU VSV were taken at the indicated time points and analyzed for IFN-α by ELISA. Results are expressed as mean ± SD (n = 2). (B) Poly(I:C)– and R-848–induced lymphopenia are dependent on IFNAR signaling. BL/6 and IFNAR–/– mice were intraperitoneally treated with poly(I:C), R-848, or PBS, and blood lymphocyte counts were determined. Representative data from 1 of 2 similar experiments are shown. (C) Poly(I:C) induces IFNAR-dependent up-regulation of CD69 in spleen. Splenocytes of PBS or poly(I:C)–treated mice were stained for CD69 and B220 and subjected to FACS analysis. Representative data of 3 similar experiments are depicted. (Di) IFN-α treatment induces lymphopenia. BL/6 mice were treated subcutaneously with 2 × 105 IU IFN-α or PBS, and blood lymphocyte counts were determined. Results are expressed as mean ± SD for 4 mice per group. (Dii) CD69 expression on B and T cells in splenocyte cultures stimulated with graded concentrations of IFN-β was determined by FACS analysis. (E) Poly(I:C)–induced lymphopenia is reversible. Syngeneic CFSE-labeled splenocytes were adoptively transferred to WT recipients. Mice were given injections of poly(I:C) or PBS, and labeled cells in blood were counted. Results are expressed as mean ± SD (n = 3) and are representative of 2 similar experiments.
Injection of IFN-α/β induces lymphopenia and stimulates lymphocytes
Because poly(I:C) and R-848 induce many cytokines in addition to IFN-α/β, we checked whether the injection of IFN-α/β alone was able to elicit lymphopenia. To this end, 2 × 105 IU IFN-α was administered subcutaneously to BL/6 mice (Figure 1Di). One day after injection with IFN-α, mice showed significantly reduced B- and T-cell numbers, unlike PBS-treated controls. Similar results were obtained with the injection of IFN-β (E.K., unpublished data, January 2005). In IFN-β–stimulated splenocyte cultures, B cells, but not T cells, showed CD69 up-regulation (Figure 1Dii). A maximal CD69 induction was observed after 8 to 10 hours of incubation with 2500 IU/mL IFN-β, an amount typically detected in the sera of VSV-infected mice. Thus, IFN-α/β stimulation was sufficient to elicit lymphopenia and to induce CD69 expression on B lymphocytes.
IFN-α/β–induced lymphopenia is fully reversible
To analyze whether the reappearance of B and T cells in blood was related to the redistribution of existing lymphocytes or to apoptosis and consecutively augmented BM output33 or to increased de novo formation, we adoptively transferred CFSE-labeled splenocytes into BL/6 recipients and monitored absolute numbers of labeled cells after treatment with poly(I:C) (Figure 1E). One day after the induction of lymphopenia, adoptively transferred lymphocytes reappeared in blood and eventually reached numbers similar to those of PBS-treated controls. Thus, lymphocytes were sequestered from blood and at later time points reappeared, demonstrating that poly(I:C)–induced lymphopenia was completely reversible and did not involve apoptosis. CFSE levels of labeled lymphocytes were not reduced after poly(I:C) treatment; therefore, cell division did not play a role in the reappearance of lymphocytes (E.K., unpublished data, April 2005).
Lymphopenia is induced by IFN-α/β stimulation of immune cells but not by triggering of stroma or endothelium. (A) BM chimeras were generated by reconstitution of congenic CD45.1+ C57BL/6 mice with CD45.2+ IFNAR–/– BM cells (IFNAR–/– > WT). Similarly, WT > IFNAR–/–, IFNAR–/– > IFNAR–/–, and WT > WT were obtained. Blood B- and T-cell numbers were counted before and after treatment with poly(I:C). Data are expressed as mean ± SD (n = 3) and are representative of 2 similar experiments. (B) IFN-α/β stimulation has a direct effect on B cells. B cells from WT and IFNAR–/– mice were labeled with TAMRA or CFSE (left panels) or vice versa (right panels) and were reinjected into WT or IFNAR–/– recipients. Labeled cells in blood were counted before and after treatment with poly(I:C) and are depicted as a percentage of total B cells (absolute numbers in parentheses). Data are representative of 1 of 3 similar experiments.
Lymphopenia is induced by IFN-α/β stimulation of immune cells but not by triggering of stroma or endothelium. (A) BM chimeras were generated by reconstitution of congenic CD45.1+ C57BL/6 mice with CD45.2+ IFNAR–/– BM cells (IFNAR–/– > WT). Similarly, WT > IFNAR–/–, IFNAR–/– > IFNAR–/–, and WT > WT were obtained. Blood B- and T-cell numbers were counted before and after treatment with poly(I:C). Data are expressed as mean ± SD (n = 3) and are representative of 2 similar experiments. (B) IFN-α/β stimulation has a direct effect on B cells. B cells from WT and IFNAR–/– mice were labeled with TAMRA or CFSE (left panels) or vice versa (right panels) and were reinjected into WT or IFNAR–/– recipients. Labeled cells in blood were counted before and after treatment with poly(I:C) and are depicted as a percentage of total B cells (absolute numbers in parentheses). Data are representative of 1 of 3 similar experiments.
IFN-α/β induces lymphopenia through the stimulation of immune cells but not through effects on endothelium or stroma
To investigate whether IFN-α/β induced lymphopenia by acting on endothelium and stroma or on immune cells, we lethally irradiated CD45.2+IFNAR–/– mice and reconstituted them 1 day later with congenic CD45.1+BL/6 wild-type (WT) BM (WT > IFNAR–/– mice). Similarly, CD45.1+ congenic WT mice were reconstituted with CD45.2+IFNAR–/– BM (IFNAR–/– > WT) (Figure 2A). With poly(I:C) treatment, only WT > WT–positive controls and WT > IFNAR–/– mice showed lymphopenia, whereas IFNAR–/– > WT mice and IFNAR–/– > IFNAR–/––negative controls did not. These data demonstrated that IFN-α/β stimulation of BM-derived immune cells, but not of radioresistant cells, including endothelium and stroma, was required for the induction of lymphopenia.
IFN-α/β directly activates B cells
To address whether IFN-α/β induces lymphopenia directly by stimulation of lymphocytes or indirectly by activating some immune cell type to secrete relevant factors, we adoptively transferred WT and IFNAR–/– B cells differentially labeled by TAMRA or CFSE into WT or IFNAR–/– recipient mice (Figure 2B). Irrespective of the recipient, only WT B cells disappeared on poly(I:C) challenge, whereas IFNAR–/– B cells remained in the blood. Reciprocal labeling of transferred cells (ie, WT B cells labeled with CFSE and IFNAR–/– cells with TAMRA) revealed comparable results, indicating that the dyes did not affect homing properties of transferred B cells. In conclusion, the data indicated that direct IFN-α/β stimulation of B cells was necessary and largely sufficient for the induction of lymphopenia.
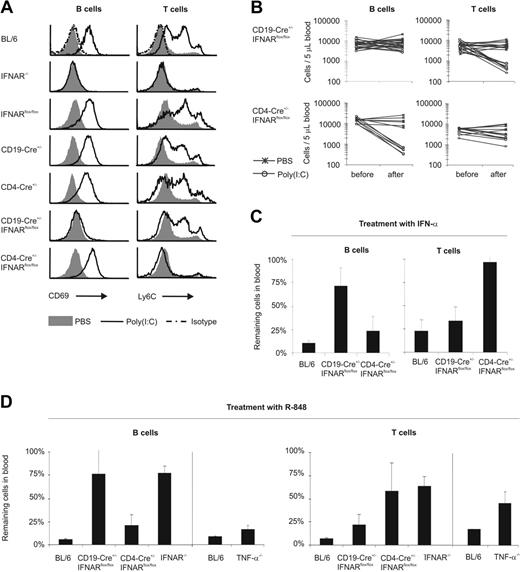
Mice with a B- or a T-cell–specific IFNAR deletion showed significantly reduced lymphopenia of B and T cells, respectively
In adoptive transfer experiments, numbers of injected B cells were limited compared with the endogenous B-cell pool. Thus, we further studied the direct effect of IFN-α/β stimulation of lymphocytes in CD19-Cre+/–IFNARflox/flox and CD4-Cre+/–IFNARflox/flox mice showing a B- or T-cell–specific IFNAR deletion (S. Roederstein, S. Indraccolo, C. Detje, M. Anzaghe, G. Esposito, S. Delorme, T. Tuting, T. Hinz, and U.K., manuscript submitted). The quantitative and selective inactivation of IFNAR was verified by analyzing the expression of the markers CD69 and Ly6C, which are IFN-α/β dependent in B and T cells, respectively (Figure 3A).53,54 After poly(I:C) treatment, controls, including BL/6, IFNARflox/flox, CD19-Cre+/–,47 and CD4-Cre+/–48 mice showed a pronounced up-regulation of CD69 on B cells and of Ly6C on T cells. Similarly, T cells of CD19-Cre+/–IFNARflox/flox revealed upregulation of Ly6C, and B cells of CD4-Cre+/–IFNARflox/flox mice exhibited increased CD69 expression. However, similar to lymphocytes of IFNAR–/– mice, B cells of CD19-Cre+/– IFNARflox/flox mice did not show an induction of CD69, and T cells of CD4-Cre+/–IFNARflox/flox mice were unable to upregulate Ly6C. These observations demonstrated that in CD19-Cre+/–IFNARflox/flox and CD4-Cre+/–IFNARflox/flox mice, B and T cells, respectively, were unresponsive to IFN-α/β, demonstrating the selective and quantitative deletion of IFNAR. With poly(I:C) treatment (Figure 3B) of CD19-Cre+/–IFNARflox/flox mice, B cells remained in blood, whereas T cells and other IFNAR-competent immune cells disappeared. In CD4-Cre+/– IFNARflox/flox mice, B cells underwent massive lymphopenia, whereas T-cell numbers were only slightly reduced (Figure 3B). After injection with IFN-α, B-cell lymphopenia was markedly reduced in CD19-Cre+/–IFNARflox/flox mice, and T-cell lymphopenia was largely abolished in CD4-Cre+/–IFNARflox/flox mice (Figure 3C). Similarly, after VSV infection, CD19-Cre+/– IFNARflox/flox and CD4-Cre+/–IFNARflox/flox mice showed selectively reduced B-cell or T-cell lymphopenia (E.K., unpublished data, May 2006). In summary, direct IFN-α/β stimulation of B and T cells was largely sufficient for the induction of lymphopenia.
Selective lymphopenia in mice with a B- or a T-cell–specific IFNAR deletion. (A) Poly(I:C) or PBS-treated splenocytes of the indicated mice were analyzed by FACS for the IFN-α/β–dependent markers CD69 on B cells and Ly6C on T cells. Note the lack of up-regulation of CD69 on B cells in CD19-Cre+/–IFNARflox/flox mice and of Ly6C on T cells in CD4-Cre+/–IFNARflox/flox mice, indicating selective and quantitative IFNAR inactivation in the respective lymphocyte subsets. Representative data of 2 experiments are shown. (B) CD19-Cre+/–IFNARflox/flox and CD4-Cre+/–IFNARflox/flox mice were given injections of poly(I:C) or PBS. Blood lymphocyte counts were determined before and after treatment. Individual results of 25 CD19-Cre+/–IFNARflox/flox and 13 CD4-Cre+/–IFNARflox/flox mice are shown. (C) BL/6, CD19-Cre+/–IFNARflox/flox, and CD4-Cre+/–IFNARflox/flox mice were treated with 2 × 105 IU IFN-α, and lymphocyte counts were determined before and after treatment. Data are expressed as mean ± SD remaining cell percentages in blood. Representative data of 3 experiments with 2 to 4 mice per group are shown. (D) BL/6, CD19-Cre+/–IFNARflox/flox, CD4-Cre+/–IFNARflox/flox, IFNAR–/–, and TNF-α–/– mice were treated with R-848. Numbers of B and T cells were counted before and after injection. Data are expressed as mean ± SD of percentages of remaining cells in blood. Experiments were performed 4 times, twice with TNF-α–/– mice, with 2 to 4 mice per group.
Selective lymphopenia in mice with a B- or a T-cell–specific IFNAR deletion. (A) Poly(I:C) or PBS-treated splenocytes of the indicated mice were analyzed by FACS for the IFN-α/β–dependent markers CD69 on B cells and Ly6C on T cells. Note the lack of up-regulation of CD69 on B cells in CD19-Cre+/–IFNARflox/flox mice and of Ly6C on T cells in CD4-Cre+/–IFNARflox/flox mice, indicating selective and quantitative IFNAR inactivation in the respective lymphocyte subsets. Representative data of 2 experiments are shown. (B) CD19-Cre+/–IFNARflox/flox and CD4-Cre+/–IFNARflox/flox mice were given injections of poly(I:C) or PBS. Blood lymphocyte counts were determined before and after treatment. Individual results of 25 CD19-Cre+/–IFNARflox/flox and 13 CD4-Cre+/–IFNARflox/flox mice are shown. (C) BL/6, CD19-Cre+/–IFNARflox/flox, and CD4-Cre+/–IFNARflox/flox mice were treated with 2 × 105 IU IFN-α, and lymphocyte counts were determined before and after treatment. Data are expressed as mean ± SD remaining cell percentages in blood. Representative data of 3 experiments with 2 to 4 mice per group are shown. (D) BL/6, CD19-Cre+/–IFNARflox/flox, CD4-Cre+/–IFNARflox/flox, IFNAR–/–, and TNF-α–/– mice were treated with R-848. Numbers of B and T cells were counted before and after injection. Data are expressed as mean ± SD of percentages of remaining cells in blood. Experiments were performed 4 times, twice with TNF-α–/– mice, with 2 to 4 mice per group.
IFNAR dependence of lymphopenia after treatment with R-848 (Figure 1B) also pointed to a major role of direct IFN-α/β stimulation of B and T cells. On injection with R-848 (Figure 3D, left part of the diagrams), IFNAR-competent B cells in BL/6 and CD4-Cre+/–IFNARflox/flox mice disappeared from peripheral blood, whereas numbers of IFNAR-deficient B cells in CD19-Cre+/–IFNARflox/flox and IFNAR–/– mice remained stable. In contrast, numbers of IFNAR-deficient T cells in CD4-Cre+/–IFNARflox/flox mice were decreased by approximately 50%. Thus, on treatment with R-848, B-cell lymphopenia was primarily mediated through direct IFN-α/β stimulation, whereas T-cell lymphopenia was only partially dependent on IFN-α/β and was probably also triggered by some other cytokine(s) or by indirect IFN-α/β stimulation of other cells. Given that TNF-α was described as inducing lymphopenia in humans,5 we investigated whether R-848–induced lymphopenia was dependent on TNF-α. After R-848 treatment of TNF-α–/– mice,49 we found almost normal B-cell lymphopenia (Figure 3D, right part of the diagrams), whereas T-cell lymphopenia was inhibited by approximately 50%. In conclusion, on R-848 injection, TNF-α partially mediated T-cell lymphopenia and exerted minor effects on B-cell lymphopenia.
During lymphopenia, B cells moderately accumulate in spleen but T cells do not
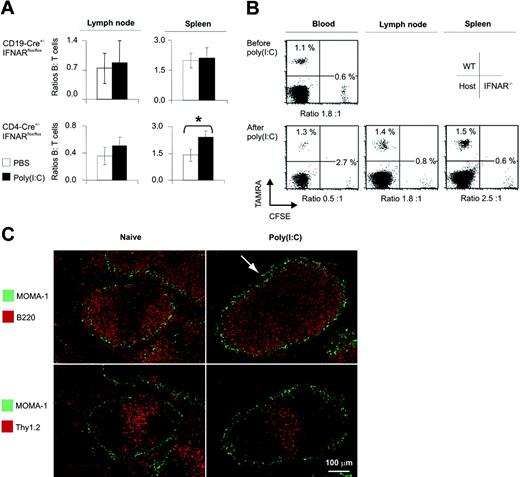
We considered it likely that virus infection recruited lymphocytes into SLOs. In line with this, several studies suggest that FTY720 causes lymphopenia by lymph node (LN) logjam.7,45,46 To investigate lymphopenic homing induced by IFN-α/β, we used 2 approaches based on the concept of changes in the ratio of lymphopenic to nonlymphopenic cells. First, we analyzed CD19-Cre+/–IFNARflox/flox mice in which B cells remained in blood after poly(I:C) treatment and T cells underwent lymphopenia (Figure 3B). Consequently, similar to changes of B/T cell ratios in blood in those tissues, where T cells preferentially home, B/T cell ratios were expected to be altered. Comparative FACS analysis of lymphocyte redistribution in LNs and spleen of CD19-Cre+/–IFNARflox/flox mice showed that B/T cell ratios remained similar, indicating that T cells did not preferentially home to LNs or spleen (Figure 4A). In CD4-Cre+/–IFNARflox/flox mice, a slight but significant increase in B/T-cell ratios was observed in spleen but not in LNs, suggesting some preferred homing or retention of B cells only in spleen during lymphopenia.
In theory, the detection of shifts in B/T cell ratios could be hampered by numbers of immigrating lymphocytes that were too low compared with constitutive B- and T-cell numbers within SLOs. To overcome this limitation, and as a second approach, we transfused mice with differentially labeled WT and IFNAR–/– B cells and analyzed SLOs by FACS for ratios of WT/IFNAR–/–cells, the latter serving as a constant reference population (Figure 4B). Compared with pretreatment levels, ratios of WT/IFNAR–/– B cells were reduced on poly(I:C) treatment in blood, remained constant in LN, and were slightly increased in spleen, suggesting that WT B cells left the circulation to preferentially accumulate in spleen. Additionally, we did not find any increased B- or T-cell trafficking in mesenteric LN (E.K., unpublished data, May 2006), and laser scan microscopy of nonlymphoid organs did not reveal major changes in ratios of differentially labeled cells (E.K., unpublished data, February 2005). Thus, only the spleen appeared to be a moderately preferred target for B-cell homing, whereas other organs were not particularly frequented. We then analyzed whether lymphocyte localization in SLOs was affected on the induction of lymphopenia. In the spleen of poly(I:C)–treated mice, marginal zone B cells disappeared (Figure 4C, arrow), and fewer B and T cells were detected in the red pulp. In conclusion, splenic lymphocytes revealed a major redistribution concomitant with lymphopenia in the blood.
Selective lymphocyte homing in mice with a B- or a T-cell–specific IFNAR deletion. (A) CD19-Cre+/–IFNARflox/flox and CD4-Cre+/–IFNARflox/flox mice were given injections of poly(I:C) or PBS. On day 1, B and T cells from spleen and from axillary and inguinal lymph nodes were subjected to FACS analysis. B/T cell ratios are depicted and are expressed as mean ± SD for 25 CD19-Cre+/–IFNARflox/flox and 13 CD4-Cre+/–IFNARflox/flox mice (as in Figure 3B). *P = .012 (PBS vs poly(I:C)). (B) B cells from WT and IFNAR–/– mice were labeled with TAMRA or CFSE and reinjected into WT recipients. Labeled cells were counted in blood before and after treatment with poly(I:C). Single-cell suspensions of spleen and LNs were analyzed by FACS. Percentages and ratios of WT/IFNAR–/– B cells are depicted. Results are representative of 1 of 3 animals in 3 similar experiments (as in Figure 2B). (C) Splenic morphology 15 hours after poly(I:C) injection. Staining of metallophilic macrophages (MOMA-1, green) delineates the marginal zone. The arrow indicates the marginal zone devoid of B cells. Images were visualized and acquired using an Olympus BX51 fluorescence microscope (Olympus, Center Valley, PA) equipped with a 10×/0.3 NA air objective. FluorSave reagent (Calbiochem, San Diego, CA) was used as imaging medium.
Selective lymphocyte homing in mice with a B- or a T-cell–specific IFNAR deletion. (A) CD19-Cre+/–IFNARflox/flox and CD4-Cre+/–IFNARflox/flox mice were given injections of poly(I:C) or PBS. On day 1, B and T cells from spleen and from axillary and inguinal lymph nodes were subjected to FACS analysis. B/T cell ratios are depicted and are expressed as mean ± SD for 25 CD19-Cre+/–IFNARflox/flox and 13 CD4-Cre+/–IFNARflox/flox mice (as in Figure 3B). *P = .012 (PBS vs poly(I:C)). (B) B cells from WT and IFNAR–/– mice were labeled with TAMRA or CFSE and reinjected into WT recipients. Labeled cells were counted in blood before and after treatment with poly(I:C). Single-cell suspensions of spleen and LNs were analyzed by FACS. Percentages and ratios of WT/IFNAR–/– B cells are depicted. Results are representative of 1 of 3 animals in 3 similar experiments (as in Figure 2B). (C) Splenic morphology 15 hours after poly(I:C) injection. Staining of metallophilic macrophages (MOMA-1, green) delineates the marginal zone. The arrow indicates the marginal zone devoid of B cells. Images were visualized and acquired using an Olympus BX51 fluorescence microscope (Olympus, Center Valley, PA) equipped with a 10×/0.3 NA air objective. FluorSave reagent (Calbiochem, San Diego, CA) was used as imaging medium.
Lymphopenia is mainly independent of GPCRs and chemokines
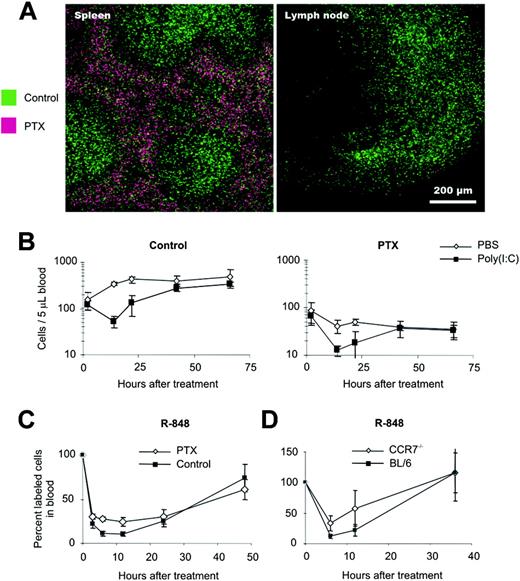
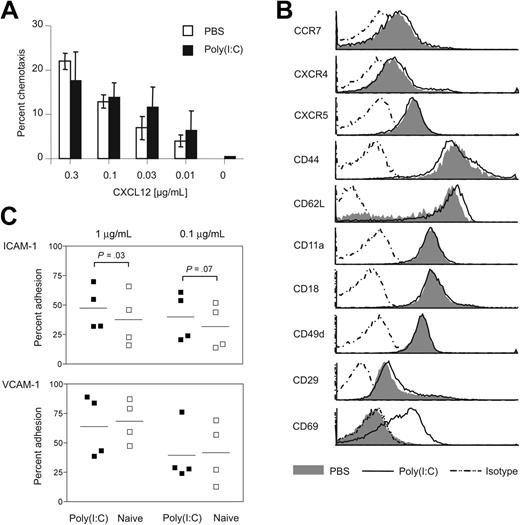
To determine whether signaling through PTX-sensitive chemokine and phospholipid receptors was involved in IFN-α/β–induced lymphopenia, we adoptively transferred differentially labeled control cells and PTX-treated splenocytes into recipients that were later given injections of PBS or poly(I:C). Effective blockade of GPCR signaling throughout the experiment was confirmed by laser scan microscopy showing that PTX-treated cells did not enter LNs and splenic white pulp (Figure 5A). On poly(I:C) challenge, control (Figure 5B, left) and PTX-treated (Figure 5B, right) lymphocytes were able to leave peripheral blood, albeit PTX-treated cells disappeared with slightly reduced efficiency. Similar results were obtained when lymphopenia was induced by R-848 (Figure 5C). In essence, these data revealed that lymphopenia was mainly independent of GPCRs. Because the homing of T and B cells is crucially controlled by the chemokine receptor CCR7,50 we cotransferred BL/6 and CCR7–/– splenocytes and induced lymphopenia by injection of R-848. Again, we observed a massive reduction of both cell populations from the bloodstream, although CCR7–/– cells disappeared slightly less efficiently (Figure 5D). Thus, CCR7 was not critical for lymphopenia. Furthermore, ex vivo B-cell chemotaxis toward CXCL12 (Figure 6A55 ), CCL19, and CCL21 (E.K., unpublished data, January 2004) was similar in B cells isolated from PBS or poly(I:C)–treated mice. In line with this, the expression of chemokine receptors, adhesion molecules, and integrins was unaffected; only L-selectin showed some minor reproducible up-regulation (Figure 6B). Because adhesion is regulated by an integrin switch from low- to high-affinity conformation, we also analyzed the integrin function on lymphocytes in an in vitro assay. Indeed, B cells of poly(I:C)–treated mice showed a slightly increased adhesion to ICAM-1, but not to VCAM-1 (Figure 6C). Therefore, enhanced lymphocyte sticking to ICAM-1–expressing endothelia probably further contributed to lymphopenia.
Lymphopenia is mainly independent of GPCRs. Splenocytes were cultured for 3 hours in medium with or without 20 ng/mL PTX (Sigma), labeled with CFSE or TAMRA, and adoptively transferred into WT mice. (A) Mice were killed after 20 hours, and spleens and LNs were analyzed. Note that in contrast to untreated cells (green), PTX-treated cells (purple) did not enter splenic white pulp and LNs, indicating quantitative blockade of GPCR signaling. Images were visualized and acquired using a Zeiss LSM 510 Meta laser scan microscope equipped with a 10×/0.3 NA Plan Neofluor objective. Moviol (Calbiochem, Darmstadt, Germany) was used as an imaging medium. (B) After treatment with poly(I:C) or PBS, transferred control cells (left) and PTX-treated lymphocytes (right) were counted in blood at the indicated time points. Data are expressed as mean ± SD (n = 3) and are representative of 3 similar experiments. (C) BL/6 splenocytes cultured for 3 hours in medium with or without PTX or (D) BL/6 and CCR7–/– cells were differentially labeled (CFSEhi or CFSElo) and transferred to BL/6 mice. After treatment with R-848, blood samples were taken at the indicated time points. Labeled cells are depicted as percentages of pretreatment values. Data are expressed as mean ± SD (n = 3).
Lymphopenia is mainly independent of GPCRs. Splenocytes were cultured for 3 hours in medium with or without 20 ng/mL PTX (Sigma), labeled with CFSE or TAMRA, and adoptively transferred into WT mice. (A) Mice were killed after 20 hours, and spleens and LNs were analyzed. Note that in contrast to untreated cells (green), PTX-treated cells (purple) did not enter splenic white pulp and LNs, indicating quantitative blockade of GPCR signaling. Images were visualized and acquired using a Zeiss LSM 510 Meta laser scan microscope equipped with a 10×/0.3 NA Plan Neofluor objective. Moviol (Calbiochem, Darmstadt, Germany) was used as an imaging medium. (B) After treatment with poly(I:C) or PBS, transferred control cells (left) and PTX-treated lymphocytes (right) were counted in blood at the indicated time points. Data are expressed as mean ± SD (n = 3) and are representative of 3 similar experiments. (C) BL/6 splenocytes cultured for 3 hours in medium with or without PTX or (D) BL/6 and CCR7–/– cells were differentially labeled (CFSEhi or CFSElo) and transferred to BL/6 mice. After treatment with R-848, blood samples were taken at the indicated time points. Labeled cells are depicted as percentages of pretreatment values. Data are expressed as mean ± SD (n = 3).
Treatment with poly(I:C) does not affect chemotaxis but increases adhesion to ICAM-1 in vitro. (A) B cells from poly(I:C)– or PBS-treated mice were allowed to migrate toward a gradient of CXCL12 for 3 hours.55 Migrated cells were counted by FACS. Results are expressed as mean ± SD for triplicates per CXCL12 concentration and are representative of 5 experiments. (B) Splenocytes of poly(I:C) or PBS-treated mice were subjected to FACS analysis for the expression of CCR7, CXCR4, CXCR5, CD44, CD62L, CD11a/CD18, CD49d/CD29, and CD69. Histograms are gated on B cells (chemokine receptors) or total lymphocytes (adhesion molecules). Representative data of at least 3 similar experiments are shown. (C) CD43– sorted B cells from naive or poly(I:C)–treated mice were adhered for 30 minutes to plates coated with the indicated concentrations of ICAM-1-Fc or VCAM-1-Fc. Results show mean values of 4 independent experiments (n = 3 each). P values were determined by paired t tests. Horizontal bars represent overall means.
Treatment with poly(I:C) does not affect chemotaxis but increases adhesion to ICAM-1 in vitro. (A) B cells from poly(I:C)– or PBS-treated mice were allowed to migrate toward a gradient of CXCL12 for 3 hours.55 Migrated cells were counted by FACS. Results are expressed as mean ± SD for triplicates per CXCL12 concentration and are representative of 5 experiments. (B) Splenocytes of poly(I:C) or PBS-treated mice were subjected to FACS analysis for the expression of CCR7, CXCR4, CXCR5, CD44, CD62L, CD11a/CD18, CD49d/CD29, and CD69. Histograms are gated on B cells (chemokine receptors) or total lymphocytes (adhesion molecules). Representative data of at least 3 similar experiments are shown. (C) CD43– sorted B cells from naive or poly(I:C)–treated mice were adhered for 30 minutes to plates coated with the indicated concentrations of ICAM-1-Fc or VCAM-1-Fc. Results show mean values of 4 independent experiments (n = 3 each). P values were determined by paired t tests. Horizontal bars represent overall means.
Discussion
Massive lymphopenia in the blood of early virus-infected patients has long been analyzed for diagnostic purposes. A reduction of blood cell counts has also been observed in treatment with several novel immunomodulatory agents. However, the respective kinetics, mechanism, involvement, and distribution of lymphocyte subsets appeared to be heterogeneous. R-848–dependent lymphopenia was proposed to be related to TLR7 stimulation of endothelia. FTY720 causes lymphopenia through S1P1-mediated LN shutdown. Hence, lymphopenia seemed to be a stereotypic reactive pattern rather than a specific reaction. The data presented here show that IFN-α/β directly mediates lymphopenia after virus infection or treatment with 2 TLR ligands, poly(I:C) and R-848.
How does IFN-α/β cause massive lymphopenia? R-848– induced lymphopenia has recently been studied; however, the role of cytokines induced by R-848 and triggering of lymphocytes have not been extensively addressed.44 Our results highlight a previously unrecognized role of direct IFN-α/β stimulation of B cells and T cells as a mechanism to cause lymphopenia. Poly(I:C)–induced lymphopenia showed pronounced IFNAR dependence in B cells but less requirement of IFN-α/β stimulation in T cells. This tendency was also found after injection with R-848 by which T-cell lymphopenia is also triggered by additional factor(s), such as TNF-α. Previous studies addressed various direct effects of IFN-α/β on lymphocytes with a focus on B- and T-cell effector functions at later stages of the immune response.20,21,26,27 In contrast, here we report an early and systemic direct effect of IFN-α/β. Lymphopenia follows the kinetics of massive IFN-α/β responses in vivo by several hours and wanes with declining cytokine levels. These kinetics are in accordance with the dose dependence and the required time to up-regulate CD69 expression on B cells in vitro. Interestingly, lymphopenia may appear more delayed if the virus is administered peripherally or if the infectious dose is relatively low (E.K., unpublished data, May 2004). This delay is probably related to the time required to induce sufficiently high systemic IFN-α/β levels. Given that certain viruses have evolved means to interfere with the induction of IFN-α/β, sufficient levels may not be achieved. Taken together, these data indicate that prominent lymphopenia might not always be observed in infected patients.
What is the role of endothelia and stromal tissues in lymphopenia? Gunzer et al44 previously suggested that R-848 directly stimulated endothelia to acquire a generalized “sticky state” characterized by increased expression of adhesion molecules. Our results reveal that R-848–induced lymphopenia critically depends on type I IFN stimulation of lymphocytes and is independent of R-848 or IFN-α/β stimulation of endothelium and stroma. Still, the slight decrease in IFNAR–/– T-cell counts in poly(I:C)–treated IFNAR–/– > WT BM chimeras could reflect lymphopenia of remnant recipient-derived WT T cells, a marginal contribution of T-cell stimulation by other cytokines induced by “pathogen-associated molecular patterns” (PAMPs), or a minor role of endothelial stimulation by PAMPs, IFN-α/β, and other cytokines. However, on injection with R-848, numbers of IFNAR–/– B cells in IFNAR–/– > WT BM chimeras did not decrease (E.K., unpublished data, August 2005), demonstrating that direct stimulation of endothelia by PAMPs is not a limiting step in the induction of lymphopenia.
Which molecular mechanism leads to lymphopenia? Considering the outstanding relevance of chemokine receptors and S1P1 for lymphocyte homing and recirculation, it was striking that PTX-inhibited adoptively transferred B and T cells, which were unable to enter splenic white pulp and LNs, still underwent lymphopenia after treatment with poly(I:C) or R-848. The extent of lymphopenia, however, was slightly reduced in PTX-treated cells compared with control lymphocytes. Hence, lymphopenia was mainly independent of GPCRs, whereas signaling through chemokine receptors and S1P1 played a minor role, if any. Given that CCR7 is essential for the organization of SLOs by controlling homeostatic B- and T-cell homing, the pronounced lymphopenia of CCR7–/– lymphocytes further corroborated the conclusion that chemokines did not play a major role in lymphopenia. Moreover, this notion was supported by our chemotaxis studies and receptor expression analyses of the homeostatic chemokine receptors CCR7, CXCR4, and CXCR5, which did not reveal differences for lymphocytes of PBS- and poly(I:C)–treated mice.
Still, an IFN-α/β–induced modulation of the S1P-S1P1 system could contribute to lymphopenia. Because the internalization of S1P156 or the disruption of S1P gradients by S1P lyase inhibition57 can lead to lymphopenia, IFN-α/β stimulation could target S1P1 or S1P metabolism, either on the level of the catabolizing enzymes S1P lyase and phosphohydrolases or the production of S1P by sphingosine kinases. So far, some cytokines and growth factors were found to activate sphingosine kinases.58 S1P-metabolizing enzymes are ubiquitously expressed, so if IFN-α/β influenced S1P metabolism, the effects would probably be cell-type specific, because only direct IFN-α/β action on lymphocytes is required to induce lymphopenia. For example, IFN-α/β could trigger B and T cells to produce factors that in turn act on S1P metabolism, either on lymphocytes or on a systemic level. Down-regulation of S1P1 and the inability to respond to S1P might even be a connecting cue between the various agents observed to cause lymphopenia. However, a direct effect of IFN-α/β stimulation on S1P1 mRNA expression levels was not observed in IFN-β–stimulated B cells (E.K., unpublished data, November 2004).
Selectin function is not inhibited by PTX36 ; therefore, our data strongly suggested increased rolling as one mechanism of lymphopenia, consistent with the observations of Gunzer et al.44 Enhanced rolling could correlate with the observed minor increase of L-selectin, with higher lectin affinity or even with a novel selectin receptor activity. Overexpression of CD69, a member of the C-type lectin–like signaling receptors, was shown to reduce the recovery of adoptively transferred thymocytes from blood,59 implicating a role in lymphopenia. We observed broad up-regulation of CD69 on B cells under all conditions tested and on T cells on virus infection and poly(I:C) challenge. However, on in vitro and in vivo treatment with IFN-α/β, no CD69 induction was found on T cells (U.K. and E.K., unpublished data, August 2000 and June 2005), although T-cell lymphopenia was induced. Hence, on B cells, IFN-α/β– induced CD69 up-regulation was likely to mediate B-cell lymphopenia. On T cells, where CD69 expression was related to indirect IFN-α/β stimulation, CD69 probably contributed to T-cell lymphopenia but was not critically involved. Ly6C, however, which was directly up-regulated on T cells by IFN-α/β stimulation, could further enhance T-cell sticking because Ly6C was suggested to intensify LFA-1–mediated adhesion.60 The slightly improved B-cell adhesion to ICAM-1 possibly reflected enhanced affinity of LFA-1 and could have contributed to lymphopenia. This effect might be related to S1P1, which can amplify integrin activation.35 Stronger interactions between LFA-1 and ICAM-1 induced by IFN-α/β could further improve immunologic synapse formation and thus play a role in enhanced priming of B and T cells. Taken together, lymphopenia seems to result from the concerted action of several different molecular mechanisms on B and T cells, which together lead to increased rolling and adhesion, with a possible contribution of S1P1 but not of chemokine receptors.
Where do lymphopenic B and T cells home? Treatment with R-848 has recently been shown to direct lymphocytes to SLOs, liver, and lung,44 whereas FTY720 is classically known to sequester lymphocytes in LNs, although the role of spleen and the homing of B cells seem to vary among studies.7,45,46 Nevertheless, in adoptively transferred or conditionally Ifnar-targeted mice treated with poly(I:C), we found some preferred B-cell, but not T-cell, accumulation in spleen. These findings are corroborated by the prominent lymphopenia in the absence of GPCR signaling, indicating that lymphoid organs indeed are not essential homing targets in lymphopenia. Similarly, in an older study, Gresser et al1 analyzed the homing of chromium-labeled lymphocytes on IFN-induced lymphopenia but did not find any increase of radioactivity in SLOs. Since Sugito et al61 demonstrated that FTY720 was able to induce lymphopenia in splenectomized aly/aly mice devoid of any SLOs, the classical model of LN logjam does not suffice to provide an explanation for experimental observations. Hence, the current concept of initial S1P1-mediated lymphocyte sequestration with lymphopenia merely a consequence of inhibited lymphocyte supply to blood should be revised. Furthermore, considering evidence for enhanced rolling and unaffected ex vivo chemotaxis, lymphocytes probably do not migrate to tissues but remain attached to the endothelium. In conclusion, the vasculature probably represents the main homing target during lymphopenia.
Previous reports2,62 have indicated that the distribution of leukocytes within spleen was altered on poly(I:C) challenge without directly addressing B- and T-cell movements. Here, we show a massive B- and T-cell depletion from splenic red pulp during poly(I:C)–induced lymphopenia and a concomitant purgation of marginal zone B cells. During lymphopenia, it is probable that the recruitment of lymphocytes into B- and T-cell zones is preparation for lymphocyte priming.
In a model of contact hypersensitivity, lymphopenia was shown to induce transient immune incompetence,44 whereas clinically relevant immunosuppression with opportunistic infections may occur on prolonged exposure to IFN-α/β, as reported in IFN-α/β– treated patients. In conclusion, our data show that IFN-α/β causes lymphopenia through direct stimulation of B and T cells. Our results extend the concept of lymphopenia and provide new insight into how IFN-α/β essentially links the innate and adaptive immune system in naturally occurring infections and clinical treatments.
Prepublished online as Blood First Edition Paper, July 25, 2006; DOI 10.1182/blood-2006-06-027599.
Supported by the Deutsche Forschungsgemeinschaft (SFB432, B15), the European Community (contract QLK2-CT-2001-02103), the Volkswagen Foundation, the Swiss National Science Foundation, and the Kanton Zürich.
The authors declare no competing financial interests.
All authors participated in designing and performing the research. E.K., T.J., and U.K. analyzed the data and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Note added in proof. During revision of this manuscript, Shiow et al63 published findings indicating that in mice given injections of poy(I:C), IFN-α/β exerted direct effects on lymphocytes to induce lymphopenia and that lymphopenia was critically driven by a selective protein/protein interaction between CD69 and S1P1 that negatively regulated GPCR surface expression.
We thank Klaus Rajewsky for the gift of CD19-Cre mice, Christopher B. Wilson for CD4-Cre mice, Michel Aguet for IFNAR–/– mice, and Manolis Pasparakis for TNF-α–/– mice. We thank Jakki Kelly-Barrett for blastocyst injection, Marion Huth, Dorothea Kreuz, and Sabine Falk for expert technical assistance, Susanne Roederstein for generation of BM chimeric mice, David Tough for providing IFN-α, Heinfried H. Radeke for providing R-848, and Sanjiv Luther for providing the CCL19-Fc fusion protein. E.K. is a PhD candidate at Justus-Liebig-University in Giessen, Germany, and this work is submitted in partial fulfillment of the requirement for the PhD.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal