To study the prevalence of the Val617Phe JAK2 mutation in familial cases of myeloproliferative disorder (MPD) and its possible implication as a predisposing genetic factor, we analyzed 72 families including 174 patients (81 polycythemia vera [PV], 68 essential thrombocythemia [ET], 11 myelofibrosis with myeloid metaplasia [MMM], 12 chronic myeloid leukemia), 1 systemic mastocytosis, and 1 chronic myelomonocytic leukemia (CMML). The JAK2 mutation was found in three quarters of patients with PV and MMM and in half of patients with ET. Among 46 families with at least 2 cases of PV, ET, or MMM, the JAK2 mutation was absent in 6 families, heterogeneously distributed in 18, and present in all MPD patients in 22. Among these 22 families, the absence of the JAK2 mutation both in purified T and B cells in 13 unrelated patients and the observation of variable ratios of the JAK2 mutant allele in patient leucocytes indicated that the Val617Phe JAK2 mutation was acquired in familial MPDs. The JAK2 mutation was present in natural killer cells in two thirds of tested patients (27 of 40), suggesting its occurrence in a multipotent hematopoietic progenitor cell. The analysis of the hematologic profile showed that the homozygous JAK2 mutation confers a proliferative advantage and is associated with the progression of the hematologic disease. (Blood. 2006;108:346-352)
Introduction
Myeloproliferative disorders (MPDs) are hematologic malignant diseases characterized by a clonal proliferation of one or several lineages.1 Typically, they include 4 clinical entities: polycythemia vera (PV), essential thrombocythemia (ET), myelofibrosis with myeloid metaplasia (MMM), and chronic myeloid leukemia (CML).2 Recently, related disorders have been classified as MPDs: idiopathic hypereosinophilic syndrome, systemic mastocytosis, and chronic myelomonocytic leukemia (CMML).3 The molecular basis for CML involves a fusion protein encoded by a chimeric hybrid Bcr-Abl gene as the result of a recurrent chromosome translocation t(9,22). This chimeric protein leads to a constitutive activation of the Abl tyrosine kinase by oligomerization through Bcr. The molecular mechanism of CML was a paradigm for the molecular basis of the other MPDs. Indeed, it was shown that some CML-like syndromes and hypereosinophilic syndromes were related to fusion proteins involving the tyrosine kinase receptor FGFR1 and PDGFRA, and systemic mastocytosis is usually associated with a gain of function in the tyrosine kinase receptor Kit.4,5 However, until recently the molecular mechanisms of non-CML MPD remained poorly understood. Deficiency of Mpl, the thrombopoietin receptor, was found on the surface of platelets and megakaryocytes of patients with PV and MMM and also in ET, suggesting a traffic defect of this receptor.6,7 Up-regulation of PRV-1 mRNA, a member of the urokinase-type plasminogen activator receptor superfamily, was observed in granulocytes of patients with PV but in the absence of changes in the level of the protein.8 Overexpression of Bcl-xL, a member of the Bcl-2 family, was observed in the erythropoietin-independent progenitor cells of PV.9 Constitutive activation of the STAT3 protein has been found in PV.10
In the last few months, several groups have reported the identification of a recurrent activating tyrosine kinase mutation, Val617Phe, in the JH2 pseudokinase domain of Janus kinase-2 (JAK2) gene in patients with sporadic MPD. This mutation was found in most patients with PV and in about half of the patients with ET or MMM.11-15 Their studies have provided evidence that the JAK2 mutation is an acquired somatic event in sporadic MPD.
However, few studies have investigated the presence of the JAK2 mutation in nonhematopoietic cells or searched for evidence of MPD in first-degree relatives of affected patients.15,16 It is presently unclear whether JAK2 mutation may be present in the germ line. Although familial clustering of MPD had been reported through case reports, biologic material from families with MPD is rarely available. To date, a single pedigree with 2 cases of PV has been screened for the JAK2 mutation and reported in the literature.17
To solve this issue, we have analyzed a large group of 72 families presenting with at least 2 cases of MPD that has been collected through a large collaborative network of hematologists. Here we describe the genetic heterogeneity of these families according to the Val617Phe JAK2 mutation. We also discuss the relationships between the JAK2 genotype and clinical and hematologic features of affected patients between and within families.
Patients, materials, and methods
Patients
A national study was conducted between 1998 and 2003 to collect families with at least 2 affected patients with MPDs to search for predisposing factors to these disorders. The study was initiated under the auspices of the French Society of Hematology. Families were recruited from 50 French and 1 Belgian hematology departments. A total of 93 families were long listed from clinical databases and, among them, 72 families (66 Europeans, 5 North Africans, and 1 African) were collected. They included 174 patients with MPDs and 1430 relatives without preexisting diagnoses of MPDs, mainly first-degree relatives (parents, siblings, and offspring). Patients with MPDs consisted of 81 PV, 68 ET, 11 MMM, 12 CML, 1 systemic mastocytosis, and 1 CMML. Medical records were obtained from all affected patients and relatives. Clinical investigations and blood samples were collected from 138 patients with MPD and 392 healthy relatives. The ethics committee of Saint Antoine Hospital, Paris, France, approved the study, and all participants gave their written informed consent.
Clinical investigations
A total of 530 participants had a standardized examination at inclusion into the study. Most of them were seen and examined by one of the authors (I.C.). The following parameters were recorded: demographic data, clinical data, complete blood-cell counts, and environmental data, including smoking, occupational hazards, and accommodation. For patients with MPD, clinical and hematologic data at diagnosis of MPD and during the course of the disease were also collected. In vitro assay of peripheral-blood erythroid-progenitor responsiveness to erythropoietin and Bcr-Abl detection was performed for all participants at inclusion into the study. Treatment complications such as thrombosis, hemorrhage, myelofibrosis, and leukemic transformation; the occurrence of other malignant diseases; and cause of death were documented. A family tree with the relevant clinical history was drawn for each case.
Inclusion criteria
The diagnoses were confirmed by a systematic review of all clinical and hematologic data (A.N.). The diagnoses were based on the criteria established by the World Health Organization.18
Molecular genetics
Genomic DNA and RNA were extracted from peripheral-blood mononuclear cells using standard procedures. Specific cell populations were isolated from peripheral-blood and bone marrow samples after centrifugation on a Ficoll gradient (Eurobio, Paris, France). T cells, B cells, and natural killer (NK) cells were sorted on a Becton Dickinson FACS DiVA flow cytometer and cell sorter (Becton Dickinson, Le Pont de Claix, France) using FITC-conjugated anti-CD3 (Becton Dickinson), PE-conjugated anti-CD19 (Becton Dickinson), and APC-conjugated anti-CD56 (Beckman Coulter, Roissy, France) antibodies.
The Val617Phe JAK2 mutation was screened by direct sequencing. Amplification by polymerase chain reaction (PCR) was performed either on genomic DNA extracted from mononuclear blood cells or directly from cell populations or EEC after heating at 95°C for 10 minutes to release genomic DNA. Primer sequences and PCR amplification conditions are available upon request. Purified PCR products were sequenced using the BigDye Terminator chemistry (Applied Biosystems, Courtaboeuf, France) and run on an Applied Biosystems 3100 capillary sequencer. The quantitation of the wild-type (G, primary peak) and mutant (T, secondary peak) alleles of the Val617Phe mutation (1849G>T) was estimated taking into account the relative area of both alleles at the coordinate of the primary peak using PolyPhred software.19 The T allele was considered as the major allele if the “area of T allele/(area T allele + area G allele)” ratio was above 50%.
Statistical analysis
Data were collected on standardized forms and encoded for computerized analysis with the use of Access for Windows software (Microsoft, Redmond, WA). Values reported for quantitative variables were median and range. Comparisons of the 2 or 3 groups (according to JAK2 genotype) were done by the χ2 test and the Kruskal-Wallis test. The Fisher exact test was used if one category contained fewer than 5 patients.
Probabilities of transformation and survival were estimated separately for each diagnosis. Cumulative incidence curves were used in a competing risks setting, death being treated as a competing event to calculate probabilities of transformation.20 Probabilities of survival after diagnosis were calculated using the Kaplan-Meier estimate.21 Statistical analyses were performed with SPSS (Chicago, IL) and Splus (MathSoft, Seattle, WA) software packages.
Results
Description of the cohort
Seventy-two families, each having at least 2 documented cases of MPD, were collected. The cohort included 174 patients (79 males, 95 females) presenting with an MPD (81 PV, 68 ET, 12 CML, 11 MMM, 1 systemic mastocytosis, and 1 CMML). The age at diagnosis of the affected patients ranged from 13 to 86 years (median, 53 years). The median age at diagnosis was the lowest in the group of patients with ET (45 years); one fourth (28%) of the patients with ET were diagnosed under the age of 35.
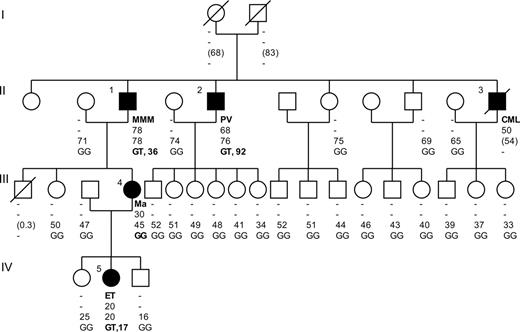
Of the 72 families, 54 had 2 affected cases. Twelve families had 3 affected cases, and 6 had more than 3 affected cases. One or several types of MPD were observed within families (Table 1). Most families (42 of 72) consisted of patients with a single type of MPD, principally PV (21 of 42) or ET (15 of 42). Two types of MPD were observed in 28 families, the main association being PV and ET (21 of 28). Two families had a more heterogeneous clinical spectrum. One had 3 affected patients, each with a distinct MPD. The other large family included 5 affected patients exhibiting the whole spectrum of MPDs (Figure 1).
Phenotypic spectrum of the 72 families with MPD
. | No. of families* . |
|---|---|
| Families with 1 type of MPD; n = 42 | |
| PV | 21 |
| ET | 15 |
| MMM | 3 |
| CML | 3 |
| Families with 2 types of MPD; n = 28 | |
| PV and ET | 21 |
| PV and MMM | 2 |
| PV and CML | 2 |
| ET and MMM | 1 |
| ET and CML | 2 |
. | No. of families* . |
|---|---|
| Families with 1 type of MPD; n = 42 | |
| PV | 21 |
| ET | 15 |
| MMM | 3 |
| CML | 3 |
| Families with 2 types of MPD; n = 28 | |
| PV and ET | 21 |
| PV and MMM | 2 |
| PV and CML | 2 |
| ET and MMM | 1 |
| ET and CML | 2 |
Two families had a complex MPD distribution consisting of 3 (PV, ET, and CML) and 5 different MPDs (PV, ET, MMM, CML, and systemic mastocytosis).
The distribution of MPD phenotypes within families was most compatible with autosomal dominant inheritance with incomplete penetrance in 39 of 72 (54%) cases; 35 family cases involved 2 generations, while 4 family cases had 3 affected generations. In the other 33 families, MPD cases were restricted to a single generation. In these latter cases, the mode of inheritance could not be decided given possible low penetrance and the lack of hematologic data for the older generations.
Genotyping of the Val617Phe JAK2 (1849G>T) mutation
A total of 284 subjects were screened for the JAK2 mutation by direct sequencing. The JAK2 mutation was found in three quarters of tested patients with PV (47 of 60) and MMM (6 of 8) and half of the patients with ET (31 of 61) (Table 2). The JAK2 mutation was not detected in the other types of MPD (8 patients with CML, 1 with systemic mastocytosis, and 1 with CMML). We analyzed the patients diagnosed with the JAK2 mutation according to the relative proportion of the mutant T allele. This parameter was estimated by taking into account the relative areas under the peaks that represent the wild-type (G allele) and mutant (T allele) sequences. Results showed that, regardless of the type of MPD, about half of patients diagnosed with the JAK2 mutation had mutant allele proportion lower than 50% (Table 2). The mutant allele was predominant in 20% and 25% of patients with PV or MMM, respectively, but only in 8% of patients with ET. The study, in 20 patients, of 4 microsatellite markers (D9S288, D9S1810, D9S286, and D9S164) located in chromosome 9 showed that patients detected with the mutant T allele proportion above 50% were homozygous for the 2 microsatellite markers (D9S1810 and D9S286) surrounding the JAK2 locus. Taking into account this observation, we found that 25% (12 of 47) and 16% (5 of 31) of patients with PV and ET were homozygous, respectively. In contrast, the mutant allele was represented in a minority of cells (proportion between 2% and 10%) in a significant number of ET patients (20%) but seldom in PV (5%) and in none of the MMM patients.
Genotyping of the Val617Phe (1849G>T) JAK2 mutation in blood mononuclear cells
. | . | JAK2 genotype . | . | . | ||
|---|---|---|---|---|---|---|
. | No. of patients . | Tnull; wild type . | Tminor; mutant allele up to 50% . | Tmajor; mutant allele more than 50% . | ||
| Polycythemia vera, no. (%) | 60 | 13 (21.7) | 35 (58.3) | 12 (20) | ||
| Essential thrombocythemia, no. (%) | 61 | 30 (49.2) | 26 (42.6) | 5 (8.2) | ||
| Myelofibrosis with myeloid metaplasia, no. (%) | 8 | 2 (25) | 4 (50) | 2 (25) | ||
| Chronic myeloid leukemia, no. (%) | 8 | 8 (100) | 0 (0) | 0 (0) | ||
| Systemic mastocytosis, no. (%) | 1 | 1 (100) | 0 (0) | 0 (0) | ||
| Chronic myelomonocytic leukemia, no. (%) | 1 | 1 (100) | 0 (0) | 0 (0) | ||
| Healthy relatives with EEC, no. (%) | 37 | 35 (94.6) | 2 (5.4) | 0 (0) | ||
| Healthy relatives, no. (%) | 201 | 200 (99.5) | 1 (0.5) | 0 (0) | ||
. | . | JAK2 genotype . | . | . | ||
|---|---|---|---|---|---|---|
. | No. of patients . | Tnull; wild type . | Tminor; mutant allele up to 50% . | Tmajor; mutant allele more than 50% . | ||
| Polycythemia vera, no. (%) | 60 | 13 (21.7) | 35 (58.3) | 12 (20) | ||
| Essential thrombocythemia, no. (%) | 61 | 30 (49.2) | 26 (42.6) | 5 (8.2) | ||
| Myelofibrosis with myeloid metaplasia, no. (%) | 8 | 2 (25) | 4 (50) | 2 (25) | ||
| Chronic myeloid leukemia, no. (%) | 8 | 8 (100) | 0 (0) | 0 (0) | ||
| Systemic mastocytosis, no. (%) | 1 | 1 (100) | 0 (0) | 0 (0) | ||
| Chronic myelomonocytic leukemia, no. (%) | 1 | 1 (100) | 0 (0) | 0 (0) | ||
| Healthy relatives with EEC, no. (%) | 37 | 35 (94.6) | 2 (5.4) | 0 (0) | ||
| Healthy relatives, no. (%) | 201 | 200 (99.5) | 1 (0.5) | 0 (0) | ||
Among the relatives, 384 of the 392 were tested for the presence of EEC; 37 (9.6%) were found to be carriers. A total of 201 subjects including the 37 healthy relatives who developed EEC in the absence of any MPD symptoms were screened for the JAK2 mutation. The mutant allele was absent from all relatives but 3. The mutant T allele was detected in a single EEC-negative subject with an allele proportion of 5%, but no follow-up of this subject could be obtained. The JAK2 mutation was detected in blood mononuclear cells in 2 EEC-positive healthy relatives with, for both, a mutant allele proportion of 15%. The JAK2 mutation was present in EEC from these 2 subjects. For one of them, the progression of hematocrit from 45.5 (45.5%) to 53.7 (53.7%) in the last 4 years after inclusion into the study is in favor of the development of PV. This result suggests that the Val617Phe JAK2 mutation is an early event preceding clinical and hematologic abnormalities.
Is the Val617Phe JAK2 mutation a predisposing factor to the occurrence of familial MPD?
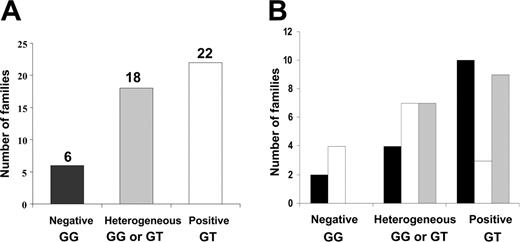
The genotyping of the JAK2 mutation in a subgroup of 46 families with at least 2 affected patients with PV, ET, or MMM revealed 3 groups of families (Figure 2A). The first subgroup (6 families; 13%) consisted of families in which the JAK2 mutation was detected in none of the MPD patients. This subgroup included 4 families with only ET patients and 2 families with only PV patients (Figure 2B). In the second subgroup, including 18 (39%) families, the distribution of the JAK2 mutation was heterogeneous. These families had at least 1 patient with the JAK2 mutation and 1 or 2 affected relatives without the JAK2 mutation. In these heterogeneous families, the absence of the JAK2 mutation was not strictly but preferentially associated with ET (13 of 18). Last, in 22 (48%) families, all affected MPD patients had the JAK2 mutation detectable in their blood mononuclear cells. This subgroup included families with a single MPD type (59%) as well as families with several types (41%). In these families, the JAK2 mutant allele quantitation demonstrated large variations, ranging from 2% to 75% in affected patients.
To assess whether the JAK2 mutation might be responsible for the familial aggregation of MPD observed in the 22 positive families, we genotyped the JAK2 mutation in purified blood-cell populations, CD3+ T cells, CD19+ B cells, and CD56+ NK cells. The study of 17 MPD patients (13 unrelated patients and 4 affected relatives) including 11 PV, 5 ET, and 1 MMM showed the absence of the JAK2 mutation in T, B, and NK cells in all patients but one. In the latter patient, belonging to a large complex MPD family (Figure 1, patient no. 2 with PV), the mutant T allele proportion was estimated at 10% in T cells, 26% in B cells, and at 27% in NK cells. Interestingly, the mutant allele proportion was estimated at 92% in blood mononuclear cells from this patient. Two other affected relatives (Figure 1, patients no. 1 with MMM and no. 5 with ET) were tested but did not reveal the JAK2 mutation in the purified T- and B-cell populations, demonstrating the absence of the JAK2 mutation in the germ line of these family members.
Genealogic tree of a large and complex family with MPD. Filled symbols indicate patients with MPD. Affected patients are numbered from 1 to 5. The type of MPD is reported on the first line under each symbol. The age at diagnosis and age at inclusion into the study (or at death in parentheses) are indicated on the second and third line, respectively. On the fourth line, the JAK2 genotype followed by the proportion, in percent, of mutant T allele is reported. - indicates not applicable; Ma, systemic mastocytosis; GG, wild-type JAK2 genotype; GT, heterozygous JAK2 genotype.
Genealogic tree of a large and complex family with MPD. Filled symbols indicate patients with MPD. Affected patients are numbered from 1 to 5. The type of MPD is reported on the first line under each symbol. The age at diagnosis and age at inclusion into the study (or at death in parentheses) are indicated on the second and third line, respectively. On the fourth line, the JAK2 genotype followed by the proportion, in percent, of mutant T allele is reported. - indicates not applicable; Ma, systemic mastocytosis; GG, wild-type JAK2 genotype; GT, heterozygous JAK2 genotype.
The JAK2 mutation was found in T and/or B cells in 13 unrelated MPD patients belonging either to the subgroup of 22 families (7 patients) or to the subgroup of 18 families (6 patients) heterogeneous for the JAK2 mutation.
A most striking observation was the detection of the JAK2 mutation in NK cells in two thirds of tested patients with MPD (27 of 40). This detection was significantly correlated with a higher proportion of the mutant T allele in blood mononuclear cells (P < .001).
Correlations between the JAK2 genotype and the hematologic and clinical profile of patients with PV or ET
To investigate whether the Val617Phe JAK2 mutation was associated with specific phenotypes, patients were classified into 3 subgroups according to the mutant allele proportion: the no mutation group (Tnull: mutant allele proportion, 0), the low mutant allele proportion group (Tminor: mutant allele proportion, up to 50%), and the high mutant allele proportion group (Tmajor: mutant allele proportion, more than 50%). The hematologic features and complications were characterized in each group.
In patients with PV (Table 3), those with the JAK2 mutation were significantly older at inclusion in the study than patients without the mutation (70 and 67 years in groups Tminor and Tmajor, respectively, versus 48 years in group Tnull; P = .01). However, the presence of the JAK2 mutation was not associated with a longer duration of the disease, the latter ranging from 69 to 88 months. The shorter duration was observed in the Tmajor group. Analysis of hematologic profiles showed that patients without the JAK2 mutation had significantly lower white blood cell and platelet counts in comparison with the 2 other groups. The identification of the JAK2 mutation was significantly associated with the detection of EEC. Only half of patients without the JAK2 mutation developed EEC compared with 80% and 84% of patients in the Tminor and Tmajor groups, respectively.
Hematologic profile and complications in patients with PV according to JAK2 genotype
. | JAK2 genotype . | . | . | P . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Tnull; wild type . | Timinor; T allele up to 50% . | Tmajor; T allele more than 50% . | Global . | Tnull vs Tminor . | Tminor vs Tmajor . | ||||
| No. of patients | 13 | 35 | 12 | — | — | — | ||||
| Age at diagnosis, y (range) | 42 (25-71) | 56 (39-86) | 63 (27-86) | .01 | <.001 | .66 | ||||
| Age at inclusion, y (range) | 48 (27-78) | 67 (41-90) | 70 (28-90) | .03 | .013 | .73 | ||||
| Duration of the disease, mo (range) | 79 (1-112) | 88 (0-366) | 69 (6-227) | .83 | .60 | .96 | ||||
| Hematologic profile | ||||||||||
| White blood cell count, × 109/L (range) | 8 (6-13) | 13 (5-26) | 12 (6-24) | .004 | .001 | .83 | ||||
| Platelet count, × 109/L (range) | 225 (142-840) | 537 (184-1208) | 549 (240-966) | .003 | .001 | .89 | ||||
| Splenomegaly, yes/no | 3/10 | 14/19 | 6/6 | .34 | .32 | .65 | ||||
| EEC, yes/no | 6/7 | 28/7 | 10/2 | .04 | .02 | 1 | ||||
| Complications | ||||||||||
| Follow-up, y (range) | 10 (0.2-19) | 8 (1-30) | 7 (1-23) | .70 | .39 | .97 | ||||
| Treatment, yes/no | 6/7 | 34/1 | 11/1 | <.001 | <.001 | .45 | ||||
| Thrombotic events, yes/no | 1/12 | 6/29 | 2/10 | .71 | .66 | 1 | ||||
| Probabilities of hematologic complications, % | ||||||||||
| 5 y after diagnosis | 0 | 4 ± 2 | 29 ± 12 | — | — | — | ||||
| 10 y after diagnosis | 0 | 10 ± 7 | 29 ± 12 | .12 | .79 | .14 | ||||
| Survival rates, % | ||||||||||
| 10 y after diagnosis | 100 | 87 ± 9 | 76 ± 15 | — | — | — | ||||
| 15 y after diagnosis | 83 ± 15 | 87 ± 9 | 38 ± 20 | .15 | .82 | .08 | ||||
. | JAK2 genotype . | . | . | P . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Tnull; wild type . | Timinor; T allele up to 50% . | Tmajor; T allele more than 50% . | Global . | Tnull vs Tminor . | Tminor vs Tmajor . | ||||
| No. of patients | 13 | 35 | 12 | — | — | — | ||||
| Age at diagnosis, y (range) | 42 (25-71) | 56 (39-86) | 63 (27-86) | .01 | <.001 | .66 | ||||
| Age at inclusion, y (range) | 48 (27-78) | 67 (41-90) | 70 (28-90) | .03 | .013 | .73 | ||||
| Duration of the disease, mo (range) | 79 (1-112) | 88 (0-366) | 69 (6-227) | .83 | .60 | .96 | ||||
| Hematologic profile | ||||||||||
| White blood cell count, × 109/L (range) | 8 (6-13) | 13 (5-26) | 12 (6-24) | .004 | .001 | .83 | ||||
| Platelet count, × 109/L (range) | 225 (142-840) | 537 (184-1208) | 549 (240-966) | .003 | .001 | .89 | ||||
| Splenomegaly, yes/no | 3/10 | 14/19 | 6/6 | .34 | .32 | .65 | ||||
| EEC, yes/no | 6/7 | 28/7 | 10/2 | .04 | .02 | 1 | ||||
| Complications | ||||||||||
| Follow-up, y (range) | 10 (0.2-19) | 8 (1-30) | 7 (1-23) | .70 | .39 | .97 | ||||
| Treatment, yes/no | 6/7 | 34/1 | 11/1 | <.001 | <.001 | .45 | ||||
| Thrombotic events, yes/no | 1/12 | 6/29 | 2/10 | .71 | .66 | 1 | ||||
| Probabilities of hematologic complications, % | ||||||||||
| 5 y after diagnosis | 0 | 4 ± 2 | 29 ± 12 | — | — | — | ||||
| 10 y after diagnosis | 0 | 10 ± 7 | 29 ± 12 | .12 | .79 | .14 | ||||
| Survival rates, % | ||||||||||
| 10 y after diagnosis | 100 | 87 ± 9 | 76 ± 15 | — | — | — | ||||
| 15 y after diagnosis | 83 ± 15 | 87 ± 9 | 38 ± 20 | .15 | .82 | .08 | ||||
Plus/minus values indicate standard deviation.
— indicates not calculated.
Distribution of the JAK2 mutation within MPD families. (A) Classification of families according to the JAK2 genotype. Families were divided into 3 groups: negative (genotype GG) for the JAK2 mutation, positive (genotype GT) for the JAK2 mutation, and heterogeneous, including patients with GG or GT genotypes within families. (B) Phenotypic spectrum within subgroups of MPD families. ▪ indicates PV or MMM; □,ET; ▦, PV or MMM and ET.
Distribution of the JAK2 mutation within MPD families. (A) Classification of families according to the JAK2 genotype. Families were divided into 3 groups: negative (genotype GG) for the JAK2 mutation, positive (genotype GT) for the JAK2 mutation, and heterogeneous, including patients with GG or GT genotypes within families. (B) Phenotypic spectrum within subgroups of MPD families. ▪ indicates PV or MMM; □,ET; ▦, PV or MMM and ET.
According to the JAK2 genotype, different clinical and hematologic profiles were also observed in patients with ET (Table 4). Patients with the JAK2 mutation were older than patients without the JAK2 mutation, but a marked difference in the duration of the disease was observed between the Tminor and the Tmajor groups (54 months versus 156 months, respectively; P = .02) As in PV, EECs were detected with a higher frequency in patients with the JAK2 mutation compared with patients without the mutation.
Hematologic profile and complications in patients with ET according to JAK2 genotype
. | JAK2 genotype . | . | . | P . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Tnull; wild type . | Tminor; T allele up to 50% . | Tmajor; T allele more than 50% . | Global . | Tnull vs Tminor . | Tminor vs Tmajor . | ||||
| No. of patients | 30 | 26 | 5 | — | — | — | ||||
| Age at diagnosis, y (range) | 42 (16-77) | 61 (20-86) | 61 (31-71) | .18 | .11 | .71 | ||||
| Age at inclusion, y (range) | 47 (16-81) | 64 (20-89) | 75 (44-78) | .05 | .07 | .26 | ||||
| Duration of the disease, mo (range) | 43 (0-287) | 54 (0-187) | 156 (79-243) | .03 | .49 | .02 | ||||
| Hematologic profile | ||||||||||
| White blood cell count, × 109/L (range) | 8 (5-16) | 9 (6-21) | 11 (7-16) | .12 | .06 | .78 | ||||
| Platelet count, × 109/L (range) | 875 (510-2000) | 818 (500-1100) | 878 (700-1144) | .52 | .37 | .29 | ||||
| Hemoglobin level, g/L (range) | 141 (113-180) | 145 (129-172) | 134 (123-140) | .03 | .06 | .012 | ||||
| Hematocrit, % (range) | 43 (34-52) | 45 (39-51) | 40 (38-43) | .05 | .05 | .06 | ||||
| Splenomegaly, yes/no | 3/25 | 1/25 | 2/3 | .05 | .61 | .06 | ||||
| EEC, yes/no | 16/14 | 22/4 | 2/3 | .02 | .02 | .06 | ||||
| Complications | ||||||||||
| Follow-up, y (range) | 6 (0.2-24) | 5 (0.6-15) | 17 (9-23) | .06 | .58 | .02 | ||||
| Treatment, yes/no | 19/10 | 21/5 | 4/0 | .21 | .24 | .99 | ||||
| Thrombotic events, yes/no | 7/23 | 8/18 | 0/5 | .33 | .53 | .29 | ||||
| Probabilities of hematologic complications, % | ||||||||||
| 5 y after diagnosis | 0 | 0 | 0 | — | — | — | ||||
| 10 y after diagnosis | 0 | 0 | 20 ± 10 | .01 | NE | .03 | ||||
| Survival rates, % | ||||||||||
| 10 y after diagnosis | 100 | 87 ± 12 | 80 ± 18 | — | — | — | ||||
| 15 y after diagnosis | 100 | 87 ± 12 | 53 ± 25 | .18 | — | — | ||||
. | JAK2 genotype . | . | . | P . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Tnull; wild type . | Tminor; T allele up to 50% . | Tmajor; T allele more than 50% . | Global . | Tnull vs Tminor . | Tminor vs Tmajor . | ||||
| No. of patients | 30 | 26 | 5 | — | — | — | ||||
| Age at diagnosis, y (range) | 42 (16-77) | 61 (20-86) | 61 (31-71) | .18 | .11 | .71 | ||||
| Age at inclusion, y (range) | 47 (16-81) | 64 (20-89) | 75 (44-78) | .05 | .07 | .26 | ||||
| Duration of the disease, mo (range) | 43 (0-287) | 54 (0-187) | 156 (79-243) | .03 | .49 | .02 | ||||
| Hematologic profile | ||||||||||
| White blood cell count, × 109/L (range) | 8 (5-16) | 9 (6-21) | 11 (7-16) | .12 | .06 | .78 | ||||
| Platelet count, × 109/L (range) | 875 (510-2000) | 818 (500-1100) | 878 (700-1144) | .52 | .37 | .29 | ||||
| Hemoglobin level, g/L (range) | 141 (113-180) | 145 (129-172) | 134 (123-140) | .03 | .06 | .012 | ||||
| Hematocrit, % (range) | 43 (34-52) | 45 (39-51) | 40 (38-43) | .05 | .05 | .06 | ||||
| Splenomegaly, yes/no | 3/25 | 1/25 | 2/3 | .05 | .61 | .06 | ||||
| EEC, yes/no | 16/14 | 22/4 | 2/3 | .02 | .02 | .06 | ||||
| Complications | ||||||||||
| Follow-up, y (range) | 6 (0.2-24) | 5 (0.6-15) | 17 (9-23) | .06 | .58 | .02 | ||||
| Treatment, yes/no | 19/10 | 21/5 | 4/0 | .21 | .24 | .99 | ||||
| Thrombotic events, yes/no | 7/23 | 8/18 | 0/5 | .33 | .53 | .29 | ||||
| Probabilities of hematologic complications, % | ||||||||||
| 5 y after diagnosis | 0 | 0 | 0 | — | — | — | ||||
| 10 y after diagnosis | 0 | 0 | 20 ± 10 | .01 | NE | .03 | ||||
| Survival rates, % | ||||||||||
| 10 y after diagnosis | 100 | 87 ± 12 | 80 ± 18 | — | — | — | ||||
| 15 y after diagnosis | 100 | 87 ± 12 | 53 ± 25 | .18 | — | — | ||||
NE indicates not evaluable; —, not calculated.
Some hematologic data indicate that the disease is more severe in patients with the JAK2 mutation. This hypothesis was confirmed by the analysis of the complications. The main complications typically observed in the course of PV and ET include thrombotic events, myelofibrosis, and leukemic transformations. In our cohort, thrombosis occurred in about one third of patients with PV or ET. Arterial and venous thrombotic occlusions were observed with the same frequency in patients with PV, whereas venous thrombotic complications were twice as frequent in ET. Our analysis showed that the occurrence of thrombotic events was independent of the presence of a JAK2 mutation in PV and ET. Vascular events were even more frequent in patients with ET and wild-type JAK2 genotype, an observation that may be related to higher levels of hemoglobin and hematocrit observed in these patients (Table 4).
In contrast to thrombotic events, hematologic complications including myelofibrosis and leukemic transformation events were more frequent in patients with the JAK2 mutation. The probabilities of hematologic complications were 29% at 5 and 10 years in PV patients with a mutant T allele proportion above 50% (Table 3). By contrast, these probabilities at 5 and 10 years were 0% in the group of PV patients without the JAK2 mutation. In patients with ET, probabilities of hematologic complications were significantly different between the group of patients with mutant T allele proportion above 50% and the 2 other subgroups (20% at 10 years in Tmajor versus 0% in Tnull and Tminor groups; P = .01) (Table 4). These results suggest that homozygous JAK2 genotype is associated with the progression of the hematologic disease.
Discussion
We studied 72 multiplex families, 42 with a single type of MPD and 30 including patients with distinct types of MPD. As compared with the observations made in the general population, our cohort revealed a major prevalence of cases with PV and ET compared with CML cases.22-24 Seven percent of the patients had CML whereas 47% of the patients had PV and 40% had ET. This marked difference may be due to a bias of diagnosis in familial cases because PV and ET may have a relatively indolent and well-tolerated course for several years and thus be underdiagnosed in sporadic cases. In contrast, CML has a more aggressive evolution with a constant transformation in acute leukemia. Conversely, the increased frequency of PV and ET observed in our cohort may be explained by unknown environmental or genetic factors that would predispose to the development of PV or ET. Several familial clusterings of MPD have been reported but, until now, cosegregation analysis and study of candidate genes failed to reliably locate or identify predisposing factors.25,26 In that context, it was of interest to investigate whether the Val617Phe JAK2 mutation, recently identified in most sporadic cases of PV, ET, and MMM, predisposes to the development of familial MPD.
The genotyping of the JAK2 mutation in our cohort revealed similar frequencies of JAK2 mutation in familial and sporadic cases of MPD.11,12,15,16 In familial cases, the JAK2 mutation was detected in three quarters of patients with PV or MMM and half of patients with ET. The frequency of homozygous patients was 25% in PV and 16% in ET. In fact, as previously shown, hematopoietic cells considered as homozygous for the JAK2 mutation have most frequently lost 1, nonmutated, JAK2 allele (as evidenced here by losses of heterozygosity at adjacent microsatellite loci) and duplicated the remaining mutant allele, thus leading to the presence of 2 mutant JAK2 alleles per hematopoietic cells.25
It was not possible in this study to directly assess whether the homozygosity of the JAK2 mutation in the familial cases was an early event or had been progressively acquired during disease evolution as recently shown in a few patients with PV.27 However, within families presenting the same type of MPD, a comparison of pairs of affected relatives showed that the homozygosity of the JAK2 mutation in PV may be acquired in a different manner. Indeed, within families, patients with homozygous JAK2 mutation in PV were characterized by their young age at diagnosis and the short duration of the disease, suggesting that is was an early event. In contrast, homozygous JAK2 mutation was observed in patients with ET who were older and had a longer duration of their disease, indicating that the homozygosity may have been acquired late in the disease evolution. However, it is possible that the proliferative advantage conferred by JAK2 is higher in PV than in ET and is dependent on interindividual genetic background.
The analysis of the distribution of the JAK2 mutation within families led to the identification of a group of 22 families in which the JAK2 mutation was present in all affected patients. This group was candidate to carry the germ-line Val617Phe JAK2 mutation. However, several observations disfavor that hypothesis: (1) the absence of the JAK2 mutation in purified T, B, and NK cells of 13 unrelated patients and 4 siblings from these families, (2) the observation of variable ratios of the JAK2 mutant allele in different cell populations, and (3) the results of a genomewide linkage analysis that excluded under a dominant genetic model (LOD score, below -4) the short arm of chromosome 9 where JAK2 is located (result not shown). This latter result corroborates with genomewide linkage analysis previously reported.25 Taken together, these observations indicate that the JAK2 mutation is acquired in sporadic MPDs as well as in familial MPDs as somatic events.
The MPD distribution in these 22 families also highlights several peculiarities. First, single or multiple types of MPD (PV, ET, and MMM) were observed within families with all MPD patients having the JAK2 mutation. These results suggest that the JAK2 mutation is not involved in the clonal proliferation of a specific lineage but is acquired upstream of the differentiation of the myeloid lineages, probably in multipotent hematopoietic progenitor cells. Among these families, 4 included 1 patient with CML, and none of the CML patients was a carrier of the JAK2 mutation. This observation is compatible with a predisposition factor common to PV, ET, MMM, and CML. Additionally, one event, such as the chromosome translocation t(9;22)(q34;q11) in CML, or several somatic events arising in hematopoietic stem cells may be responsible for the onset of the disease and the specific characteristics of the proliferation. This hypothesis supports the model of multiple mutational somatic events proposed by Kralovics and colleagues for the pathogenesis of familial PV.26
Secondly, the observation of 18 heterogeneous families shows that the Val617Phe JAK2 mutation is not a prerequisite to the occurrence of the MPD. Other somatic mutations involving either other JAK2 mutations or another group of genes may be implicated in the development of MPDs in patients negative for the Val617Phe JAK2 mutation.
Finally, 7 families were negative for the Val617Phe JAK2 mutation. This group was characterized by patients with a significantly younger age at diagnosis and a different hematologic profile. For these patients, we have excluded the implication of several genes known to be mutated in congenital polycythemia and thrombocytemia (EpoR, VHL, TPO, MPL; data not shown). We hypothesize that these patients may have developed distinct clinical entities characterized by mutations in other genes to be discovered.
Several studies have analyzed the relationships between JAK2 genotype and clinical profile.11,15,16,28 A correlation between the presence of JAK2 mutation and the occurrence of complications (secondary fibrosis and vascular events) has been reported in a single study.16 Recently, correlations have been described between the presence of the JAK2 mutation and increased hemoglobin, neutrophil counts, and a higher rate of polycythemic transformation.28 In the present study, we observed that hematologic complications including myelofibrosis and myeloid acute leukemia were more frequently associated with the homozygous JAK2 mutation. This observation contrasts with the recent report that in sporadic cases AML secondary to MPD was essentially associated with nonmutated JAK2 cases or was independent of the presence of the JAK2 mutation.29,30 In our study, progression of the hematologic disease was associated with a predominant T allele both in PV and ET. These observations concur with the hypothesis that cells with 2 mutant JAK2 alleles have an increased proliferative advantage and thus may be more tightly associated with transformation.12,16 This hypothesis is also corroborated with our observations in T, B, and NK cells. The detection of the JAK2 mutation in these cells was significantly correlated with the higher proportion of the mutant JAK2 allele. These observations are also in favor of the occurrence of the JAK2 mutation in a multipotent hematopoietic stem cell with a combined lymphoid and myeloid potential.25,31 In sporadic cases, the presence of the Val617Phe JAK2 mutation in a myelolymphoid progenitor both in PV and MMM has been directly demonstrated (F. C., Sabrina Dupont, Carole Tonetti, Aline Massé, Isabelle Godin, Jean-Pierre Le Couedic, Najet Debili, Patrick Saulnier, Yann Lécluse, Frédéric Larbret, N. C., W. V., Stéphane Giraudier; Evidence that the JAK2 V617F mutation occurs in a lymphomeyloid progenitor in polycythemia vera and idiopathic myelofibrosis, manuscript in preparation).
In summary, the study of a large cohort of families with MPD does not support the existence of germ-line Val617Phe JAK2 mutation as a predisposing factor. By providing a refined classification, determination of the JAK2 genotype may contribute to the search for genetic determinants favoring the development of MPD. The homozygous Val617Phe JAK2 mutation appears to confer a proliferative advantage, and its presence is correlated with the progression of the hematologic disease; patients with a homozygous JAK2 genotype are at higher risk for hematologic complications. These observations need to be replicated on a large scale in sporadic cases and may be of major importance for the follow-up of patients.
Appendix
The following institutions and investigators participated in the study: AP-HP Saint Antoine, Paris, A. Najman, N. C. Gorin, F. Isnard, J. Ph. Laporte, N. Cheron, L. Garderet, D. Jaulmes, S. Lesage; Centre Hospitalo-Universitaire (CHU) de Lille, F. Bauters, J. P. Jouet, M. T. Caulier, B. Lerche, N. Trillot; CHU de Dijon, P. M Carli, H. Guy, M. Flesch; AP-HP Lariboisière-Saint Louis (AP-HP), Paris, J. D. Rain, A. C. Braud, S. Bellucci, P. Malphettes, W. Vainchenker; Centre Hospitalier (CH) de Metz, V. Dorvaux; AP-HP Necker-Enfants Malades, Paris, B. Varet, A. Buzyn; CHU de Poitiers, F. Guilhot, V. Delwail, P. Roblot; CH de Versailles, S. Castaigne; CHU de Bordeaux, P. Cony-Makloul, K. Bouabdallah, M. Boiron, G. Marit; CHU de Marseille, G. Sebahoun, F. Harlé; CHU de Brest, J. F. Abgrall; Institut Bergonié, Bordeaux, H. Eghbali; AP-HP Hôtel-Dieu, N. Casadevall, C. M. Blanc, F. Cymbalista; Université de Bruxelles, Belgique, J. Delannoy; CH de Chambéry, M. Blanc; Institut Curie, Paris, J. Dumont, D. Stoppa-Lyonnet; CHU de Grenoble, J. J. Sotto; CH de Lens, M. Simon, B. Dupriez, A. Stalnikiewicz; CHU de Reims, A. M. Blaise, D. Ramage; CHU de Rennes, P. Grosbois, P. Jego, P. Y. Le Prisé; CHU de Toulouse, J. Pris; CHU d'Angers, M. Gardembas-Pain; CH D'Angouleme, M. Bonnefoy; CH d'Annecy, Cl. Martin; CH de Bastia, H. Zanaldi; AP-HP Beaujon, Clichy, J. Brière; CHU de Besançon, J. Y. Cahn, E. Deconnink, J. M. de Sède, J. Vuillier; AP-HP Kremlin-Bicêtre, G. Tchernia; CHU de Caen, M. Leporrier; CH de Chalons sur Saone, B. Salles; CH de Corbeil, A. Devidas; Clinique Pasteur, Evreux, N. Albin; CH de Laval, D. Jacomy; CH de Libourne, J. Ceccaldi; CH Université Catholique de Lille, J. L. Demory; CHU de Limoges, D. Bordessoule; CHU de la Martinique, G. Panelatti; CH de La Rochelle, B. Gombert; CH de Meaux, Ch. Allard; CHU de Montpellier, M. Navarro, T. Lavabre-Bertrand; CH de Mulhouse, Ph. Hénon; CHU de Nancy, A. P. Guerci; Centre Antoine Lacassagne, Nice, A. Thyss; AP-HP Pitié-Salpétrière, Paris, Z. Azgui, S. Choquet; AP-HP Trousseau, Paris, G. Leverger, J. Landman-Parker, A. Auvrignon; CH de Pontoise, J. Facquet-Danis; CH de Roubaix, I. Plantier; CH Becquerel, Rouen, P. Lenain; CH de Saint-Brieuc, P. Morice; CH de Fréjus-Saint Raphaël, J. Gutnecht; CH de Troyes, G. Dine; CH de Vannes, H. Jardel; Institut Gustave Roussy, Villejuif, A. Turhan.
Prepublished online as Blood First Edition Paper, March 14, 2006; DOI 10.1182/blood-2005-12-4852.
A list of the institutions and investigators that participated in the study appears in the “Appendix.”
Supported by research grants 1998-9037 and 2000-5136 (A.N.) and 2001-5181 (G.T.) from the Association pour la Recherche sur le Cancer (ARC) and a grant from the Association pour la Recherche sur la Moelle Osseuse (ARMO) I.C. was a fellow of ARC, and F.B. is a fellow of ARMO.
C.B.-C. designed the study, analyzed data, and wrote the manuscript; I.C. recruited patients, conducted the endogenous erythroid colony (EEC) assays, and recorded all clinical and hematologic data; M.L. did all the statistical analysis and reviewed the manuscript; F.B., V.B., and C.D.T. contributed to molecular analysis; F.D. performed the flow cytometry assay and reviewed the manuscript; N.C. and W.V. contributed to the recruitment of patients and critically reviewed the manuscript; G.T. designed the study, contributed to data analysis, and critically reviewed the manuscript; and A.N. initiated this study, recruited patients, contributed to data analysis, and wrote the manuscript.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to all families for contributing to the study. We thank all physicians who contributed to the recruitment of patients. We thank the Société Francaise d'Hématologie for supporting this study. We thank Marie-Fernande Legrand, Gwendoline Leroy, Aline Massé, and Patrick Saulnier for their technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal