We retrospectively surveyed the data of 233 patients who underwent myeloablative allogeneic hematopoietic stem cell transplantation (allo-HSCT) for non-Hodgkin lymphoma (NHL). Donors were HLA-matched relatives in 154 patients (66%) or unrelated volunteers in 60 (26%). Ninety patients (39%) were in complete remission. One hundred ninety-three (83%) received a total body irradiation (TBI)-based regimen, and 40 (17%) received a non-TBI-based regimen. Acute graft-versus-host disease (GVHD) occurred in 155 (67%) of the 233 evaluable patients; grade II to IV in 90 (39%), and grade III to IV in 37 (16%). Treatment-related mortality (TRM) was observed in 98 patients (42%), and 68% of them were related to GVHD. In a multivariate analysis, chemoresistance, prior autograft, and chronic GVHD were identified as adverse prognostic factors for TRM. Relapse or progression of lymphoma was observed in 21%. The 2-year overall survival rates of the patients with indolent (n = 38), aggressive (n = 111), and lymphoblastic lymphoma (n = 84) were 57%, 42%, and 41%, respectively. In a multivariate analysis, chemoresistance, prior autograft, and prior radiotherapy were identified as adverse prognostic factors for overall survival. Although myeloablative allo-HSCT represents an effective therapeutic option for patients with NHL, more work is still needed to decrease TRM and relapse. (Blood. 2006;108:382-389)
Introduction
Hematopoietic stem cell transplantation (HSCT) for patients with non-Hodgkin lymphoma (NHL) has been mainly focused on an autograft strategy. High-dose therapy with autologous HSCT (auto-HSCT) can increase remission rates and possibly prolong disease-free survival and overall survival (OS) in patients with chemotherapy-sensitive NHL at relapse.1 This is also effective as first-line therapy for those with advanced aggressive lymphoma.2 Nevertheless, relapse is a frequent cause of treatment failure after auto-HSCT.1,3
Allogeneic HSCT (allo-HSCT) has several advantages over auto-HSCT, because the former can avoid the reinfusion of malignant cells and can also be associated with a graft-versus-lymphoma (GVL) effect, which might reduce the risk of relapse. Most physicians believe that a small fraction of patients with end-stage aggressive lymphoma can still achieve prolonged lymphoma-free survival with the application of allo-HSCT. However, the high incidence of treatment-related mortality (TRM) (up to 55%) associated with allogeneic HSCT with a myeloablative regimen has prevented the wider application of this strategy.4-8 Several reports on allo-HSCT for refractory or advanced lymphoma, as well as studies comparing autoversus allo-HSCT for NHL, have been published over the past decade.8-10 However, most of these studies were small and nonrandomized, and incorporated patients who had heterogeneous backgrounds. Thus, the role of allo-HSCT in the treatment of NHL remains controversial. Moreover, the outcome of allo-HSCT in each histologic subtype has not been fully determined. Previous studies have suggested that allo-HSCT improves the prognosis of patients with advanced follicular lymphoma (FL),7,10,11 whereas few reports have been published on its benefit in aggressive lymphoma.12,13 In particular, there has been very little information available on subtypes, including mantle-cell lymphoma11,14 ; peripheral T-cell lymphoma, unspecified (PTCL)15 ; natural killer (NK) cell lymphoma16 ; and anaplastic large cell lymphoma.
The application of reduced-intensity stem cell transplantation (RIST) or “nonmyeloablative” HSCT has been reported to decrease TRM.17-19 Additionally, the recent development of supportive treatments may have decreased the risk of TRM and facilitated the application of allo-HSCT to NHL.20 Therefore, we conducted a retrospective nationwide survey on Japanese patients with NHL who had undergone conventional allo-HSCT to establish a benchmark of myeloablative allo-HSCT in the treatment of NHL.
Patients, materials, and methods
Data sources
This survey collected the data of 233 consecutive patients who received myeloablative allo-HSCT for NHL between 1990 and 2001 in 56 participating hospitals. Data were derived from questionnaires distributed to each hospital. Additional questionnaires were sent to confirm the follow-up data, including the occurrence of graft-versus-host disease (GVHD). The indications for allo-HSCT were left to the discretion of each institution. The patients included in this study received a conditioning regimen with an intensity that was equivalent to that of total body irradiation (TBI) plus cyclophosphamide or busulfan plus cyclophosphamide. Patients who had previously received monoclonal antibody therapy or T-cell-depleted transplantation, those younger than 14 years, and those who received RIST were not included. Additionally, those with adult T-cell leukemia/lymphoma were excluded because their clinical course differed from that of other types of lymphoma. The minimum data required for the inclusion of a patient in this study were age, sex, histologic diagnosis, prior treatment details, status at transplantation, donor information, conditioning regimen, date of transplantation, therapy-related complications, date of last follow-up, disease status at follow-up, date of disease progression/death, and cause of death. Approval was obtained from the institutional review board. Informed consent was provided according to the Declaration of Helsinki.
Definitions
The initial institutional histologic diagnosis was further reviewed by a pathologist (K. Takeuchi) using the WHO classification.21 Briefly, NHL was divided into 3 clinical subtypes: indolent, aggressive, and lymphoblastic lymphoma. Indolent lymphoma included all grades of FL and extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). Aggressive lymphoma included all lymphomas except for indolent and lymphoblastic lymphoma. Transformed indolent lymphoma and Burkitt lymphoma were classified as aggressive lymphoma. Furthermore, because most of the patients were evaluated before publication of the WHO classification, this analysis only included those who had tumors that formed lesions, such as T-cell lymphoblastic lymphoma (T-LBL), and all other patients who had features of leukemia were excluded. Those with chemosensitive disease included all patients who had shown a response to the last chemotherapy prior to transplantation (partial remission [PR], complete remission [CR] unconfirmed, and CR), whereas chemoresistant disease included those with primary refractory disease or refractory relapse prior to transplantation. Acute and chronic GVHD was graded according to the consensus criteria.22,23 Patients who survived 100 days were evaluable for the assessment of chronic GVHD. OS was measured as the time from the day of transplantation until death from any cause, and progression-free survival (PFS) was the time from the day of transplantation until disease progression (PD)/relapse or death from any cause. Patients who died from transplantation-related causes were classified as TRM regardless of their disease status.
Statistical analysis
OS and PFS were calculated using the Kaplan-Meier method.24 Surviving patients were censored on the last day of follow-up, in July 2002. The associations among patient-, disease-, and transplantation-related factors and OS were assessed by using univariate and multivariate Cox proportional hazards models. The associations between these factors and TRM were assessed by using univariate and multivariate logistic models. The variables analyzed included age, clinical subtype, histologic diagnosis, chemosensitivity, history of autograft or radiotherapy, years of transplantation, donor, source of stem cells, TBI-containing regimen, GVHD prophylaxis, and acute and chronic GVHD. Acute GVHD was treated as a time-dependent covariate in the Cox model. Stepwise variable selection at a significance level of .05 was used to identify covariates associated with outcomes. TRM and disease progression/relapse were calculated by using cumulative incidence. The statistical analysis was performed with the SAS 8.2 program package (SAS Institute, Cary, NC).
Results
Patients' characteristics
The patients' characteristics are listed in Table 1. All patients were younger than 60 years at the time of transplantation, with a median age of 31 years. Thirty-eight patients (16%) had indolent lymphoma, 111 (48%) had aggressive lymphoma (diffuse large B-cell, n = 44; PTCL, n = 22; extranodal NK/T-cell, n = 19; anaplastic large cell, n = 7; mantle cell, n = 5; Burkitt, n = 4; angioimmunoblastic T cell, n = 2; blastic NK cell, n = 2; hepatosplenic T-cell, n = 2; subcutaneous panniculitis like T cell, n = 2; mycosis fungoides with visceral dissemination, n = 2), and 84 (36%) had lymphoblastic lymphoma. Ninety patients (39%) were in CR, 38 (16%) were in PR, 42 (18%) were in primary refractory, and 63 (27%) had refractory relapse at the time of allo-HSCT. Ninety patients (39%) had received 4 or more chemotherapy regimens before allo-HSCT. Forty patients (17%) had received prior autograft, and 81 (35%) had received prior radiotherapy. One hundred fifty-four patients (66%) received a transplant from a human leukocyte antigen (HLA)-matched related donor, 19 (8%) from a 1-antigen-mismatched related donor, 43 (19%) from a matched unrelated donor, and 17 (7%) from a 1-antigen-mismatched unrelated donor. One hundred fifty-nine (68%) patients received bone marrow (60 from an unrelated donor) and 70 (30%) received granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood. One hundred ninety-three patients (83%) received TBI-based myeloablative regimens, including TBI 12 Gy plus cyclophosphamide (n = 60); a combination of TBI, cyclophosphamide, and etoposide (n = 47); or TBI, cyclophosphamide, and cytarabine (n = 40). Forty patients (17%) received a non-TBI-based myeloablative regimen, including a combination of busulfan and cyclophosphamide with or without other agents (n = 27); melphalan, thiotepa, and busulfan (n = 3); cytarabine, ranimustine, carboplatin, cyclophosphamide, and total lymphoid irradiation (n = 2); or cytarabine, etoposide, and busulfan (n = 2). The remaining 6 patients received individualized regimens. GVHD prophylaxis included a combination of cyclosporin and methotrexate in 204 (88%) or tacrolimus and methotrexate in 22 (9%). Two hundred twenty-six patients (97%) were treated with G-CSF, starting at days +1to +6 after graft infusion until engraftment.
Patient-, disease-, and transplantation-related characteristics
Variable . | No. (%)* . |
|---|---|
| Patient characteristics | |
| Younger than 40 y | 158 (68) |
| 40 y or older | 75 (32) |
| Male sex | 150 (64) |
| Disease characteristics at diagnosis | |
| Histology | |
| Indolent | 38 (16) |
| Follicular | 37 (16) |
| MALT | 1 (0) |
| Aggressive | 111 (48) |
| Diffuse large B cell | 44 (19) |
| Peripheral T cell, unspecified | 22 (9) |
| Extranodal NK/T cell, nasal type | 19 (8) |
| Anaplastic large cell | 7 (3) |
| Mantle cell | 5 (2) |
| Others | 14 (6) |
| Lymphoblastic | 84 (36) |
| Precursor B cell | 7 (3) |
| Precursor T cell | 77 (33) |
| Stage I | 9 (4) |
| Stage II | 25 (11) |
| Stage III | 30 (13) |
| Stage IV | 150 (64) |
| No data | 19 (8) |
| Disease characteristics at transplantation | |
| Response to chemotherapy† | |
| Sensitive | 128 (55) |
| Complete remission‡ | 90 (39) |
| Partial remission | 38 (16) |
| Resistant | 104 (45) |
| Primary refractory disease | 41 (18) |
| Refractory relapse | 63 (27) |
| No. of prior chemotherapy regiments† | 3 (0-11) |
| Fewer than 4 regimens | 143 (61) |
| At least 4 regimens | 90 (39) |
| Prior autograft | 40 (17) |
| Prior radiotherapy | 81 (35) |
| Transplantation characteristics | |
| Year of transplantation | |
| 1990-1995 | 46 (20) |
| 1996-2001 | 187 (80) |
| No. of patients receiving a transplant per hospital | |
| Fewer than 9 patients | 146 (63) |
| At least 9 patients | 87 (37) |
| Donor | |
| HLA-matched related | 154 (66) |
| HLA-1 antigen-mismatched related | 19 (8) |
| HLA-matched unrelated | 43 (19) |
| HLA-1 antigen-mismatched unrelated | 17 (7) |
| Donor-recipient sex match | |
| Male-male | 80 (34) |
| Male-female | 66 (28) |
| Female-male | 33 (14) |
| Female-female | 46 (20) |
| Donor-recipient CMV status§ | |
| +/+ | 131 (57) |
| −/+ | 14 (6) |
| +/− | 14 (6) |
| −/− | 11 (5) |
| Source of stem cells | |
| Bone marrow | 159 (68) |
| Peripheral blood cells | 70 (30) |
| Bone marrow + peripheral blood cells | 2 (1) |
| Cord blood | 2 (1) |
| Conditioning regimen | |
| TBI-containing | 193 (83) |
| Non-TBI | 40 (17) |
| GVHD prophylaxis | |
| Cyclosporin + methotrexate | 204 (88) |
| Tacrolimus + methotrexate | 22 (9) |
| Others | 7 (3) |
Variable . | No. (%)* . |
|---|---|
| Patient characteristics | |
| Younger than 40 y | 158 (68) |
| 40 y or older | 75 (32) |
| Male sex | 150 (64) |
| Disease characteristics at diagnosis | |
| Histology | |
| Indolent | 38 (16) |
| Follicular | 37 (16) |
| MALT | 1 (0) |
| Aggressive | 111 (48) |
| Diffuse large B cell | 44 (19) |
| Peripheral T cell, unspecified | 22 (9) |
| Extranodal NK/T cell, nasal type | 19 (8) |
| Anaplastic large cell | 7 (3) |
| Mantle cell | 5 (2) |
| Others | 14 (6) |
| Lymphoblastic | 84 (36) |
| Precursor B cell | 7 (3) |
| Precursor T cell | 77 (33) |
| Stage I | 9 (4) |
| Stage II | 25 (11) |
| Stage III | 30 (13) |
| Stage IV | 150 (64) |
| No data | 19 (8) |
| Disease characteristics at transplantation | |
| Response to chemotherapy† | |
| Sensitive | 128 (55) |
| Complete remission‡ | 90 (39) |
| Partial remission | 38 (16) |
| Resistant | 104 (45) |
| Primary refractory disease | 41 (18) |
| Refractory relapse | 63 (27) |
| No. of prior chemotherapy regiments† | 3 (0-11) |
| Fewer than 4 regimens | 143 (61) |
| At least 4 regimens | 90 (39) |
| Prior autograft | 40 (17) |
| Prior radiotherapy | 81 (35) |
| Transplantation characteristics | |
| Year of transplantation | |
| 1990-1995 | 46 (20) |
| 1996-2001 | 187 (80) |
| No. of patients receiving a transplant per hospital | |
| Fewer than 9 patients | 146 (63) |
| At least 9 patients | 87 (37) |
| Donor | |
| HLA-matched related | 154 (66) |
| HLA-1 antigen-mismatched related | 19 (8) |
| HLA-matched unrelated | 43 (19) |
| HLA-1 antigen-mismatched unrelated | 17 (7) |
| Donor-recipient sex match | |
| Male-male | 80 (34) |
| Male-female | 66 (28) |
| Female-male | 33 (14) |
| Female-female | 46 (20) |
| Donor-recipient CMV status§ | |
| +/+ | 131 (57) |
| −/+ | 14 (6) |
| +/− | 14 (6) |
| −/− | 11 (5) |
| Source of stem cells | |
| Bone marrow | 159 (68) |
| Peripheral blood cells | 70 (30) |
| Bone marrow + peripheral blood cells | 2 (1) |
| Cord blood | 2 (1) |
| Conditioning regimen | |
| TBI-containing | 193 (83) |
| Non-TBI | 40 (17) |
| GVHD prophylaxis | |
| Cyclosporin + methotrexate | 204 (88) |
| Tacrolimus + methotrexate | 22 (9) |
| Others | 7 (3) |
The study included 233 patients. The median age was 31 years (range, 15-59 years). Age was a continuous variable.
MALT indicates extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue; NK, natural killer; HLA, human leukocyte antigen; CMV, cytomegalovirus; TBI, total body irradiation; GVHD, graft-versus-host disease.
Categoric variable.
One patient with mediastinal B-LBL did not receive prior chemotherapy for an unknown reason but did receive prior radiotherapy.
Includes 2 patients in complete remission, unconfirmed.
Sixty-three pairs were not evaluated for CMV status.
GVHD
Acute GVHD occurred in 155 (67%) of the 233 patients: grade I in 65 (28%), grade II to IV in 90 (39%), and grade III to IV in 37 (16%) patients. Of the 165 patients who survived the initial 100 days after allo-HSCT, chronic GVHD occurred in 79 (48%), with extensive type in 48 (29%). In allo-HSCT from related (n = 173) and unrelated (n = 60) donors, grade II to IV acute GVHD occurred, respectively, in 61 (35%) and 30 (50%), grade III to acute GVHD occurred in 25 (15%) and 12 (20%), chronic GVHD occurred in 54 (31%) and 25 (42%) patients, and chronic extensive GVHD occurred in 33 (19%) and 16 (27%). In allo-HSCT from HLA-matched (n = 197) and mismatched (n = 36) donors, grade II to IV acute GVHD occurred, respectively, in 76 (39%) and 15 (42%), grade III to IV acute GVHD occurred in 30 (15%) and 7 (19%), chronic GVHD occurred in 65 (33%) and 14 (39%), and chronic extensive GVHD occurred in 41 (21%) and 7 (19%). The distribution pattern of the incidences of acute and chronic GVHD by background factors was analyzed by using a chi-square test. Although none of the factors correlated with acute GVHD, the incidence of chronic GVHD was higher in patients who had GVHD prophylaxis with tacrolimus plus methotrexate than in those with cyclosporin plus methotrexate (P = .015, chi-square test; P = 0.023, Fisher exact test).
Disease response
Of the 143 patients who had measurable disease at allo-HSCT, 89 (62%) achieved CR, 7 (5%) PR, 6 (4%) stable disease (SD), and 12 (8%) PD, whereas 29 (20%) were not evaluable because of early death. Of the 90 patients who were in CR at transplantation, 80 (89%) maintained CR, 4 (4%) showed PD, and 6 (7%) were not evaluable because of early death. Thirty-five patients died before the first response evaluation, with a median survival of 29 days (range, 0-72 days) after allo-HSCT. In the 27 patients with indolent lymphoma who had measurable disease at allo-HSCT, 22 (81%) achieved CR or PR. In the 72 patients with aggressive lymphoma who had measurable disease at allo-HSCT, 49 (68%) achieved CR or PR. In the 41 patients with lymphoblastic lymphoma who had measurable disease at allo-HSCT, 26 (63%) achieved CR.
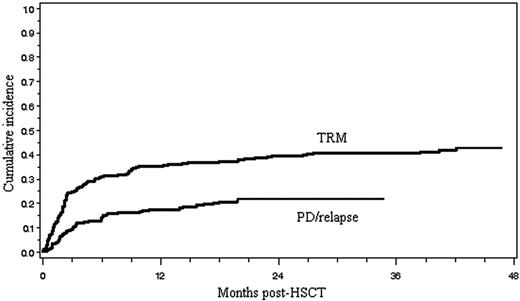
TRM, disease relapse, and progression
Ninety-eight patients (42%) died of TRM, and its cumulative incidence is shown in Figure 1. Of the 98 patients who died of therapy-related complications, 60 (61%) died within day 100 of transplantation and 38 (39%) died thereafter. The major causes of TRM included GVHD (n = 11), infection (n = 29), interstitial pneumonitis (n = 16), venoocclusive disease of the liver (n = 11), thrombotic microangiopathy (n = 8), heart failure (n = 7), hemorrhage (n = 4), renal failure (n = 3), and others (n = 9), as shown in Table 2. The causes of infection-related mortality (n = 29) were bacterial (n = 13), fungal (n = 11), or viral (n = 5). Seventeen (59%) of 29 patients died of infections within 100 days of allo-HSCT, 7 (24%) from 101 days to 1 year and 5 (17%) thereafter. Fourteen patients died of TRM before engraftment. Of the 98 patients who died of TRM, 67 (68%) had GVHD, and 11 of these died of GVHD (6 acute, 5 chronic) itself. The 14 factors shown in Table 3 were assessed with regard to their relation to TRM. A univariate analysis revealed that 6 factors, including older patient age, chemoresistant disease, prior autograft, prior radiotherapy, aggressive lymphoma other than PTCL, and chronic GVHD, were associated with a significantly increased risk of TRM. In a multivariate analysis using a logistic model, chemoresistant disease, prior autograft, and chronic GVHD remained significant.
Causes of treatment-related mortality
Causes of TRM . | Patients, no. (%) . | No. of patients with GVHD . | No. of patients without GVHD . | Early death, no.* . |
|---|---|---|---|---|
| GVHD | 11 (11) | |||
| Infection | 29 (30) | 15 | 8 | 6 |
| Interstitial pneumonitis | 16 (17) | 15 | 0 | 1 |
| Venoocclusive disease | 11 (11) | 5 | 4 | 2 |
| Thrombotic microangiopathy | 8 (8) | 7 | 1 | 0 |
| Heart failure | 7 (7) | 3 | 1 | 3 |
| Hemorrhage | 4 (4) | 3 | 1 | 0 |
| Renal failure | 3 (3) | 2 | 1 | 0 |
| Others† | 9 (9) | 6 | 1 | 2 |
| Total | 98 (100) | 56 | 17 | 14 |
Causes of TRM . | Patients, no. (%) . | No. of patients with GVHD . | No. of patients without GVHD . | Early death, no.* . |
|---|---|---|---|---|
| GVHD | 11 (11) | |||
| Infection | 29 (30) | 15 | 8 | 6 |
| Interstitial pneumonitis | 16 (17) | 15 | 0 | 1 |
| Venoocclusive disease | 11 (11) | 5 | 4 | 2 |
| Thrombotic microangiopathy | 8 (8) | 7 | 1 | 0 |
| Heart failure | 7 (7) | 3 | 1 | 3 |
| Hemorrhage | 4 (4) | 3 | 1 | 0 |
| Renal failure | 3 (3) | 2 | 1 | 0 |
| Others† | 9 (9) | 6 | 1 | 2 |
| Total | 98 (100) | 56 | 17 | 14 |
GVHD indicates graft-versus-host disease.
Early death was defined as treatment-related death before engraftment.
Others (n = 9) were acute respiratory distress syndrome (n = 2), hepatic failure (n = 2), leukoencephalopathy (n = 1), secondary solid cancer (n = 1), suicide (n = 1), and unknown cause (n = 2).
Univariate and multivariate analyses of treatment-related mortality
. | . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|---|
| Variable . | No. . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| Age at transplantation | .035 | — | |||||
| Younger than 40 y | 158 | 1.00 | — | ||||
| 40 y or older | 75 | 1.82 (1.04-3.17) | — | ||||
| Clinical subtype | .349 | — | |||||
| Indolent | 38 | 1.00 | — | ||||
| Lymphoblastic | 84 | 1.47 (0.66-3.32) | — | ||||
| Clinical subtype | .103 | — | |||||
| Indolent | 38 | 1.00 | — | ||||
| Aggressive | 111 | 1.91 (0.88-4.16) | — | ||||
| Aggressive lymphoma | .045 | — | |||||
| PTCL | 22 | 1.00 | — | ||||
| Non-PTCL | 89 | 2.85 (1.02-7.94) | — | ||||
| Response to chemotherapy | < .001 | < .001 | |||||
| Sensitive | 128 | 1.00 | 1.00 | ||||
| Resistant | 105 | 3.41 (1.97-5.88) | 2.95 (1.66-5.25) | ||||
| Prior autograft | < .001 | < .001 | |||||
| No | 193 | 1.00 | 1.00 | ||||
| Yes | 40 | 4.74 (2.23-10.07) | 4.09 (1.85-9.04) | ||||
| Prior radiotherapy | .010 | — | |||||
| No | 152 | 1.00 | — | ||||
| Yes | 81 | 2.05 (1.18-3.55) | — | ||||
| Years of transplantation | .225 | — | |||||
| 1996-2001 | 187 | 1.00 | — | ||||
| 1990-1995 | 46 | 1.49 (0.78-2.86) | — | ||||
| Donor | .295 | — | |||||
| HLA-matched | 197 | 1.00 | — | ||||
| HLA-mismatched | 36 | 1.46 (0.72-2.98) | — | ||||
| HLA-matched donor | .437 | — | |||||
| Related | 154 | 1.00 | — | ||||
| Unrelated | 43 | 1.24 (0.72-2.15) | — | ||||
| Source of stem cells* | .544 | — | |||||
| BM | 159 | 1.00 | — | ||||
| PBSCs | 70 | 1.09 (0.82-1.46) | — | ||||
| Conditioning regimen | .144 | — | |||||
| TBI-containing | 193 | 1.00 | — | ||||
| Others | 40 | 1.67 (0.84-3.30) | — | ||||
| GVHD prophylaxis† | .169 | — | |||||
| Cyclosporin + methotrexate | 204 | 1.00 | — | ||||
| Tacrolimus + methotrexate | 22 | 1.86 (0.77-4.51) | — | ||||
| Acute GVHD | .537 | — | |||||
| No | 78 | 1.00 | — | ||||
| Yes | 155 | 1.19 (0.69-2.06) | — | ||||
| Chronic GVHD | < .001 | .029 | |||||
| No | 79 | 1.00 | 1.00 | ||||
| Yes | 154 | 2.76 (1.53-4.98) | 2.02 (1.07-3.77) | ||||
. | . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|---|
| Variable . | No. . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| Age at transplantation | .035 | — | |||||
| Younger than 40 y | 158 | 1.00 | — | ||||
| 40 y or older | 75 | 1.82 (1.04-3.17) | — | ||||
| Clinical subtype | .349 | — | |||||
| Indolent | 38 | 1.00 | — | ||||
| Lymphoblastic | 84 | 1.47 (0.66-3.32) | — | ||||
| Clinical subtype | .103 | — | |||||
| Indolent | 38 | 1.00 | — | ||||
| Aggressive | 111 | 1.91 (0.88-4.16) | — | ||||
| Aggressive lymphoma | .045 | — | |||||
| PTCL | 22 | 1.00 | — | ||||
| Non-PTCL | 89 | 2.85 (1.02-7.94) | — | ||||
| Response to chemotherapy | < .001 | < .001 | |||||
| Sensitive | 128 | 1.00 | 1.00 | ||||
| Resistant | 105 | 3.41 (1.97-5.88) | 2.95 (1.66-5.25) | ||||
| Prior autograft | < .001 | < .001 | |||||
| No | 193 | 1.00 | 1.00 | ||||
| Yes | 40 | 4.74 (2.23-10.07) | 4.09 (1.85-9.04) | ||||
| Prior radiotherapy | .010 | — | |||||
| No | 152 | 1.00 | — | ||||
| Yes | 81 | 2.05 (1.18-3.55) | — | ||||
| Years of transplantation | .225 | — | |||||
| 1996-2001 | 187 | 1.00 | — | ||||
| 1990-1995 | 46 | 1.49 (0.78-2.86) | — | ||||
| Donor | .295 | — | |||||
| HLA-matched | 197 | 1.00 | — | ||||
| HLA-mismatched | 36 | 1.46 (0.72-2.98) | — | ||||
| HLA-matched donor | .437 | — | |||||
| Related | 154 | 1.00 | — | ||||
| Unrelated | 43 | 1.24 (0.72-2.15) | — | ||||
| Source of stem cells* | .544 | — | |||||
| BM | 159 | 1.00 | — | ||||
| PBSCs | 70 | 1.09 (0.82-1.46) | — | ||||
| Conditioning regimen | .144 | — | |||||
| TBI-containing | 193 | 1.00 | — | ||||
| Others | 40 | 1.67 (0.84-3.30) | — | ||||
| GVHD prophylaxis† | .169 | — | |||||
| Cyclosporin + methotrexate | 204 | 1.00 | — | ||||
| Tacrolimus + methotrexate | 22 | 1.86 (0.77-4.51) | — | ||||
| Acute GVHD | .537 | — | |||||
| No | 78 | 1.00 | — | ||||
| Yes | 155 | 1.19 (0.69-2.06) | — | ||||
| Chronic GVHD | < .001 | .029 | |||||
| No | 79 | 1.00 | 1.00 | ||||
| Yes | 154 | 2.76 (1.53-4.98) | 2.02 (1.07-3.77) | ||||
CI indicates confidence interval; PTCL, peripheral T-cell lymphoma; HLA, human leukocyte antigen; BM, bone marrow; GVHD, graft-versus-host disease; and —, not applicable.
Those who received cord blood (n = 2) or BM + PBSC (n = 2) were excluded because of the small number of patients.
Seven patients using other GVHD prophylaxis were excluded.
Cumulative incidences of treatment-related mortality (TRM) and disease relapse/progression (PD/relapse).
Cumulative incidences of treatment-related mortality (TRM) and disease relapse/progression (PD/relapse).
The cumulative incidence of relapse and PD is shown in Figure 1. Relapse or progression of lymphoma after allo-HSCT was observed in 49 patients (21%; 5 indolent, 19 aggressive, 25 LBL), and 32 (14%; 3 indolent, 13 aggressive, and 16 LBL) died of PD. Of the 105 patients with chemoresistant disease before allo-HSCT, 61 (58%) died of treatment-related complications, 19 (18%) died of PD, and 25 (24%) are alive with a median follow-up of 20.9 months (range, 1.8-136.0 months). Of the 128 patients with chemosensitive disease before allo-HSCT, 37 (29%) died of treatment-related complications, 12 (9%) died of PD, and 79 (62%) are alive with a median follow-up of 35.2 months (range, 4.4-140.2 months). Eight (16%) of the 49 patients who showed PD died of treatment-related complications such as infection (n = 4), interstitial pneumonitis (n = 3), and GVHD (n = 1). Only 6 of the 70 patients who had passed 2 years after transplantation developed relapse thereafter.
Donor lymphocyte infusion
Donor lymphocyte infusions (DLIs) were given after the withdrawal of immunosuppressive therapy to those who relapsed or showed evidence of disease progression or persistent disease without any sign of GVHD. A total of 7 patients, including 5 with T-LBL, received DLI after allo-HSCT from an HLA-matched related donor (n = 6) or a -matched unrelated donor (n = 1). Two patients who received DLI from an HLA-matched related donor developed grade II acute GVHD, which subsequently extended to extensive chronic GVHD; one of them with T-LBL died without a response, whereas the other with T-cell lymphoma is still alive without disease progression 3.8 years after allo-HSCT. Five patients did not develop GVHD following DLI; 3 patients subsequently died of disease progression, but 2 patients with T-LBL are still alive without disease progression at 361 and 783 days after allo-HSCT.
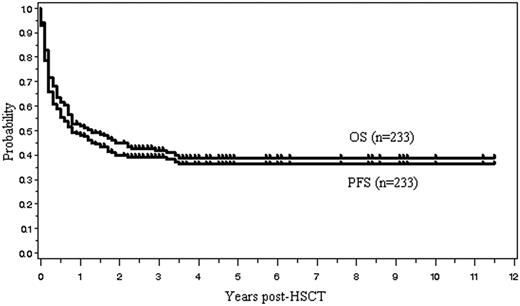
OS and PFS
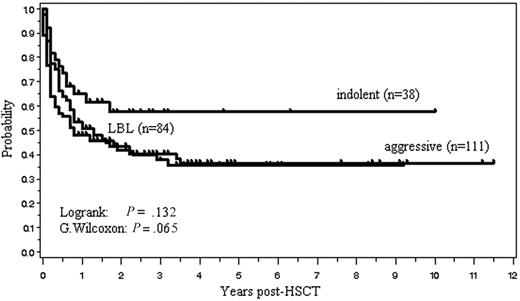
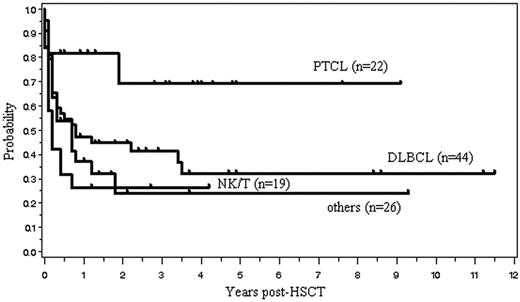
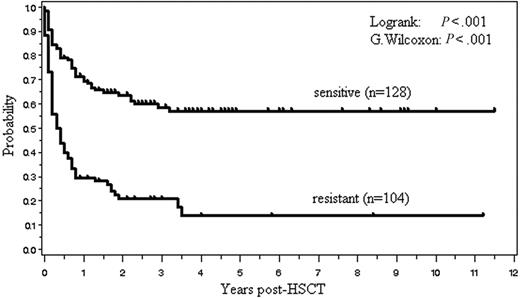
One hundred four (45%) of the 233 patients are currently alive with a median follow-up of 31 months (range, 1.8-138 months). The OS and PFS are, respectively, 45% and 40% at 2 years, and 39% and 36% at 5 years after allo-HSCT (Figure 2). Median OS and PFS are, respectively, 15.6 months (95% confidence interval, 9.6-27.6 months) and 9.6 months (6-18 months). The 2-year OS of those with indolent, aggressive, and lymphoblastic lymphoma was, respectively, 57%, 42%, and 41%. Patients with indolent lymphoma tended to have a better survival (P = .131, log rank test; P = .064, G. Wilcoxon test) (Figure 3). Kaplan-Meier estimates of OS of patients with 4 histologic subtypes of aggressive lymphoma, including diffuse large B-cell lymphoma (n = 44), PTCL (n = 22), extranodal NK/T-cell lymphoma, nasal type (n = 19), and others (n = 26), are shown in Figure 4.
The 14 clinical factors shown in Table 4 were assessed with regard to their relation to OS. A univariate analysis revealed that 5 factors, including chemoresistant disease, prior autograft, prior radiotherapy, aggressive lymphoma other than PTCL, and clinical subtype (aggressive versus indolent), were associated with a significantly worse OS. In a multivariate analysis using Cox proportional hazard models, chemoresistant disease, prior autograft, and prior radiotherapy were associated with a worse OS (Table 4). Acute GVHD, which was treated as a time-dependent variable, was not a significant factor for OS in both univariate and multivariate models. The relation between OS and response to chemotherapy is shown in Figure 5.
Univariate and multivariate analyses of overall survival
. | . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|---|
| Variable . | No. . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| Age at transplant | .134 | — | — | ||||
| younger than 40 y | 158 | 1.00 | — | — | |||
| 40 y or older | 75 | 1.32 (0.92-1.90) | — | — | |||
| Clinical subtype | .126 | — | — | ||||
| Indolent | 38 | 1.00 | — | — | |||
| Lymphoblastic | 84 | 1.57 (0.88-2.80) | — | — | |||
| Clinical subtype | .045 | — | — | ||||
| Indolent | 38 | 1.00 | — | — | |||
| Aggressive | 111 | 1.77 (1.01-3.11) | — | — | |||
| Aggressive lymphoma | .004 | — | — | ||||
| PTCL | 22 | 1.00 | — | — | |||
| Non-PTCL | 89 | 3.45 (1.47-7.69) | — | — | |||
| Response to chemotherapy | <.001 | — | — | ||||
| Sensitive | 128 | 1.00 | — | — | |||
| Resistant | 105 | 3.31 (2.30-4.76) | 3.12 (2.16-4.51) | <.001 | |||
| Prior autograft | < .001 | — | — | ||||
| No | 193 | 1.00 | — | — | |||
| Yes | 40 | 2.59 (1.73-3.87) | 2.18 (1.43-3.30) | < .001 | |||
| Prior radiotherapy | < .001 | — | — | ||||
| No | 152 | 1.00 | — | — | |||
| Yes | 81 | 1.99 (1.41-2.83) | 1.47 (1.02-2.11) | .037 | |||
| Years of transplantation | .932 | — | — | ||||
| 1996-2001 | 187 | 1.00 | — | — | |||
| 1990-1995 | 46 | 1.02 (0.67-1.54) | — | — | |||
| Donor | .076 | — | — | ||||
| HLA-matched | 197 | 1.00 | — | — | |||
| HLA-mismatched | 36 | 1.50 (0.96-2.33) | — | — | |||
| HLA-matched donor | .769 | — | — | ||||
| Related | 154 | 1.00 | — | — | |||
| Unrelated | 43 | 0.93 (0.58-1.50) | — | — | |||
| Source of stem cells* | .095 | — | — | ||||
| BM | 159 | 1.00 | — | — | |||
| PBSCs | 70 | 1.37 (0.95-2.00) | — | — | |||
| Conditioning regimen | .107 | — | — | ||||
| TBI-containing | 193 | 1.00 | — | — | |||
| Others | 40 | 1.42 (0.93-2.17) | — | — | |||
| GVHD prophylaxis† | .227 | — | — | ||||
| Cyclosporin + methotrexate | 204 | 1.00 | — | — | |||
| Tacrolimus + methotrexate | 22 | 1.40 (0.81-2.40) | — | — | |||
| Acute GVHD-time‡ | — | 1.25 (0.85-1.84) | .264 | 1.28 (0.87-1.90) | .213 | ||
. | . | Univariate analysis . | . | Multivariate analysis . | . | ||
|---|---|---|---|---|---|---|---|
| Variable . | No. . | Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | ||
| Age at transplant | .134 | — | — | ||||
| younger than 40 y | 158 | 1.00 | — | — | |||
| 40 y or older | 75 | 1.32 (0.92-1.90) | — | — | |||
| Clinical subtype | .126 | — | — | ||||
| Indolent | 38 | 1.00 | — | — | |||
| Lymphoblastic | 84 | 1.57 (0.88-2.80) | — | — | |||
| Clinical subtype | .045 | — | — | ||||
| Indolent | 38 | 1.00 | — | — | |||
| Aggressive | 111 | 1.77 (1.01-3.11) | — | — | |||
| Aggressive lymphoma | .004 | — | — | ||||
| PTCL | 22 | 1.00 | — | — | |||
| Non-PTCL | 89 | 3.45 (1.47-7.69) | — | — | |||
| Response to chemotherapy | <.001 | — | — | ||||
| Sensitive | 128 | 1.00 | — | — | |||
| Resistant | 105 | 3.31 (2.30-4.76) | 3.12 (2.16-4.51) | <.001 | |||
| Prior autograft | < .001 | — | — | ||||
| No | 193 | 1.00 | — | — | |||
| Yes | 40 | 2.59 (1.73-3.87) | 2.18 (1.43-3.30) | < .001 | |||
| Prior radiotherapy | < .001 | — | — | ||||
| No | 152 | 1.00 | — | — | |||
| Yes | 81 | 1.99 (1.41-2.83) | 1.47 (1.02-2.11) | .037 | |||
| Years of transplantation | .932 | — | — | ||||
| 1996-2001 | 187 | 1.00 | — | — | |||
| 1990-1995 | 46 | 1.02 (0.67-1.54) | — | — | |||
| Donor | .076 | — | — | ||||
| HLA-matched | 197 | 1.00 | — | — | |||
| HLA-mismatched | 36 | 1.50 (0.96-2.33) | — | — | |||
| HLA-matched donor | .769 | — | — | ||||
| Related | 154 | 1.00 | — | — | |||
| Unrelated | 43 | 0.93 (0.58-1.50) | — | — | |||
| Source of stem cells* | .095 | — | — | ||||
| BM | 159 | 1.00 | — | — | |||
| PBSCs | 70 | 1.37 (0.95-2.00) | — | — | |||
| Conditioning regimen | .107 | — | — | ||||
| TBI-containing | 193 | 1.00 | — | — | |||
| Others | 40 | 1.42 (0.93-2.17) | — | — | |||
| GVHD prophylaxis† | .227 | — | — | ||||
| Cyclosporin + methotrexate | 204 | 1.00 | — | — | |||
| Tacrolimus + methotrexate | 22 | 1.40 (0.81-2.40) | — | — | |||
| Acute GVHD-time‡ | — | 1.25 (0.85-1.84) | .264 | 1.28 (0.87-1.90) | .213 | ||
CI indicates confidence interval; PTCL, peripheral T-cell lymphoma; HLA, human leukocyte antigen; BM, bone marrow; GVHD, graft-versus-host disease; and —, not applicable.
Those who received cord blood (n = 2) or BM + PBSCs (n = 2) were excluded because of the small number of patients.
Seven patients using other GVHD prophylaxis were excluded.
Acute GVHD was treated as time-dependent variable.
Discussion
This report describes the general outcome of patients with NHL who underwent modern allo-HSCT with a myeloablative regimen in Japan, focusing on the background problems of myeloablative therapy and the identification of risk factors for TRM and OS. We showed that long-term, lymphoma-free survival could be achieved in approximately 40% of patients. Patients with FL had a better prognosis, consistent with previous reports.8,10 Even in patients with aggressive lymphoma or LBL, long-term survival of 35% was identified, consistent with previous reports.8,9 However, there were no significant differences between clinical subtypes (eg, aggressive versus indolent or PTCL versus non-PTCL) in a multivariate analysis. Because rituximab became commercially available after 2001 in Japan, patients with B-cell NHL who received anti-CD20 antibody therapy were not included in this study. The clinical effect of the introduction of rituximab on outcome after allogeneic transplantation should be carefully evaluated in a future study.
Our study confirmed a high TRM rate (42%) after conventional allo-HSCT with a myeloablative regimen, consistent with previous reports.4-8,25 One of the major causes of death was severe regimen-related toxicities, which included interstitial pneumonitis, venoocclusive disease, cardiac and renal toxicity, and organ hemorrhage. Although TBI-based regimens are frequently chosen because lymphoma cells are considered to be sensitive to irradiation, they have also been associated with long-term complications, including interstitial pneumonitis.26,27 Because most patients received TBI-based regimens as reported,4,5,7 we failed to detect any significant differences in TRM between those who received or did not receive TBI.
Overall survival (OS) and progression-free survival (PFS) for all 233 patients.
Overall survival (OS) and progression-free survival (PFS) for all 233 patients.
Another major cause of death in our study was GVHD and/or infection. Of the 98 patients who died of treatment-related complications in our study, 29 (30%) died of infection. At least half of the patients (15 of 29) who died of infectious complications also had GVHD. In a prospective trial of allo-HSCT for patients with NHL, infection accounted for 63% of all TRM,28 whereas other studies, including ours, have reported an incidence of 25% to 30%.4,6 In practical transplantation procedures, complications are usually multifactorial, and it is always very difficult to define the exact cause of death, which may account for the wide variations in the incidence of infections among those who died of TRM (18%-63%) in previous reports.4,5,28,29
Overall survival stratified according to the clinical subtype. Indolent lymphoma included all grades of FL and extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Aggressive lymphoma included all lymphomas except for indolent and lymphoblastic lymphoma (LBL).
Overall survival stratified according to the clinical subtype. Indolent lymphoma included all grades of FL and extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Aggressive lymphoma included all lymphomas except for indolent and lymphoblastic lymphoma (LBL).
In this study, the incidence of chronic GVHD was high (48%), and chronic GVHD was a risk factor for TRM. The reason for the higher incidence of chronic GVHD in our study compared with the IBMTR report9,30 was that the IBMTR study included data of patients who died within 100 days after allo-HSCT, whereas we excluded these patients. Unexpectedly, the incidence of chronic GVHD was higher in patients who had GVHD prophylaxis with tacrolimus plus methotrexate than in those with cyclosporin plus methotrexate. In Japan, there is a clear tendency to select tacrolimus rather than cyclosporine for GVHD prophylaxis in unrelated or HLA-mismatched transplantation.31,32 In addition, PBSCT is not yet permitted for unrelated transplantation. Altogether, the higher incidence of GVHD observed in the tacrolimus group may simply reflect that patients with a higher risk of GVHD were selected to receive tacrolimus.
Overall survival for patients with 4 histologic subtypes of aggressive lymphoma. PTCL indicates peripheral T-cell lymphoma, unspecified; DLBCL, diffuse large B-cell lymphoma; NK/T, extranodal NK/T-cell lymphoma, nasal type.
Overall survival for patients with 4 histologic subtypes of aggressive lymphoma. PTCL indicates peripheral T-cell lymphoma, unspecified; DLBCL, diffuse large B-cell lymphoma; NK/T, extranodal NK/T-cell lymphoma, nasal type.
We found that the incidence of disease relapse/progression of NHL was low (21%). High TRM in the early phase of the transplantation course may mask later disease relapse/progression, and this made it difficult to estimate the relapse rate in this study. OS and PFS were not affected by the severity of acute GVHD. Our limited analysis failed to confirm a GVL effect after myeloablative allo-HSCT. Although the risk of relapse for patients with acute or chronic GVHD was not significantly different from that of patients without acute or chronic GVHD in previous studies with malignant lymphoma,8,10,30 a study from the Japan Marrow Donor Program showed that the development of grade II to IV acute GVHD was associated with a lower incidence of disease progression after unrelated HSCT.31 It has been reported that a low level of acute GVHD was associated with improved OS, and all levels of acute GVHD were associated with a decrease in the relapse rate for intermediate-grade NHL.8 High levels of acute GVHD had a deleterious effect on OS but were associated with an improved relapse rate for LBL.8 Thus, our study confirmed that greater effort is required to reduce GVHD-related complications after myeloablative allo-HSCT.
We confirmed that chemoresistance before allo-HSCT and prior autograft were significant risk factors for both OS and TRM. RIST or a less organ-toxic myeloablative allo-HSCT using a combination of fludarabine plus intravenous busulfan may be applied more safely in this population to reduce TRM.19-21,33,34 However, further studies are needed to determine whether reduced-intensity conditioning could control activity of chemoresistant disease. In contrast to previous studies, we showed that prior radiotherapy was associated with a significantly worse OS, which may be related to the fact that 44 (54%) of the 81 patients who had a history of local radiotherapy had refractory disease at transplantation. Hence, it might be that prior radiotherapy was a marker of survival for more advanced and refractory disease.
In conclusion, we confirmed that myeloablative allo-HSCT is a curative therapeutic option in a subset of patients with NHL, but it carries a high risk of toxicities and TRM. Chemoresistant disease and a history of previous autograft are risk factors for both OS and TRM. Whether the introduction of a reduced-intensity transplantation procedure results in reduction of TRM should be evaluated, and more effective GVHD prophylaxis while maintaining a GVL effect should be developed.
Appendix
The following institutions contributed data to this study: Asahikawa Medical College Hospital, Hokkaido University Hospital, Sapporo Hokuyu Hospital, Akita University Hospital, Gunma Saiseikai Maebashi Hospital, Jichi Medical School Hospital, Suifu Hospital, Saitama Cancer Center Hospital, Jikei University Kashiwa Hospital, Chiba Aoba Municipal Hospital, Tokyo Metropolitan Komagome Hospital, National Cancer Center Hospital, Keio University Hospital, Toranomon Hospital, Yokohama City University Hospital, Kanagawa Cancer Center, Tokai University Hospital, Kurobe City Hospital, Kanazawa University Hospital, Ishikawa Prefectural Central Hospital, Nagoya City University Hospital, Japanese Red Cross Nagoya First Hospital, Nagoya Daini Red Cross Hospital, Meitetsu Hospital, JA Aichi Showa Hospital, Kyoto University Hospital, Kyoto Prefectural University of Medicine Hospital, Osaka University Hospital, Osaka City University Hospital, Kansai Medical University Hospital, Kinki University Hospital, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka City General Hospital, Rinku General Medical Center Izumisano Hospital, Hyogo College of Medicine Hospital, Hyogo Medical Center for Adults, Okayama University Hospital, Okayama Medical Center, Shimane Prefectural Central Hospital, Takamatsu Red Cross Hospital, Ehime Prefectural Central Hospital, University of Occupational and Environmental Health Hospital, Kitakyushu Municipal Medical Center, Kyushu Cancer Center, Kokura Memorial Hospital, Fukuoka University Hospital, Hamanomachi Hospital, Harasanshin Hospital, Saga Prefectural Hospital Koseikan, Sasebo Municipal General Hospital, Miyazaki Prefectural Miyazaki Hospital, Imamura Bun-in Hospital, and Ryukyu University Hospital.
Prepublished online as Blood First Edition Paper, March 7, 2006; DOI 10.1182/blood-2005-02-0596.
Supported in part by grants from the Ministry of Health, Labor and Welfare, Japan.
Presented in part as a poster presentation at the 44th annual meeting of the American Society of Hematology, Philadelphia, PA, December 2002.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the many physicians in the participating teams who contributed to this survey.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal