Phosphorylation of transcription factors is important in posttranslational control of protein function. The indispensable zinc-finger transcription factor, Gata1, is phosphorylated constitutively at 6 serine residues (26, 49, 72, 142, 178, 187), and at a seventh (310) following induction of erythroid differentiation. However, the biologic consequences of phosphorylation with respect to function are unclear. To address this issue, we generated mice with serine-to-alanine mutations at the inducibly phosphorylated serine 310 alone or at conserved serine residues 72, 142, and 310 together. The peripheral blood parameters of the mice were normal, as was their response to acute erythropoietic stress. Analysis of hematopoietic progenitor populations during ontogeny and into adulthood showed a moderate decrease in erythroid burst-forming unit (BFU-E) and erythroid colony-forming unit (CFU-E) numbers only in the adult bone marrow of the triple mutant. Yet, later stage erythropoiesis was not perturbed. This suggests that any molecular consequences associated with loss of phosphorylation at residues 72, 142, and 310 can be compensated for in the in vivo environment.
Introduction
Gata1 is expressed abundantly in the erythroid, megakaryocytic, eosinophilic, and mast cell lineages and at lower levels in the multipotent hematopoietic progenitors. Gene targeting in mice has demonstrated an essential role for Gata1 in the differentiated lineages in which it is expressed (reviewed in Crispino1 ). Disruption of the Gata1 locus results in embryonic lethality at the yolk sac stage due to extreme anemia. In vitro differentiation of embryonic stem (ES) cells demonstrates that definitive erythroid progenitors arrest at the proerythroblast stage and undergo apoptosis; megakaryocytes that lack Gata1 fail to mature normally and proliferate abnormally. Thus, Gata1 is required for the normal balance among proliferation, cell death, and differentiation.
The expression of transcription factors is under tight spatial and temporal regulation. Posttranslational modifications, such as phosphorylation, provide a further level of control and allow transcription factor activity to be modulated in response to environmental signals. The mechanism by which the activity of a transcription factor is modified varies, but examples include structural changes permissive to cofactor binding, DNA binding affinity, and transcriptional activation activity.2 In the mouse erythroleukemia cell line, MEL, Gata1 is phosphorylated constitutively on 6 serine residues and at a seventh residue following DMSO-induced differentiation.3 However, the effects of phosphorylation on Gata1 remain unclear; phosphorylation of Gata1 in MEL cells did not change DNA binding efficiency or transcriptional activation,3 whereas in K562 cells phosphorylation was suggested to increase Gata1 DNA binding efficiency.4 Thus, cellular context may define the consequences of Gata1 phosphorylation.
To address an in vivo requirement for Gata1 phosphorylation, we generated mice that express Gata1 with serine-to-alanine replacements at specific residues. One mouse line harbored a single replacement at serine 310, the phosphorylation of which parallels erythroid differentiation. In a second line, replacements at 2 additional serines, residues 72 and 142, were generated. The residues that were replaced are highly conserved among Gata1 proteins of diverse species. No major hematopoietic abnormalities were observed in these mice, even in response to acute erythropoietic stress. This suggests that phosphorylation of residues 72, 142, and 310 is largely dispensable for the function of Gata1 in hematopoiesis.
Study design
Generation of mice bearing the Gata1S310A and Gata13SA alleles
Construction of targeting vectors and generation of the mice bearing the Gata1S310A and Gata13SA alleles are depicted and described in Figure S1 (available on the Blood website; see the Supplemental Figure link at the top of the online article).
Hematologic blood parameters
Blood was obtained by retroorbital sinus bleeding and analyzed using an automated system (Advia 120; Bayer, Leverkursen, Germany). Embryonic hematocrits were determined by capillary tube centrifugation. Images were acquired with a Nikon Eclipse E800 microscope (Nikon, Tokyo, Japan) with a 60 ×/0.40 NA oil objective lens or a 100 ×/1.30 NA oil objective lens and an RT Slider digital camera (Diagnostic Instruments, Sterling Heights, MI). Images were processed with Spot 3.5.6 for Mac 05 (Diagnostic Instruments) and Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Induction of anemia with phenylhydrazine
Mice received intraperitoneal injections of 60 mg phenylhydrazine (PHZ)/kg body weight on days 1 and 2. Blood was taken for analysis on days 1, 3, 6, 9, and 14.
In vitro colony-forming assays
Bone marrow or fetal liver cells (2 × 105 cells/mL-5 × 105 cells/mL) were cultured in methylcellulose (M3234; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with erythropoietin alone (2 U/mL) or with Kit Ligand (10 ng/mL) from R&D Systems (Minneapolis, MN).
FACS analysis
Bone marrow or fetal liver cells were immunostained simultaneously with CD71 (fluorescein isothiocyanate [FITC]–conjugated; Pharmingen, San Diego, CA) and Ter119 (phycoerythrin [PE]–conjugated; Pharmingen). Flow cytometry was performed using a FACSCalibur (Becton Dickinson, San Jose, CA).
Results and discussion
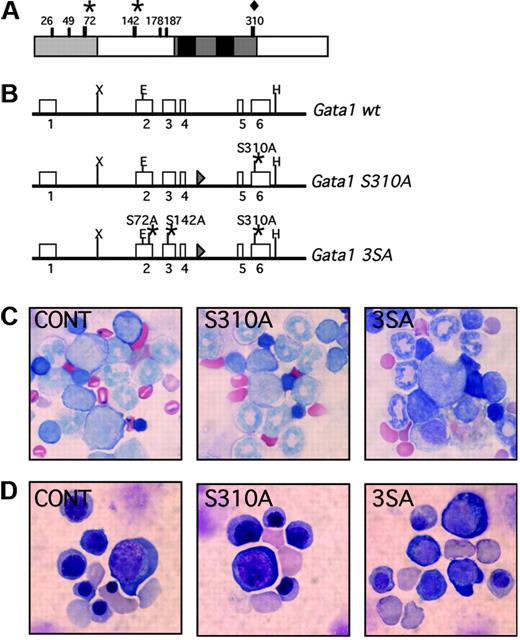
The sites at which Gata1 is phosphorylated are shown in Figure 1A. We generated knock-in mice that harbor serine-to-alanine replacements at residue 310 alone (Gata1S310A) or residues 72, 142, and 310 (Gata13SA) (Figure 1B). After Cre-excision of the neomycin resistance gene, the Gata1 allele was altered only by the addition of a loxP site between exons IV and V and the point mutation(s) that convert the serine to alanine.
Frequency of birth of affected pups was ascertained by crossing Gata1S310A and Gata13SA heterozygous females with C57BL/6 males. Gata1S310A and Gata13SA hemizygous males were born at approximately Mendelian frequency, were fertile, and had a normal life expectancy. We compared the peripheral blood parameters of young (5-6 weeks, Table 1) and older (11-12 months, data not shown) mice. Gata1S310A and Gata13SA mice had white and red blood cell counts comparable to their wild-type littermates. In particular, there was no evidence of red blood cell abnormalities and unlike the Gata1ΔneoΔHS mice in which expression of Gata1 is decreased specifically in megakaryocytes, the Gata1S310A and Gata13SA mice were not thrombocytopenic.5 Indeed, platelet number, volume, and distribution width were normal. Furthermore, bone marrow and spleen cellularities were normal and red cells of various stages of maturation were seen in May-Gruenwald-Giemsa–stained cytospins of the bone marrow (Figure 1C). Thus, the mice have no overt hematopoietic abnormalities, indicating that phosphorylation at residues 72, 142, and 310 is dispensable for steady-state hematopoiesis.
Hematopoietic indices of the Gata1S310A and Gata13SA mice
Genotype . | No. mice . | WBCs, × 106 cells/mL . | Eos, × 106 cells/mL . | RBCs, × 109 cells/mL . | HGB, g/dL . | HCT, % . | MCV, fL . | MCHC, g/dL . | Retics, % . | Platelets, × 106 cells/mL . | MPV, fL . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | 7 | 9.0 ± 1.1 | 2.2 ± 0.5 | 9.6 ± 0.3 | 15.5 ± 0.5 | 53.7 ± 1.9 | 56.2 ± 1.4 | 28.7 ± 0.8 | 2.6 ± 0.9 | 1268 ± 113 | 7.8 ± 0.2 |
| S310A KI | 8 | 7.9 ± 1.2 | 2.0 ± 0.3 | 9.0 ± 0.5 | 14.7 ± 0.9 | 51.6 ± 2.9 | 57.6 ± 1.1 | 28.4 ± 0.6 | 3.9 ± 0.8 | 1318 ± 208 | 7.9 ± 0.3 |
| WT | 10 | 7.6 ± 1.5 | 2.2 ± 1.1 | 9.6 ± 0.5 | 15.9 ± 1.1 | 53.6 ± 2.4 | 55.9 ± 1.8 | 29.6 ± 1.3 | 3.3 ± 1.2 | 1204 ± 103 | 7.4 ± 0.2 |
| 3SA KI | 8 | 7.0 ± 1.7 | 2.2 ± 1.0 | 9.5 ± 0.4 | 15.7 ± 0.4 | 53.9 ± 0.7 | 56.4 ± 2.3 | 29.3 ± 0.6 | 3.6 ± 0.8 | 1503 ± 133 | 7.8 ± 0.3 |
Genotype . | No. mice . | WBCs, × 106 cells/mL . | Eos, × 106 cells/mL . | RBCs, × 109 cells/mL . | HGB, g/dL . | HCT, % . | MCV, fL . | MCHC, g/dL . | Retics, % . | Platelets, × 106 cells/mL . | MPV, fL . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | 7 | 9.0 ± 1.1 | 2.2 ± 0.5 | 9.6 ± 0.3 | 15.5 ± 0.5 | 53.7 ± 1.9 | 56.2 ± 1.4 | 28.7 ± 0.8 | 2.6 ± 0.9 | 1268 ± 113 | 7.8 ± 0.2 |
| S310A KI | 8 | 7.9 ± 1.2 | 2.0 ± 0.3 | 9.0 ± 0.5 | 14.7 ± 0.9 | 51.6 ± 2.9 | 57.6 ± 1.1 | 28.4 ± 0.6 | 3.9 ± 0.8 | 1318 ± 208 | 7.9 ± 0.3 |
| WT | 10 | 7.6 ± 1.5 | 2.2 ± 1.1 | 9.6 ± 0.5 | 15.9 ± 1.1 | 53.6 ± 2.4 | 55.9 ± 1.8 | 29.6 ± 1.3 | 3.3 ± 1.2 | 1204 ± 103 | 7.4 ± 0.2 |
| 3SA KI | 8 | 7.0 ± 1.7 | 2.2 ± 1.0 | 9.5 ± 0.4 | 15.7 ± 0.4 | 53.9 ± 0.7 | 56.4 ± 2.3 | 29.3 ± 0.6 | 3.6 ± 0.8 | 1503 ± 133 | 7.8 ± 0.3 |
All data are shown as mean plus or minus standard deviation.
WT indicates wild-type; WBC, white blood cell; Eos, eosinophils; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCHC, mean corpuscular hgb concentration; Retics, reticulocytes; MPV, mean platelet volume.
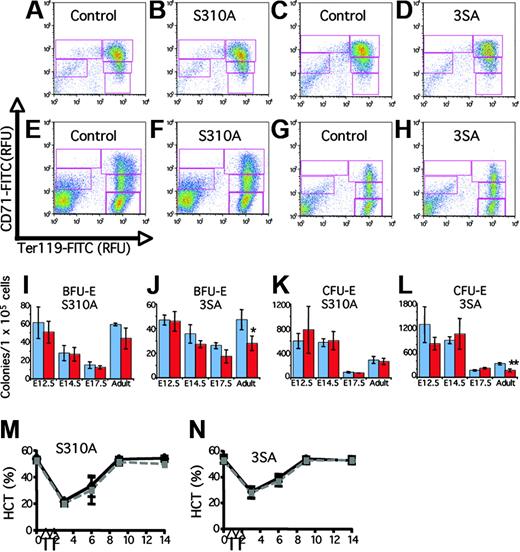
To determine whether the response of Gata1S310A and Gata13SA mice to erythroid stress is attenuated, anemia was induced by injection on 2 consecutive days with PHZ. Analysis of the peripheral demonstrated that PHZ induced anemia by day 3 and recovery was complete within 14 days. As evidenced by the hematocrit, Gata1S310A and Gata13SA mice recover from PHZ induced anemia at the same rate as their littermates (Figure 2M-N).
The hematopoietic system has remarkable feedback systems that compensate for abnormalities in the bone marrow such that the peripheral blood is not adversely affected. To determine whether the progenitor populations in the bone marrow were affected, colony assays were performed. In both lines, no differences were seen in the number of myeloid, IL-7–dependent, or megakaryocyte colonies. A moderate decrease in both erythroid burst-forming unit (BFU-E) and erythroid colony-forming unit (CFU-E) progenitor populations was observed in the bone marrow from the triple mutant, Gata13SA (Figure 2J,L). Progenitor numbers in the bone marrow of the Gata1S310A mutant were not markedly affected. Erythroid progenitor populations did not display an altered sensitivity to erythropoietin dosage (data not shown).
Analysis of later stages of erythropoiesis was achieved by fluorescence-activated cell sorting (FACS)–based profiling of cells using the markers CD71 and Ter119 that allows quantitative evaluation of erythroid differentiation.6 The profile is divided into 5 subpopulations of cells with increasing maturity, R1-R5. The CD71/Ter119 profiles of the bone marrow from the knock-in mice were normal, indicating that there was not a block in differentiation (Figure 2E-H). No significant quantitative differences in the proportion of cells in each subpopulation in either the bone marrow or the spleen were seen. Thus, changes in the progenitor numbers are compensated for within the bone marrow.
Generation of the Gata1S310A and Gata13SA knock-in mice. (A) Schematic representation of the mouse Gata1 protein. The N-terminal activation domain, amino acids 1-83, is shaded in gray and the highly conserved zinc fingers and surrounding domains are drawn in black. The serine residues that are phosphorylated in MEL cells are indicated. Serine 310 (diamond) was targeted alone or together with serines 42 and 172 (asterisks). (B) The Gata1S310A and Gata13SA knock-in alleles generated by homologous recombination. Point mutations were introduced that changed the serine to alanine at residue 310 (Gata1S310A) or residues 72, 142, and 310 (Gata13SA) to abolish phosphorylation at these sites. (C,D) Erythropoiesis is preserved in Gata1S310A and Gata13SA mice. Cells at various stages of erythropoiesis were seen in fetal livers at 14.5 dpc (C) and in the bone marrow of adult mice (D) harboring Gata1S310A and Gata13SA mutations. Original magnification ×1000.
Generation of the Gata1S310A and Gata13SA knock-in mice. (A) Schematic representation of the mouse Gata1 protein. The N-terminal activation domain, amino acids 1-83, is shaded in gray and the highly conserved zinc fingers and surrounding domains are drawn in black. The serine residues that are phosphorylated in MEL cells are indicated. Serine 310 (diamond) was targeted alone or together with serines 42 and 172 (asterisks). (B) The Gata1S310A and Gata13SA knock-in alleles generated by homologous recombination. Point mutations were introduced that changed the serine to alanine at residue 310 (Gata1S310A) or residues 72, 142, and 310 (Gata13SA) to abolish phosphorylation at these sites. (C,D) Erythropoiesis is preserved in Gata1S310A and Gata13SA mice. Cells at various stages of erythropoiesis were seen in fetal livers at 14.5 dpc (C) and in the bone marrow of adult mice (D) harboring Gata1S310A and Gata13SA mutations. Original magnification ×1000.
To ascertain whether the earlier progenitors, the megakaryocyte/erythroid progenitors, and the common myeloid progenitors were affected, we used a FACS analysis protocol described by Akashi et al7 to determine the relative proportions of these progenitors within the bone marrow. No significant differences were observed between the Gata1S310A or Gata13SA mice and their wild-type littermates (data not shown).
Other lineages in which Gata1 is highly expressed include megakaryocytes, eosinophils, and mast cells.8 No evidence of abnormalities in any of these lineages was observed in either the Gata1S310A or Gata13SA mice. In addition to normal peripheral blood platelet parameters (Table 1), no change in the percentage of CD41-expressing cells in the bone marrow was observed and acetylcholinesterase-positive megakaryocyte colonies were seen at the same frequency as wild-type in the bone marrow and fetal liver (data not shown). Furthermore, the number of eosinophils present in the peripheral blood of all mice was normal (Table 1). Finally, mast cells were cultured from adult bone marrow from both Gata1S310A and Gata13SA mice and FACS analysis was used to demonstrate that these cells coexpressed the mature mast cell marker, the high-affinity IgE receptor, and c-kit (data not shown).
Analysis of Gata1S310A and Gata13SA fetal and adult hematopoiesis. (A-H) Erythroid differentiation profiles of fetal livers at 14.5 dpc (A-D) and adult bone marrow (E-H) from Gata1S310A and Gata13SA mice, and wild-type controls. Freshly isolated cells were stained simultaneously with FITC-conjugated CD71 and PE-conjugated Ter119. Shown are representative density plots. Relative number of cells in regions R1-R5 (CD71medTer119lo, CD71hiTer119lo, CD71hiTer119hi, CD71medTer119lo, and CD71loTer119hi populations, respectively) were assessed. (I-L) Erythroid progenitor cell numbers in the fetal liver at 12.5, 14.5, and 17.5 dpc and adult bone marrow of wild-type (blue) and knock-in (red) mice. Mean and standard deviation of BFU-E and CFU-E colony numbers per 1 × 105 cells are shown from representative assays performed in triplicate. Statistically significant differences are marked: *P = .05; **P = .001. (M,N) Acute anemia was induced in 12- to 15-week-old mice by the administration of phenylhydrazine (60 mg/kg body weight) on days 1 and 2 (indicated by arrows). Peripheral blood was analyzed prior to administration and on days 3, 6, 9, and 14. Hematocrits (HCT) showing the induction of anemia and recovery over 14 days is shown for knock-in mice (gray) and their wild-type littermates (black).
Analysis of Gata1S310A and Gata13SA fetal and adult hematopoiesis. (A-H) Erythroid differentiation profiles of fetal livers at 14.5 dpc (A-D) and adult bone marrow (E-H) from Gata1S310A and Gata13SA mice, and wild-type controls. Freshly isolated cells were stained simultaneously with FITC-conjugated CD71 and PE-conjugated Ter119. Shown are representative density plots. Relative number of cells in regions R1-R5 (CD71medTer119lo, CD71hiTer119lo, CD71hiTer119hi, CD71medTer119lo, and CD71loTer119hi populations, respectively) were assessed. (I-L) Erythroid progenitor cell numbers in the fetal liver at 12.5, 14.5, and 17.5 dpc and adult bone marrow of wild-type (blue) and knock-in (red) mice. Mean and standard deviation of BFU-E and CFU-E colony numbers per 1 × 105 cells are shown from representative assays performed in triplicate. Statistically significant differences are marked: *P = .05; **P = .001. (M,N) Acute anemia was induced in 12- to 15-week-old mice by the administration of phenylhydrazine (60 mg/kg body weight) on days 1 and 2 (indicated by arrows). Peripheral blood was analyzed prior to administration and on days 3, 6, 9, and 14. Hematocrits (HCT) showing the induction of anemia and recovery over 14 days is shown for knock-in mice (gray) and their wild-type littermates (black).
In mice that express low levels of Gata1, irregularities are apparent in fetal erythropoiesis that are not seen in surviving adults.9 We investigated fetal erythropoiesis at 12.5, 14.5, and 17.5 days postcoitum (dpc) to determine whether a transitory anemia during development might be occurring. Gata1S310A and Gata13SA embryos were normal in color and size and the fetal liver was prominent at all stages examined. Hematocrits of peripheral blood from embryos 17.5 to 18.5 dpc were normal (data not shown). Erythroid colony assays were performed on cells from the fetal liver from embryos 12.5, 14.5, and 17.5 dpc. Neither BFU-E nor CFU-E progenitor activities were consistently affected in the Gata1S310A and Gata13SA fetal livers (Figure 2I-L). FACS analysis of CD71 and Ter119 expression indicated no block in differentiation; a population of mature erythrocytes (CD71lo/Ter119hi) was present in increasing percentages during ontogeny, signifying normal maturation. No difference in the proportions of cells in each of the R1-R5 subpopulations was identified. Profiles are shown for fetal liver 14.5 dpc as an illustration (Figure 2A-D). An absence of irregularities in fetal liver erythropoiesis was consistent with the normal response to stress that we observed.
In conclusion, we have shown that hematopoiesis is ostensibly normal in mice with Gata1 alleles with serine-to-alanine replacements at residues 71, 142, and 310. While the number of BFU-E and CFU-E progenitor cells in the bone marrow of Gata13SA mice is decreased, peripheral blood parameters are normal, indicating that other mechanisms exist that compensate for this decrease. Similarly, the response to developmental and acute erythroid stress is not blunted.
Recent reports have demonstrated that Gata1 is phosphorylated at serine 310 by AKT in response to erythropoietin.10,11 This phosphorylation is required for maximal induction of erythroid differentiation by Gata1 and transactivation of the Gata1 target genes b-globin and Alas2,10 and TIMP1 is poorly expressed in Gata1S310A fetal liver erythroid cells.11 In addition, in Ba/F3 cells, phosphorylation at serine 26 by ERK is important for expression of the survival genes, Bcl-XL and E4bp4.12 Thus, a picture emerges in which multiple pathways converge on Gata1 and modulate itstranscriptional activity through phosphorylation. A cumulative effect of progressive loss of Gata1 phosphorylation might be anticipated and this was not fully addressed by our studies in which only 3 serines were targeted. However, it is clear that while phosphorylation of Gata1 at key residues governs the optimal expression of specific genes, phosphorylation at residues 72, 142, and 310 is dispensable for the critical nonredundant activities of Gata1 in erythropoiesis and megakaryopoiesis.
Prepublished online as Blood First Edition Paper, January 3, 2006; DOI 10.1182/blood-2005-10-4309.
Supported in part by grants from the National Institutes of Health, Bethesda, MD (S.H.O.). S.H.O. is an Investigator of the Howard Hughes Medical Institute, Boston, MA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank H. Hock, A. Cantor, T. Schlaeger, and Z. Li for suggestions and technical assistance. Thanks also to Y. Fujiwara and C. Browne for invaluable help with generation of the transgenic mice. We have benefited greatly from conversations with S. Ghaffari and H. Lodish.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal