Hepatocyte growth factor (HGF) has previously been reported to act as a hemangiogenic factor, as well as a mitogenic factor for a variety of tumor cells. Here, we demonstrate that HGF is a lymphangiogenic factor, which may contribute to lymphatic metastasis when overexpressed in tumors. In a mouse corneal lymphangiogenesis model, implantation of HGF induces sprouting and growth of new lymphatic vessel expressing the lymphatic vessel endothelial specific marker hyaluronan receptor-1 (Lyve-1). Unlike blood vessels, the Lyve-1–positive structures consist of blunt-ended vessels of large diameters that generally lack expression of CD31. The growth of HGF-induced lymphatic vessels can be partially blocked by a soluble VEGFR-3, suggesting that HGF may stimulate lymphatic vessel growth through an indirect mechanism. Consistent with this finding, the HGF receptor (c-Met) is only localized on corneal blood vessels but is absent on lymphatic vessels in a mouse corneal assay. In a transgenic mouse model that expresses HGF under the control of the whey acidic protein (WAP) gene promoter, transgenic females develop tumors in the mammary glands after several pregnancies. Interestingly, dilated Lyve-1–positive lymphatic vessels accumulate in the peritumoral area and occasionally penetrate into the tumor tissue. Our findings indicate that HGF may play a critical role in lymphangiogenesis and potentially contribute to lymphatic metastasis.
Introduction
Similar to hemangiogenesis, the process of lymphangiogenesis may be regulated by multiple growth factors. In the adult, the lymphatic vasculature remains quiescent with the exception of situations of tissue and organ repair or regeneration, as well as during pathologic conditions such as tumor growth and metastasis.1,2 The quiescent nature of the lymphatic system suggests that in most tissues production of lymphangiogenesis inhibitors is predominant over that of lymphangiogenic factors. Although endogenous lymphangiogenesis inhibitors remain to be discovered, several lymphangiogenic factors have been identified. These include members of the vascular endothelial growth factor (VEGF),1,3 fibroblast growth factor (FGF),4 angiopoietin (Ang),5-8 platelet-derived growth factor (PDGF),9 hepatocyte growth factor (HGF),10 and IGF families.11 It appears that in certain types of malignant tissues these factors are overexpressed and may cause peritumoral or intratumoral lymphangiogenesis, which may mediate lymphatic metastasis. Indeed, a positive correlation between tumor expression of lymphangiogenic factors, such as VEGF-C or VEGF-D, and lymphatic metastasis has been established using clinical samples,12,13 and the existence of peritumoral lymphatic vessels has been suggested a sensitive markers for lymph node metastasis.14,15
Because the genome of tumor cells is unstable, multiple growth factors are switched on during malignant progression.2 Among these tumor-derived growth factors, HGF and its receptor c-Met are frequently expressed at high levels in most types of solid tumors, and the levels of c-Met expression have also been correlated with the degree of tumor invasiveness.16-19 HGF is a growth factor that belongs to the plasminogen-prothrombin gene superfamily, which includes macrophage-stimulating protein and plasminogen, among others. HGF consists of 6 domains; an amino-terminal domain, 4 kringle domains within the α-chain, and a serine proteinase homology domain within the β-chain. One of the characteristics of the plasminogen-prothrombin family is that they all contain multiple kringle domains that are identified by triple disulfide loop structures in the N-terminal domain of the α chain.20 Unlike other members in the family, HGF has no proteolytic activity despite the presence of a serine proteinase homology domain in the β-chain.21 The growth promoting activity of HGF requires proteolytic cleavage by extracellular serine proteinases such as urokinase plasminogen activator and tissue-type plasminogen activator.22,23 In addition to its direct role in promoting tumor cell growth and invasion, HGF is also a potent hemangiogenic factor that also contributes to tumor angiogenesis.24,25 HGF plays a direct role in stimulating blood vessel growth in vitro and in vivo by signaling through the c-Met receptor, which is expressed on endothelial cells.26,27 Thus, previously published reports indicate that HGF contributes to tumor growth and metastasis via its direct stimulatory effects on tumor cells and angiogenesis. In the present work, we show that HGF acts as a lymphangiogenic factor with an indirect mechanism of action,
Materials and methods
Animals
Female and male 7- to 8-week-old C57Bl/6 mice were caged in groups of 6 or less. Animals were anesthetized by an injection of a mixture of Dormicum and Hypnorm (1:1) before all procedures and killed by a lethal dose of CO2 followed by cervical dislocation. All animal studies were reviewed and approved by the animal care and use committee of the North Stockholm Animal Board. WAP-HGF FVB/N transgenic mice were generated as previously described.28
Mouse corneal hemangiogenesis and lymphangiogenesis assay
The mouse corneal assay was performed according to procedures previously described.29 Briefly, micropellets (0.35 × 0.35 mm) of sucrose and aluminum sulfate coated with Hydron polymer type NCC (IFN Sciences, New Brunswick, NJ) containing 280 ng HGF, 160 ng VEGF-A (R&D Systems, Minneapolis, MN), 320 ng PDGF-BB (PeproTech, Rocky Hill, NJ), or 80 ng FGF-2 (Phamacia & UpJohn, Milan, Italy) were implanted into corneal micropockets created by surgical operations in 7- to 8-week-old C57Bl/6 male mice. In another experiment, 280 ng HGF was implanted together with 500 ng soluble VEGFR-3-Fc (R&D Systems). The pellet was positioned 1.0 to 1.4 mm from the corneal limbus. The corneal inflammatory neovascularization model was performed using a modified suture method.30 A single and penetrating suture (8-0 silk) was fastened in the center of the cornea of anesthetized C57Bl mice using a C-3 needle. After implantation or suture, erythromycin ophthalmic ointment was applied to each eye. The eyes were examined on day 5 or day 14 after pellet implantation or after suture implanation, vessel length and clock hours of circumferential neovascularization were measured, and vascularization areas were calculated. The enucleated eyes were also used for immunohistochemical analysis.
Whole mount immunostaining and confocal analysis
Growth factor– or suture-implanted mouse eyes were enucleated at day 14 after implantation. The corneal tissue was dissected and flattened before fixation with 3% paraformaldehyde (PFA) overnight. The tissues were digested with proteinase K (20 μg/mL), followed by staining overnight at 4°C with a mixture of a rat anti–mouse CD31 monoclonal antibody (Pharmingen, San Diego, CA) and a rabbit anti–mouse polyclonal LYVE-1 (kindly provided by Dr David Jackson), or a mixture of a rat anti–mouse monoclonal LYVE-1 and a rabbit anti–mouse c-Met polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Following rigorous rinsing, blood and lymphatic vessels were detected with a mixture of goat anti–rat Alexa-555 Red (Molecular Probes, Eugene OR) and goat anti–rabbit Cy5 (Chemicon, Temecula, CA) antibodies. After washing, slides were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and analyzed under a confocal microscope (Zeiss Confocal LSM510 microscope; Carl Zeiss, Jena, Germany). The positive LYVE-1 and c-Met structures presented in Figure 3 were converted to blue and red signals, respectively. The same protocol was used for whole mount staining of mammary glands from WAP-HGF-FVB/N mice. By scanning multiple sections of tumor or healthy mammary tissue samples, 3-dimensional projections were assembled. The images were further analyzed with Adobe Photoshop 7.0 (Adobe, San Diego, CA).
Proliferation assay
Primary human lymphatic endothelial cells (LECs) were isolated as previously described9 and were seeded at a density of 1 × 104 cells/well in 24-well plates in EMB-2 medium supplemented with 5% FCS and 100 ng/mL recombinant HGF or VEGF-C. Nontreated cells served as a negative control. The cells were incubated at 37°C for 72 hours before trypsinized, resuspended in Isoton II solution (Beckman Coulter, Bromma, Sweden), and counted in a Coulter Counter. Triplicates were used for each sample, and the experiment was repeated twice.
Lymphatic endothelial cell migration assay
The motility responses of human LECs to recombinant HGF were assayed using a modified Boyden chamber technique previously described.9 Briefly, the ability of isolated LECs to migrate through a micropore nitrocellulose filter (8-μm pore size) was measured as a criterion for chemotactic stimuli. Serum-free EBM-2 medium supplemented with 0.2% BSA and increasing concentrations of recombinant HGF (0, 0.1, 1, 10, and 100 ng/mL) or VEGF-C (100 ng/mL) were added to the lower chambers. Nontreated cells served as negative controls. Cells were detached using Accutase (PAA Laboratories GmbH, Pasching, Austria) and resuspended in serum-free medium (0.2% BSA) at a concentration of 0.6 × 106 cells/mL, and to each well in the upper chamber 30 000 cells were added. After 4 hours incubation at 37°C, the Boyden chamber was disassembled, and cells attached to the filter were fixed in methanol and stained with a Giemsa solution. Quadruplicates of each sample were used, and all experiments were performed twice. The cells that had migrated through the filter were counted using a light microscope, and plotted as numbers of migrating cells per optic field (× 32).
For more details on materials and methods, see Document S1, available on the Blood website (see the Supplemental Materials link at the top of the online article).
Results
HGF induced hemangiogenesis in the mouse cornea
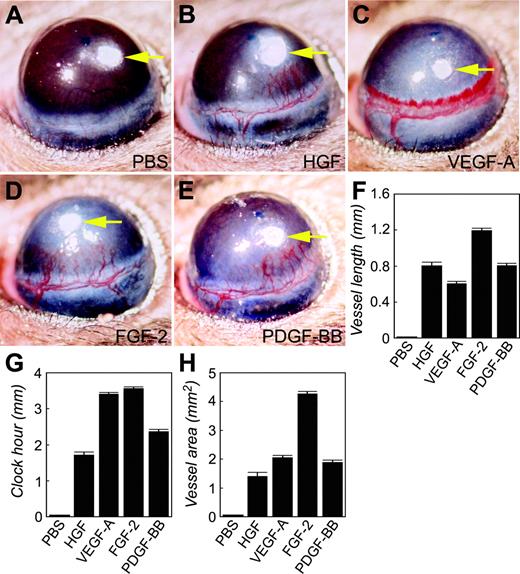
We chose the mouse corneal angiogenesis model to study whether HGF was able to induce hemangiogenesis and lymphangiogenesis. At day 5 after implantation, HGF at a dose of 280 ng was able to induce the growth of new blood vessels sprouting from the preexisting corneal limbus (Figure 1B). The HGF-induced vasculature consisted of well-defined microvessels separated from each other that grew toward the implanted pellet. The corneal vascular network induced by HGF is reminiscent of that induced by FGF-2 or PDGF-BB, but strikingly different from the VEGF-A–induced nascent vasculature, which was composed of disorganized vascular plexuses as a result of fusion of multiple capillaries (Figure 1B-E). A sinusoidal and pseudo-hemorrhagic phenotype was seen at the leading edge of the VEGF-A–induced vasculature. Quantification analysis shows that, although the HGF-induced blood vessels were longer than those induced by VEGF-A, the clock hours of corneal neovascularization (the degrees of neovascularization if the cornea is considered as a clock) stimulated by HGF were approximately half of the VEGF-A–induced clock hours (Figure 1F-G). FGF-2–induced vessel length and clock hours were larger than those induced by HGF, whereas the angiogenic response induced by PDGF-BB was highly similar to that induced by HGF. A comparative analysis of hemangiogenic potency is shown in Figure 1H.
HGF stimulated lymphangiogenesis in vivo
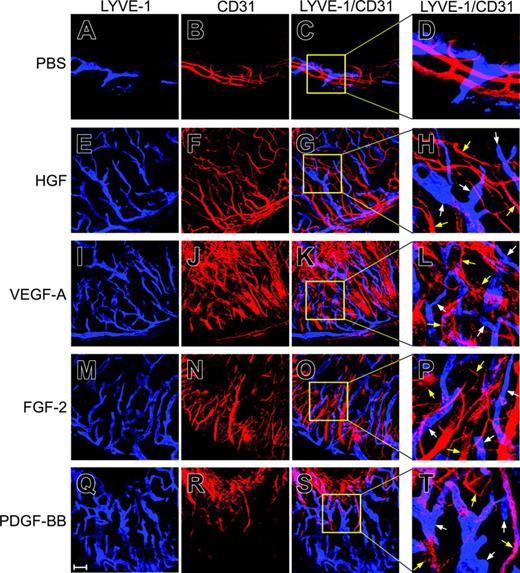
At approximately 2 weeks after pellet implantation, growth factor–implanted corneas were used for immunohistochemical analysis for detection of lymphatic vessels. Although a few cell surface molecules have been localized on LECs, Lyve-1 is generally accepted as a marker specifically expressed on LECs.31 Lyve-1–positive structures as detected with a rabbit anti–mouse Lyve-1 polyclonal antibody were present in all corneas implanted with HGF, VEGF-A, FGF-2, or PDGF-BB (Figure 2E-T). These Lyve-1–positive structures generally exhibited nonoverlapping staining with CD31+ signals and they consisted of larger vascular structures as compared with the newly formed blood vessels. The HGF-induced lymphatic vessels contained relatively well-defined lymphatic vascular networks (Figure 2E-H). Similarly, FGF-2–induced lymphatic vessels also consisted of well-defined vasculatures (Figure 2M-P). Interestingly, although the VEGF-A– and PDGF-BB–stimulated blood vessels were not well separated and rather fused into large vascular plexuses at the leading edge, the lymphatic vessels induced by these 2 factors were almost indistinguishable from those induced by HGF and FGF-2 (Figure 2I-L, Q-T). These data demonstrate that HGF is a novel lymphangiogenic factor.
Stimulation of corneal angiogenesis. HGF (B), VEGF-A (C), FGF-2 (D), or PDGF-BB (E) was implanted into the micropocket of the mouse cornea. At day 5 after implantation, corneal neovascularization was photographed and measured as vessel length (F), clock hour (G), and vascularization area (H). Corneas implanted with phosphate-buffered saline were used as negative controls (A, F-H). Six to 10 corneas were used in each group. Error bars indicate SEM.
Stimulation of corneal angiogenesis. HGF (B), VEGF-A (C), FGF-2 (D), or PDGF-BB (E) was implanted into the micropocket of the mouse cornea. At day 5 after implantation, corneal neovascularization was photographed and measured as vessel length (F), clock hour (G), and vascularization area (H). Corneas implanted with phosphate-buffered saline were used as negative controls (A, F-H). Six to 10 corneas were used in each group. Error bars indicate SEM.
Expression of HGF receptor c-Met on blood vessels but not on lymphatic vessels
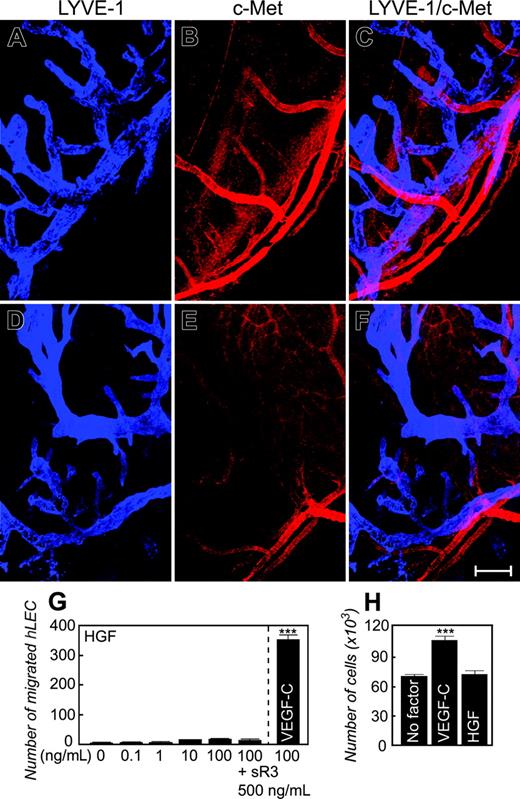
To study whether c-Met is expressed on the newly formed lymphatic vessels, we used an anti–c-Met specific antibody and an anti–Lyve-1 antibody for colocalization. Surprisingly, c-Met protein was only localized to newly formed blood vessels but not to lymphatic vessels (Figure 3A-C). No overlapping staining signals were detected with both antibodies. The distinct expression patterns of c-Met on blood vessels, but not lymphatic vessels, indicate that HGF may stimulate lymphangiogenesis indirectly. Consistent with c-Met expression, isolated LECs lacked HGF-stimulated responses of cell chemotaxis and proliferation (Figure 3G-H). In contrast, LECs significantly responded to VEGF-C in migration and proliferation assays (Figure 3G-H). These data demonstrate that HGF might indirectly induce lymphangiogenesis.
To further study the expression of c-Met in newly formed blood and lymphatic vessels, we performed a well-established inflammatory neovascularization assay in the mouse cornea.30 A single 8-0 silk suture thread through the center of the mouse cornea led to a robust angiogenic response after 14 days of suturing. Similar to growth factor–induced vasculatures, the expression of c-Met protein was only limited to the inflammation-induced blood vessels, and no c-Met positive signals were detected on lymphatic vessels (Figure 3D-F). These findings further support our conclusion that HGF may act as an indirect lymphangiogenic factor.
Soluble VEGFR-3 receptor significantly blocked HGF-induced lymphangiogenesis
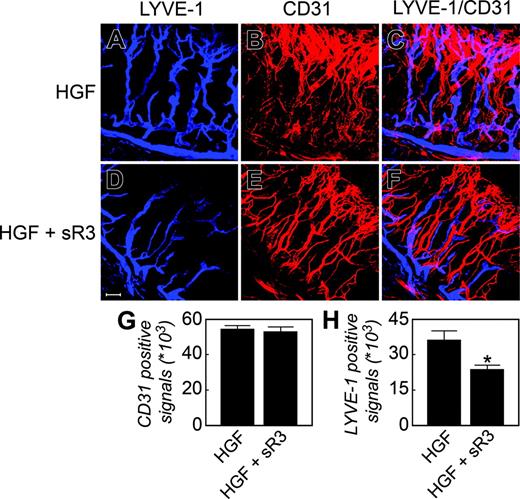
The differential distribution patterns of c-Met on blood and lymphatic vessels suggest that HGF may display its lymphangiogenic activity via other lymphangiogenic factors. Among the known lymphangiogenic factors, VEGF-C and VEGF-D are the most potent and specific growth factors acting directly on LECs.32 The lymphangiogenic activity of VEGF-C and VEGF-D is mediated via VEGFR-3.33 It is also known that VEGF-A– and FGF-2–induced lymphangiogenesis is mediated at least in part via the VEGF-C/-D/VEGFR-3 pathway. To study whether VEGFR-3 antagonists could inhibit HGF-induced lymphangiogenesis, we coimplanted a neutralizing soluble VEGFR-3 receptor (sR-3) with HGF in the mouse corneas. We chose a high dose of sR-3, which is known to effectively block VEGF-C–induced lymphangiogenesis in this mouse cornea.9 Interestingly, the growth of HGF-induced Lyve-1–positive structures was significantly inhibited by sR-3 (Figure 4A-F, H, Lyve-1 staining), although HGF did not significantly induce VEGF-C/-D and PDGF-B expression (Figure S1). In contrast, HGF-stimulated blood vessel growth was not affected by sR-3 (Figure 4A-F, G, CD31 staining). These data indicate that VEGFR-3 signaling is implemented in HGF-induced lymphangiogenesis.
Stimulation of lymphangiogenesis. At day 14 after pellet implantation, corneal tissues were used for whole mount double staining with antibodies against Lyve-1 (A, E, I, M, and Q) and CD31 (B, F, J, N, and R). Lyve-1–positive signals are in blue color and CD31+ signals are in red color. Lyve-1–positive and CD31+ signals in the same areas did not show overlapping staining (C, D, G, H, K, L, O, P, S, and T). Panels D, H, L, P, and T represent enlargement of the boxed areas of panels C, G, K, O, and S, respectively. Bar = 100 μm. White arrows point to Lyve-1–positive structures and yellow arrows point to CD31+ structures. Images were captured with a Zeiss LSM 510 confocal laser-scanning microscope (Carl Zeiss, Jena, Germany) that includes an Axiovert 100M confocal microscope and a camera, and with Image Browser 5 LSM ver3.2 (Carl Zeiss). Images were taken with Plan Neofluar 10 ×/0.3 NA objective lenses. Figure panels were prepared with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Stimulation of lymphangiogenesis. At day 14 after pellet implantation, corneal tissues were used for whole mount double staining with antibodies against Lyve-1 (A, E, I, M, and Q) and CD31 (B, F, J, N, and R). Lyve-1–positive signals are in blue color and CD31+ signals are in red color. Lyve-1–positive and CD31+ signals in the same areas did not show overlapping staining (C, D, G, H, K, L, O, P, S, and T). Panels D, H, L, P, and T represent enlargement of the boxed areas of panels C, G, K, O, and S, respectively. Bar = 100 μm. White arrows point to Lyve-1–positive structures and yellow arrows point to CD31+ structures. Images were captured with a Zeiss LSM 510 confocal laser-scanning microscope (Carl Zeiss, Jena, Germany) that includes an Axiovert 100M confocal microscope and a camera, and with Image Browser 5 LSM ver3.2 (Carl Zeiss). Images were taken with Plan Neofluar 10 ×/0.3 NA objective lenses. Figure panels were prepared with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Expression of c-Met on corneal blood vessels but not lymphatic vessels. A rabbit anti–mouse c-Met specific antibody (B) together with a rat anti–mouse Lyve-1 antibody (A) was used for double staining of corneal tissues with implanted growth factors (A-C) or with suture (D-F). Lyve-1–positive signals are in blue and c-Met–positive signals are in red. No overlapping signals were detected between c-Met and Lyve-1 (C,F). Bar = 100 μm. (G) LEC migration stimulated by various concentrations of HGF or VEGF-C). (H) LEC proliferation stimulated by 100 ng HGF or VEGF-C. Numbers represent averages of 3 determinants for proliferation experiments and 4 determinants for chemotactic experiments (± SEM). Images were captured as described in Figure 2.
Expression of c-Met on corneal blood vessels but not lymphatic vessels. A rabbit anti–mouse c-Met specific antibody (B) together with a rat anti–mouse Lyve-1 antibody (A) was used for double staining of corneal tissues with implanted growth factors (A-C) or with suture (D-F). Lyve-1–positive signals are in blue and c-Met–positive signals are in red. No overlapping signals were detected between c-Met and Lyve-1 (C,F). Bar = 100 μm. (G) LEC migration stimulated by various concentrations of HGF or VEGF-C). (H) LEC proliferation stimulated by 100 ng HGF or VEGF-C. Numbers represent averages of 3 determinants for proliferation experiments and 4 determinants for chemotactic experiments (± SEM). Images were captured as described in Figure 2.
HGF induced tumor hemangiogenesis and lymphangiogenesis
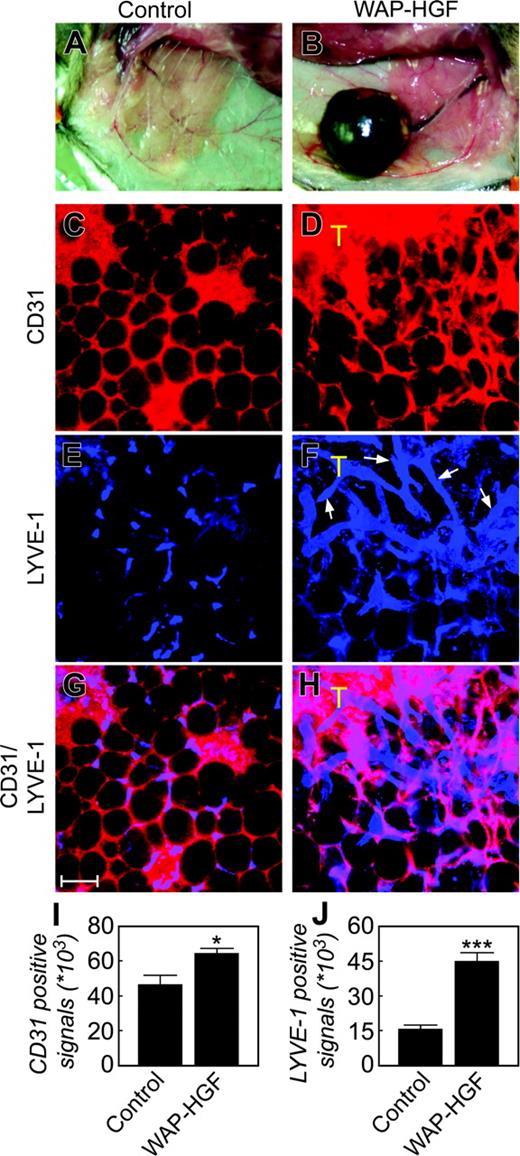
To study the role of HGF in promoting tumor hemangiogenesis and lymphangiogenesis, we have used a transgenic mouse model of HGF-driven mammary tumorigenesis (Figure S2).28 HGF-overexpressing mouse tumor tissues were immunohistochemically double-stained with antibodies against CD31 and Lyve-1. In the healthy mammary adipose tissue (Figure 5A), CD31 staining detected well-organized and uniform honeycomb-like structures, indicating that the microvessels are distributed around each adipocyte (Figure 5C). This type of vasculature was also found in the peritumoral adipose tissue, except that the number of microvessels seemed to be increased in the peritumoral adipose tissue (Figure 5C-D). In the tumor tissue, the CD31+ structures exhibited a disorganized staining pattern because of possible fusion of multiple microvessels into large vascular plexuses with no distinction among individual microvessels (Figure 5D). Similar to blood vessels, the Lyve-1 staining revealed an incomplete honeycomb vascular pattern, suggesting that microlymphatic vessels are also distributed around adipocytes in the healthy mammary glands (Figure 5E). Interestingly, disorganized, large, and relatively tortuous lymphatic vasculatures with high density were detected at the borders of the tumors and were found to occasionally invade the tumor tissue (Figure 5F). As expected, an overlapping staining pattern between CD31+ and Lyve-1–positive signals were present in the healthy mammary adipose tissue because both blood and lymphatic vessel were distributed in the periadipocyte space (Figure 5G). However, nonoverlapping CD31+ and Lyve-1–positive signals were found in the peritumoral areas (Figure 5H). Quantification analysis showed that WAP-HGF tumor tissues contained significantly high numbers of CD31+ and LYVE-1–positive signals as compared with those of healthy control mammary gland tissues (Figure 5I-J). These data demonstrate that HGF produced by tumor cells is able to stimulate peritumoral lymphatic vessel growth.
Discussion
In the present study, we show that HGF acts as a lymphangiogenic factor with an indirect mechanism of action. In a genetic mouse tumor model, overexpression of HGF induces lymphatic vessel growth in the peritumoral region. The role of HGF in lymphatic metastasis needs to be further investigated.
Similar to hemangiogenesis, recent studies show that several known hemangiogenic factors are able to induce lymphangiogenesis in various in vivo models.2,34 In addition to the most well-studied VEGF-C and VEGF-D, VEGF-A, FGF-2, PDGF-BB, Ang-1, Ang-2, IGF-1, and IGF-2 are able to stimulate lymphangiogenesis.7-9,35-40 It appears that these factors either directly or indirectly act on LECs to promote lymphatic vessel growth. For example, VEGF-A– and FGF-2–induced lymphangiogenesis is at least partially mediated by the VEGF-C/-D/VEGFR-3 signaling pathway,35,36,39 although their direct effects cannot be excluded.3,10,38,41 PDGFRs, Tie-2, and IGF receptors have been localized on LECs, and their respective ligands are able to promote cellular activity of isolated LECs.5,7-9,11 These findings demonstrate that PDGFs, IGFs, and Ang-1/Ang-2 act as direct lymphangiogenic factors, although their indirect roles in stimulation of lymphangiogenesis cannot be excluded. Our present study shows that the HGF receptor, c-Met, is not expressed on the newly formed lymphatic vessels in the mouse cornea, indicating that HGF probably indirectly induces lymphangiogenesis. In support of this notion, we have also found that c-Met mRNA is not expressed in isolated LECs, which also lack responses of cell migration and proliferation on stimulation with HGF. Consistent with these findings, a soluble VEGFR-3 significantly blocks the HGF-induced lymphangiogenesis in the mouse cornea. Taken together with previous studies, it seems that the VEGFR-3 signaling system is a common pathway for several other lymphangiogenic factors, including VEGF-A, FGF-2, and HGF. Thus, blockage of the VEGFR-3–mediated signaling pathway is crucial for inhibition of lymphangiogenesis under pathologic conditions such as malignant diseases. Particularly during malignant progression, these angiogenic factors are frequently expressed at high levels.9,35,39,40 In contrast to our data, a very recent study showed that HGF is a direct lymphangiogenic factor in vitro and in vivo.10 Although the discrepancy of this study and our present results remains to be clarified, the in vitro and in vivo lymangiogenic models or the cell assay systems seem to be different. For example, it has been found that c-Met receptor is barely detectable in healthy lymphatic vasculature, but it is highly expressed in the lymphatics of VEGF-A–induced inflammatory mouse model, suggesting that inflammation plays a critical role in mediating HGF-induced lymphangiogenesis. In addition, HGF receptor is also expressed in growing lymphatics during embryonic development and in activated lymphatics of flamed skin during wound healing.10 In the present study, we have chosen a mouse corneal inflammation model to study whether inflammation plays a role in up-regulation of c-Met expression in lymphatic vessels. Interestingly, inflammation-induced corneal lymphatic vessels also lack the expression of c-Met. These studies suggest that c-Met might be differentially expressed in various tissues and HGF may directly or indirectly stimulate lymphangiogenesis depending on the availability of c-Met in lymphatic vessels of various tissues.
Inhibition of HGF-induced corneal lymphangiogenesis by a soluble VEGFR-3. HGF alone (A-C) or HGF/sVEGFR-3-Fc (D-F) implanted corneal tissues were double stained with a rat anti-CD31 antibody (red) and a rabbit anti–LYVE-1 antibody (blue) (A-F). Quantification of CD31+ (G) and LYVE-1–positive (H) signals (n = 7 different optical fields). *P < .05. Scale bar = 100 μm. Images were captured as described in Figure 2.
Inhibition of HGF-induced corneal lymphangiogenesis by a soluble VEGFR-3. HGF alone (A-C) or HGF/sVEGFR-3-Fc (D-F) implanted corneal tissues were double stained with a rat anti-CD31 antibody (red) and a rabbit anti–LYVE-1 antibody (blue) (A-F). Quantification of CD31+ (G) and LYVE-1–positive (H) signals (n = 7 different optical fields). *P < .05. Scale bar = 100 μm. Images were captured as described in Figure 2.
Whole mount staining of healthy mammary glands and mammary tumors. A healthy mammary gland of a WAP-HGF mouse served as a control (A), and a mammary tumor from a WAP-HGF transgenic mouse (B) was used for double staining with rat anti-CD31 (C-D, red) and rabbit anti–Lyve-1 (E-F, blue) antibodies. Panels G and H show CD31 and LYVE-1 double-positive signals of panels C and E, and D and F, respectively. T marks the tumor. Bar = 100 μm. Quantification analysis of CD31+ (I) and LYVE-1–positive (J) signals as μm2. *P < .05; ***P < .001. Error bars indicate SEM. Images in panels C-H were captured as described in Figure 2. Images in panels A-B were captured with a Nikon SMZ-2T microscope and a Nikon FDX-35 camera (Nikon, Tokyo, Japan) with a 15 × objective.
Whole mount staining of healthy mammary glands and mammary tumors. A healthy mammary gland of a WAP-HGF mouse served as a control (A), and a mammary tumor from a WAP-HGF transgenic mouse (B) was used for double staining with rat anti-CD31 (C-D, red) and rabbit anti–Lyve-1 (E-F, blue) antibodies. Panels G and H show CD31 and LYVE-1 double-positive signals of panels C and E, and D and F, respectively. T marks the tumor. Bar = 100 μm. Quantification analysis of CD31+ (I) and LYVE-1–positive (J) signals as μm2. *P < .05; ***P < .001. Error bars indicate SEM. Images in panels C-H were captured as described in Figure 2. Images in panels A-B were captured with a Nikon SMZ-2T microscope and a Nikon FDX-35 camera (Nikon, Tokyo, Japan) with a 15 × objective.
In contrast to lymphatic vessels, c-Met protein is localized on blood vessels in the cornea, pointing to the direct role of HGF in promoting hemangiogenesis. Although the relation between hemangiogenesis and lymphangiogenesis is poorly understood, we may speculate that lymphatic vessels grow after blood vessels, which may cross-communicate with lymphatic endothelium. Another possibility is that the outgrowth of blood vessels will supply inflammatory cells that produce VEGF-C/-D or other cytokines to induce lymphangiogenesis. Indeed, in the case of VEGF-A–induced lymphangiogeneis, infiltration of inflammatory cells is critical for lymphatic vessel growth.36 A similar observation of infiltration of inflammatory cells has also been found in the FGF-induced lymphangiogenesis. However, it seems that blood vessel growth is not a prerequisite for lymphatic vessel growth because under certain circumstances lymphatic vessel can grow in the absence of blood vessels.35
The mouse cornea model provides us a unique opportunity to study lymphangiogenesis induced by various growth factors simply because this organ is avascular for blood and lymphatic vessels. Consistent with previous reports, HGF is sufficiently potent to induce new blood vessel growth in the mouse cornea, although its angiogenic activity is less potent as compared with those of FGF-2 or VEGF-A. In addition to its angiogenic activity, HGF is a known potent growth factor that contributes to invasiveness of variety of different tumors. Thus, the expression levels of HGF and c-Met is tightly linked to tumor metastasis.42-44 High expression levels of HGF in the mammary epithelial cells not only lead to the formation and growth of mammary gland tumors, but also lead to pulmonary metastasis in 20% of female mice.28
We have also observed enlargement of regional lymph nodes in mice bearing mammary gland tumors, although lymphatic metastases in lymph nodes need to be further studied. Transformation of normal epithelial cells to malignant cells by HGF may not necessarily initiate tumor growth unless an angiogenic phenotype is switched on. Indeed, histologic analyses of blood vessels demonstrates a very high density of blood vessels in the HGF-overexpressing tumor tissue, suggesting that HGF also contributes to the switch of angiogenesis in this genetic tumor model. Consistent with findings of corneal lymphangiogenesis, tumor-produced HGF also seems to induce lymphatic vessel growth, although the lymphatic vessels are concentrated in the peritumoral regions. The presence of peritumoral lymphatic vessels has also been correlated with clinical metastasis.14,15
Taken together, our present findings add a new member to the growing list of direct and indirect lymphangiogenic factors. Similar to hemangiogenesis, our and other results demonstrate that lymphangiogenesis is a complex process, which is regulated by multiple growth factors.2 Although the interplay between these lymphangiogenic factors is not understood at the present time, they may collectively contribute to promoting tumor lymphangiogenesis and lymphatic metastasis. Thus, development of therapeutic agents targeting a common pathway of lymphangiogenesis should be considered for the treatment of lymphatic metastasis.
Prepublished online as Blood First Edition Paper, January 19, 2006; DOI 10.1182/blood-2005-06-2538.
Supported by the Swedish Research Council, the Swedish Heart and Lung Foundation, the Swedish Cancer Foundation, the Karolinska Institute Fund, the EU integrated projects of Angiotargeting (contract No. 504743), VascuPlug (contract No. STRP 013811), and the Söderberg Foundation (laboratory of Y.C.).
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Yuan Xue for helping us with some artistic work. We thank Dr David Jackson at the John Radcliffe Hospital, Oxford University, for sharing his anti–Lyve-1 antibody and LECs with us for this study. Troma 1 monoclonal antibody developed by Philippe Brulet and Rolf Kemler was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child and Human Development (NICHD) and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal