Abstract
We have previously demonstrated that plasmin acts as a potent proinflammatory activator of human peripheral monocytes. Here we identify the annexin A2 heterotetramer, composed of annexin A2 and S100A10, as a receptor for the plasmin-induced signaling in human monocytes. Monocytes express the annexin A2 heterotetramer on the cell surface as shown by flow cytometry, fluorescence microscopy, and coimmunoprecipitation of biotinylated cell surface proteins. Binding of plasmin to annexin A2 and S100A10 on monocytes was verified by biotin transfer from plasmin labeled with a trifunctional cross-linker. Antibodies directed against annexin A2 or S100A10 inhibited the chemotaxis elicited by plasmin, but not that induced by fMLP. Further, down-regulation of annexin A2 or S100A10 in monocytes by antisense oligodeoxynucleotides impaired the chemotactic response to plasmin, but not that to fMLP. Antisense oligodeoxynucleotides similarly decreased the TNF-α release by plasmin-stimulated, but not by LPS-stimulated, monocytes. At the molecular level, stimulation with plasmin, but not with catalytically inactivated plasmin, induced cleavage of annexin A2 and dissociation of the heterotetramer complex. Substitution of lysine to alanine in position 27 abolished the cleavage of recombinant annexin A2 in vitro. Together, these data identify the annexin A2 heterotetramer as a signaling receptor activated by plasmin via proteolysis.
Introduction
Most cells are able to bind plasmin(ogen) with low affinity.1-3 Generally, the membrane binding of plasmin(ogen) has been mainly regarded in terms of membrane-associated activation of fibrinolytic or proteolytic activity.4-7 However, plasmin may elicit profound functional changes in various cells implying receptor-mediated signaling. Plasmin induces neutrophil aggregation, biosynthesis of platelet-activating factor in endothelial cells, and Ca2+ signaling in platelets and endothelial cells as well as proliferation of hepatocytes.1,2 In monocytes plasmin triggers release of proinflammatory lipid mediators,8 chemotaxis,9 and expression of different proinflammatory genes such as tumor necrosis factor α (TNF-α), interleukin-1, monocyte chemoattractant protein-1, CD40, and tissue factor through activation of nuclear factor κB (NF-κB),10 JAK/STAT, and p38 MAPK signaling cascades.11 All these effects are critically dependent on the proteolytic activity and cannot be mimicked by either plasminogen or catalytically inactivated plasmin.8-10
Several proteins, such as α-enolase,12,13 annexin A2, and TIP49a on monocytic cell lines,14-16 annexin A2 on endothelial cells, macrophages, and monocytes,16-18 S100A10 in colorectal cancer and fibrosarcoma cells,19,20 gp330 on kidney epithelial cells,21 and several others1 have been identified as plasmin(ogen) binding sites. Occasionally these binding sites have been termed receptors, yet mostly in terms of membrane-associated proteolytic activity without signal transduction function. Protease-activated receptor 1 (PAR1) and PAR4 have been proposed to mediate plasmin signaling in fibroblasts, platelets,22,23 and transfected CHO cells.24 Others proposed that PARs are not activated but rather truncated and inactivated by plasmin.25 Available evidence indicates that on human monocytes the signaling plasmin receptor is distinct from PARs,26 yet its identity remains obscure.
Annexin A2 belongs to the family of calcium-binding proteins; it is found in the cytoplasm and complexed with S100A10 (annexin A2 light chain, p11) as annexin A2 heterotetramer in the membrane.3,7,27 Annexin A2 controls endosomal membrane dynamics including multivesicular endosome biogenesis28 as well as exocytosis in endothelial cells.29 Nuclear annexin A2 appears to inhibit growth of LNCaP cells30 and overexpression of annexin A2 inhibits migration of prostate cancer cells.31 The recently demonstrated binding of annexin A2 to c-myc mRNA implicates a novel regulatory role for this protein.32,33 Cell surface annexin A2 had been shown to facilitate binding of plasminogen to endothelial cells and macrophages.16,18,34 On the other hand, in tumor cells plasminogen binding and activation appear to be dependent primarily on the cell surface expression of S100A10, but not of annexin A2.19,20 S100A10 might also have some additional regulatory functions because it facilitates the sensory neuron-specific sodium channel expression.35
Here we analyzed the role of annexin A2 and S100A10 in the plasmin-mediated activation of human peripheral monocytes and demonstrate that the annexin A2 heterotetramer is not only important for binding of plasmin to monocytes but it is indeed required for the plasmin-induced signaling. We also show that activation of the plasmin receptor involves proteolysis of annexin A2 and dissociation of the annexin A2 heterotetramer. Our data demonstrate that this event initiates the signaling cascade in plasmin-stimulated monocytes leading finally to full-scale proinflammatory activation.
Materials and methods
Materials
Anti-S100A10 and CD47 mouse monoclonal antibodies (mAbs) were from BD Biosciences (San Diego, CA) and RDI (Flanders, NJ), anti-annexin A2 mAbs were from BD Biosciences or Biodesign International (Saco, ME), anti-PAR1 mAbs (WEDE15) and anti-CD41 were from Immunotech (Marseille, France), CD14 mAbs were from BD Biosciences. Control mouse and rabbit IgG and PE-conjugated donkey anti-mouse and anti-rabbit IgG F(ab)2 were from Dianova (Hamburg, Germany). BSA, N-formyl-Met-Leu-Phe (fMLP), lysine-free RPMI 1640, trans-4-(aminomethyl)cyclohexane-1-carboxylic acid (t-AMCA), and lipopolysaccharide (LPS; Escherichia coli serotype 055:B5) were from Sigma (St Louis, MO). Purified human plasmin (lot 328081) was from Fluka (Deisenhofen, Germany). Human plasminogen and D-Val-Phe-Lys chloromethyl ketone (VPLCK) were from Calbiochem (San Diego, CA), human urokinase was from Medac (Hamburg, Germany). The multifunctional cross-linker, sulfosuccinimidyl-2-[6-(biotinamido)-2-(P-azidobenzamido]hexanoamido]ethyl-1,3′-dithiopropionate (sulfo-SBED), sulfosuccinimidyl-2-(biotinamido) ethyl-1,3-dithiopropionate (sulfo-NHS-SS-biotin), and AminoLink coupling gel were from Pierce (Rockford, IL). The plasmin substrate S-2251 (H-d-valyl-leucyl-l-lysine-P-nitroanilide dihydrochloride) was from Chromogenix (Milan, Italy). Tissue culture media were from Invitrogen (Carlsbad, CA). Other chemicals were of analytic grade.
Cell preparations
Monocytes and neutrophils were isolated by autologous plasma-Percoll gradient centrifugation as described9,10,26 ; preparations with at least 94% CD14+ cells were used. Contaminating cells were lymphocytes. Flow cytometric analysis (FACScan, BD Biosciences, San Jose, CA) with anti-CD41 mAb did not reveal any platelets associated with monocytes.
Immunoprecipitation
Freshly isolated human monocytes (7 × 106 cells/assay) were lysed at 4°C in 1000 μL buffer containing 20 mM HEPES, pH 7.5, 150 mM NaCl, 1.5 mM CaCl2, 0.5% NP-40, 10% glycerol, and mammalian protease and phosphatase inhibitor cocktails (Calbiochem). Proteins were immunoprecipitated with 2.5 μg anti-S100A10 antibodies and 40 μL protein A/G agarose (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C with constant rotation. After extensive washing with lysis buffer, immunoprecipitated proteins were solubilized in sample loading buffer and resolved by 15% Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).11
Cross-linking experiments
To prevent the proteolytic autodegradation of plasmin, the cross-linker was synthesized in 2 steps. First, plasminogen (2 mg) was conjugated with a 6-fold molar excess of sulfo-SBED cross-linker reagent in PBS for 30 minutes at 20°C. The plasminogen-cross-linker conjugate was purified using a PD-10 column (Amersham Pharmacia Biotech, Freiburg, Germany). Fractions, containing the plasminogen cross-linker, were detected by protein assay and by blotting using streptavidin-horseradish peroxidase (HRP; Calbiochem). The plasminogen cross-linker was activated using immobilized urokinase. Human urokinase (1 mg) was coupled to agarose beads using AminoLink coupling gel. Formation of plasmin was monitored using the chromogenic substrate S-2251.9 The optimal conditions for the plasminogen activation were 5 minutes at 37°C. Urokinase was removed by centrifugation, and monocytes (20 × 106 cells/200 μL medium) were treated with freshly generated plasmin cross-linker (equivalent to a final concentration of 0.143 CTA U/mL) for 7 minutes at 37°C. The monocytes were then exposed to UV light 0.04 J/cm2 to activate the cross-linker. The cells were washed and lysed with buffer containing 1% Triton X-100.11 The biotin-labeled proteins were precipitated with streptavidin-agarose beads (Sigma) and extensively washed. The disulfide bond between plasmin and the binding proteins was cleaved by reduction in the sample loading buffer leaving the receptor proteins solely biotin-tagged. The proteins were finally separated by SDS-PAGE and identified with avidin-HRP or specific mAbs against annexin A2 or S100A10.
Analysis of protein expression
For Western blot analysis 2 × 106 monocytes/sample were lysed and resolved by 15% Tricine-SDS-PAGE.11 Gels were blotted onto PVDF membranes and immunostained for annexin A2 and S100A10 (BD Biosciences). The same membranes were subsequently stained with mAbs against actin (Chemicon, Temecula, CA) to ensure equal loading.
Cell surface proteins were biotinylated for 30 minutes at 4°C using sulfo-NHS-SS-biotin (0.25 mg/mL). Cells were lysed and the biotinylated proteins were recovered by precipitation with immobilized neutravidin. After extensive washing, the precipitated proteins were solubilized in lysis buffer containing 50 mM DTT for 1 hour at 20°C. The proteins were then immunoprecipitated with anti-S100A10 mAbs and protein A/G agarose, and analyzed by immunoblotting for annexin A2 and S100A10.
For flow cytometry, cells were incubated with 5 μg/mL rabbit antibodies against annexin A2 (Biodesign) or mAb against S100A10 (RDI), or control rabbit or mouse IgG (Dianova) at 4°C for 1 hour. After washing, the cells were incubated with PE-conjugated anti-mouse or anti-rabbit IgG F(ab)2 (1:100) for 45 minutes at 4°C. After final washing, the cells were analyzed using a FACScan (BD Biosciences, San Jose, CA).
Immunofluorescence microscopy
Monocytes (2 × 106) resuspended in 800 μL RPMI were permitted to adhere on chamber slides (Discovery Labware, Bedford, MA) for 30 minutes; then they were fixed with paraformaldehyde and stained with Hoechst 33342 (DNA marker), rabbit anti-annexin A2 and mouse anti-S100A10 antibodies. The antibodies were visualized with anti-mouse rhodamine red-labeled IgG F(ab)2 and anti-rabbit FITC-labeled IgG F(ab)2 and analyzed using a Nikon Diaphot fluorescence microscope and a Nikon 100 ×/1.30 numeric aperture oil objective (Nikon, Düsseldorf, Germany). Images were acquired using an ORCA.ER camera (Hamamatsu Photonics, Hamamatsu, Japan), SimplePCI version 5.2 acquisition software (Compix, Imaging Systems, Cranberry Township, PA) and Adobe Photoshop (Adobe Systems, San Jose, CA).
Antisense experiments
For in vitro knockdown of annexin A2 and S100A10, phosphorothioate-modified oligodeoxynucleotides (ODNs; ThermoHybaid, Ulm, Germany) were used. The ODNs were selected on the basis of the major predicted secondary structures.36 The antisense ODNs used to down-regulate the annexin A2 expression correspond to the nucleotides 380-401 of annexin A2 mRNA (Genbank accession no. NM_004039): 5′-CTTTTAGCTCAGAAGCGTCATA-3′. The antisense ODNs used against S100A10 target the nucleotides 94-112 of S100A10 mRNA (Genbank accession no. BC015973): 5′-GAGATGGCATTTTGGTGTG-3′. The sense sequences served as control ODNs. The selected sequences for either annexin A2 or S100A10 did not show any similarity to any other mRNA sequence.
Monocytes were treated for 48 hours with 10 μM of the specific sense or antisense ODN in DMEM supplemented with 10% FCS. After treatment, cells were allowed to recover for 12 hours in RPMI 1640 without FCS. For expression of TNF-α, 5 × 105 cells in 100 μL were stimulated with either 100 ng/mL LPS in the presence of 1% human AB serum or 0.43 CTA U/mL plasmin for 4 hours. TNF-α was analyzed by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).9,10,37
Migration assays
Cell migration was evaluated using 24-well Transwell plates (Costar, Cambridge, MA) with polycarbonate membranes (pore size 5 μm). Monocytes were suspended in HBSS containing 0.4% BSA at a concentration of 2 × 106 cells/mL, and 100 μL was added to the upper compartment of each well.9,38 For the experiments with inhibitory antibodies, monocytes were pretreated with antibodies (10 μg/mL), which were added to the upper and lower compartments 20 minutes before addition of the respective chemoattractant. PAR1-blocking mAb WEDE15 was used at a concentration of 50 μg/mL.26
Analysis of the plasmin-mediated cleavage of annexin A2
The cDNA encoding annexin A2 was generated by reverse transcription-polymerase chain reaction (RT-PCR) amplification using primers 5′-ATGTCTACTGTTCACGAAATCC-3′ (sense) and 5′-TCAGTCATCTCCACCACACAG-3′ (antisense) and subcloned into pcDNA 3.1 expression vector (Invitrogen). The annexin A2 mutant K27A was generated by insertion of point mutations into the cDNA using the QuickChange mutagenesis kit (Stratagene, La Jolla, CA). All sequences were checked by sequencing using an ABI 310 automated sequencer (Applied Biosystems, Foster City, CA). Annexin A2 wild type (WT) or mutant (K27A) was translated in vitro in the presence of 35S-methionine with a TNT T7 quick-coupled transcription/translation system (Promega, Madison, WI). The proteins were incubated with increasing concentrations of plasmin for 10 minutes at 37°C and resolved by 15% Tricine-SDS-PAGE; bands were visualized using a PhosphoImager (Molecular Dynamics, Sunnyvale, CA). For the in vivo experiments including the immunoprecipitation, 2 × 106 monocytes were stimulated with 0.43 CTA U/mL plasmin or equivalent amounts of plasmin catalytically inactivated by 25 μM VPLCK (VPLCK-plasmin)10 for 30 minutes at 37°C. The cell lysates were resolved on 15% Tricine-SDS-PAGE and analyzed by immunoblotting.
Statistical analysis
Values shown represent mean ± SEM. Statistical significances were calculated with the Newman-Keuls test. Differences were considered significant for P values less than .05.
Results
Monocytes, but not neutrophils, express the annexin A2 heterotetramer
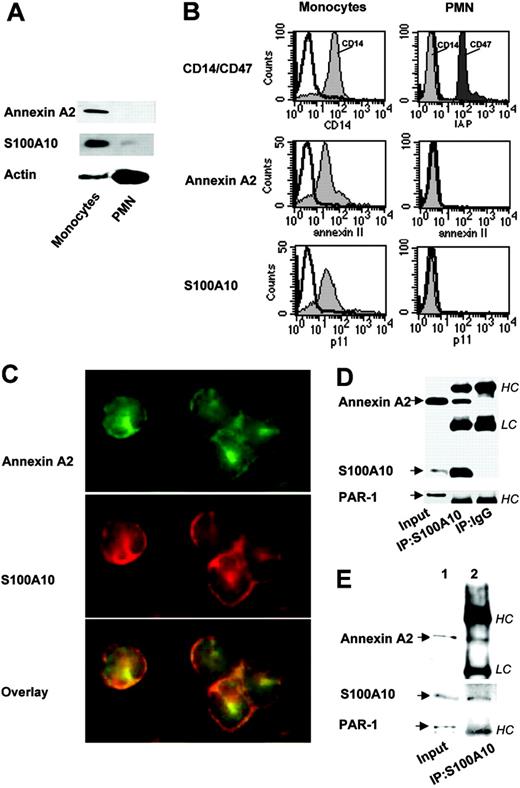
To assess the role of the annexin A2 heterotetramer in the plasmin-induced monocyte activation, we analyzed the expression of annexin A2 and S100A10 in monocytes. We had previously demonstrated that plasmin triggers a true chemotaxis in monocytes but not in neutrophils.9 Consistent with this observation, immunoblotting of monocyte cell lysates showed both annexin A2 and S100A10 (Figure 1A), whereas neutrophils expressed S100A10 only faintly and annexin A2 not at all (Figure 1A). Flow cytometric analysis confirmed that both annexin A2 and S100A10 are expressed on the monocyte but not on the neutrophil membrane (Figure 1B).
Immunofluorescence microscopy of nonpermeabilized cells showed that annexin A2 and S100A10 are colocalized on the monocyte membrane (Figure 1C). The physical interaction between annexin A2 and S100A10 was confirmed by antibodies directed against S100A10, which coimmunoprecipitated annexin A2 indicating that in monocytes both proteins form the annexin A2 heterotetramer (Figure 1D).
Biotinylation of the cell surface proteins with a sequential 2-step precipitation was used to identify formation of the annexin A2 heterotetramer at the monocyte membrane. Here we combined the selective detection of biotinylated surface proteins with coimmunoprecipitation. These data demonstrate that S100A10 located at the monocyte membrane coimmunoprecipitated with annexin A2 indicating formation of the annexin A2 heterotetramer at the monocyte surface (Figure 1E).
Human peripheral monocytes but not neutrophils (polymorphonuclear cells) express the annexin A2 heterotetramer. (A) Western blotting. Monocyte and neutrophil cell (PMN) lysates were separated by SDS-PAGE and analyzed by immunoblotting with antibodies against annexin A2, S100A10, and α-actin as control. (B) Flow cytometry. Cells were stained with control rabbit or mouse IgG (open histograms), annexin A2 and S100A10 antibodies (filled histograms). Primary antibodies were visualized by PE-conjugated IgG F(ab)2 and cells were analyzed by FACScan. CD14 and CD47 served as controls. (C) Immunofluorescence microscopy. Monocytes were fixed and stained with mouse antibody against S100A10 followed by anti-mouse rhodamine red-labeled IgG F(ab)2, and rabbit anti-annexin A2 antibody followed by anti-rabbit FITC-labeled IgG F(ab)2. Cells were analyzed by fluorescence microscopy. Overlay picture shows colocalization of annexin A2 and S100A10. Immunostaining for annexin A2 and S100A10 was not imitated by antibodies against p38 MAPK or actin (data not shown). (D) Coimmunoprecipitation. S100A10 and annexin A2 were immunoprecipitated from monocyte lysates using anti-S100A10 antibodies and protein A/G agarose. Immunoprecipitates were analyzed by immunoblotting using mAbs against annexin A2 and S100A10. Input is the loading control, that is, cell lysate. IgG indicates negative control; IP, immunoprecipitation. HC and LC indicate IgG heavy and light chain, respectively. PAR1 immunostaining served as control. (E) Localization of the annexin A2 heterotetramer at the monocyte surface. Monocytes were surface biotinylated and lysed. The biotinylated proteins were precipitated with immobilized neutravidin and the cross-linker was removed by DTT. From these recovered surface proteins, annexin A2 and S100A10 were coimmunoprecipitated using anti-S100A10 mAbs and analyzed by Western blotting. Loading control (input, lane 1) and the immunoprecipitate (lane 2) were stained using mAbs against annexin A2 and S100A10. PAR1 immunostaining was used as control. IP indicates immunoprecipitation. All results are representative of at least 3 experiments.
Human peripheral monocytes but not neutrophils (polymorphonuclear cells) express the annexin A2 heterotetramer. (A) Western blotting. Monocyte and neutrophil cell (PMN) lysates were separated by SDS-PAGE and analyzed by immunoblotting with antibodies against annexin A2, S100A10, and α-actin as control. (B) Flow cytometry. Cells were stained with control rabbit or mouse IgG (open histograms), annexin A2 and S100A10 antibodies (filled histograms). Primary antibodies were visualized by PE-conjugated IgG F(ab)2 and cells were analyzed by FACScan. CD14 and CD47 served as controls. (C) Immunofluorescence microscopy. Monocytes were fixed and stained with mouse antibody against S100A10 followed by anti-mouse rhodamine red-labeled IgG F(ab)2, and rabbit anti-annexin A2 antibody followed by anti-rabbit FITC-labeled IgG F(ab)2. Cells were analyzed by fluorescence microscopy. Overlay picture shows colocalization of annexin A2 and S100A10. Immunostaining for annexin A2 and S100A10 was not imitated by antibodies against p38 MAPK or actin (data not shown). (D) Coimmunoprecipitation. S100A10 and annexin A2 were immunoprecipitated from monocyte lysates using anti-S100A10 antibodies and protein A/G agarose. Immunoprecipitates were analyzed by immunoblotting using mAbs against annexin A2 and S100A10. Input is the loading control, that is, cell lysate. IgG indicates negative control; IP, immunoprecipitation. HC and LC indicate IgG heavy and light chain, respectively. PAR1 immunostaining served as control. (E) Localization of the annexin A2 heterotetramer at the monocyte surface. Monocytes were surface biotinylated and lysed. The biotinylated proteins were precipitated with immobilized neutravidin and the cross-linker was removed by DTT. From these recovered surface proteins, annexin A2 and S100A10 were coimmunoprecipitated using anti-S100A10 mAbs and analyzed by Western blotting. Loading control (input, lane 1) and the immunoprecipitate (lane 2) were stained using mAbs against annexin A2 and S100A10. PAR1 immunostaining was used as control. IP indicates immunoprecipitation. All results are representative of at least 3 experiments.
Plasmin binds to the annexin A2 heterotetramer on the monocyte membrane
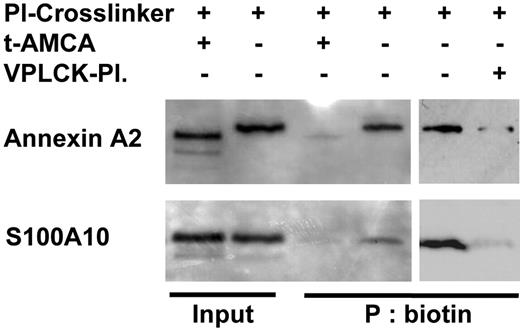
In vitro data suggest that plasmin can bind both phospholipid-associated annexin A2 as well as S100A10.17 We therefore tested whether such an interaction occurs also under in vivo conditions. We synthesized a bifunctional photo-activatable plasmin cross-linker and analyzed the transfer of the biotin tag from the cross-linker to the interacting proteins. After incubation of the monocytes with the plasmin cross-linker, the cells were lysed, and the biotinylated proteins were precipitated with streptavidin and analyzed by Western blotting. Under the conditions used, staining of the membranes with avidin-HRP showed 2 major bands of about 36 and 11 kDa (data not shown). Immunostaining of annexin A2 and S100A10 demonstrated that the biotinylated proteins are, indeed, annexin A2 and S100A10 (Figure 2). Thus, plasmin interacts in vivo with both annexin A2 and S100A10. As expected,8-10 the lysine analog t-AMCA (2 mM) and excess of catalytically inactivated plasmin inhibited binding of plasmin to annexin A2 and S100A10 and the subsequent biotin transfer (Figure 2).
Plasmin binds annexin A2 and S100A10 expressed on the monocyte surface. Monocytes (20 × 106) were exposed to plasmin cross-linker 0.143 CTA U/mL in the presence or absence of the inhibitor of plasmin binding, t-AMCA 2 mM, for 7 minutes. The cross-linker was activated by UV light, and after solubilization, the biotinylated proteins were precipitated using streptavidin beads. Precipitated proteins were analyzed by immunoblotting with mAbs against annexin A2 and S100A10. VPLCK-plasmin indicates catalytically inactivated plasmin generated by pretreatment with 25 μM VPLCK; a 35-fold molar excess of VPLCK-plasmin was used. Results of one of 3 independent experiments are shown.
Plasmin binds annexin A2 and S100A10 expressed on the monocyte surface. Monocytes (20 × 106) were exposed to plasmin cross-linker 0.143 CTA U/mL in the presence or absence of the inhibitor of plasmin binding, t-AMCA 2 mM, for 7 minutes. The cross-linker was activated by UV light, and after solubilization, the biotinylated proteins were precipitated using streptavidin beads. Precipitated proteins were analyzed by immunoblotting with mAbs against annexin A2 and S100A10. VPLCK-plasmin indicates catalytically inactivated plasmin generated by pretreatment with 25 μM VPLCK; a 35-fold molar excess of VPLCK-plasmin was used. Results of one of 3 independent experiments are shown.
Inhibition of plasmin-induced chemotaxis by neutralizing antibodies
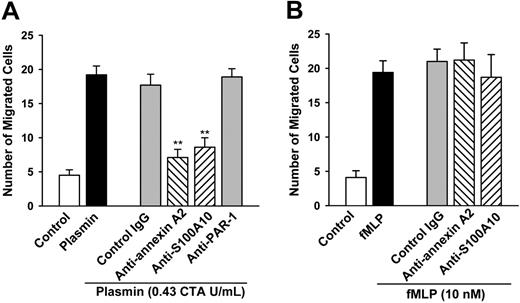
To characterize the functional role of annexin A2 and S100A10 in plasmin-induced signaling, we analyzed the monocyte chemotaxis toward 0.43 CTA U/mL plasmin or 10 nM fMLP in the absence and presence of antibodies directed against annexin A2 or S100A10. Pretreatment of monocytes with plasmin for 30 minutes prior to chemotaxis led to desensitization (14.2% ± 3.3% of positive controls, n = 3). The monocyte chemotaxis induced by plasmin remained unaffected by preincubation with 10 μg/mL unspecific IgG (Figure 3). However, the cell migration was significantly reduced by preincubation of the monocytes with 10 μg/mL specific antibodies against annexin A2 and S100A10 by 59.9% ± 6.8% and by 51.4% ± 7.9%, respectively (Figure 3A).
Recent data from CHO cells cotransfected with α9β1 and PAR1 suggested that PAR1 might mediate the plasmin-induced CHO cell migration.24 However, WEDE15, a mAb that blocks PAR1 signaling,26 had no effect on the plasmin-induced migration of monocytes suggesting that PAR1 is not involved in the plasmin-mediated signaling in human monocytes (Figure 3A).
We also investigated whether various integrins might be involved in the plasmin-induced monocyte migration. However, none of the neutralizing mAbs (12.5 μg/mL) directed against α1, αv, αL, αM, β1, β2, β3, and αvβ3 (Chemicon, BD PharMingen, San Diego, CA) had any significant effect on the plasmin-mediated monocyte chemotaxis (data not shown) suggesting that plasmin does not use any of those integrins for chemotactic signaling in human monocytes.
In contrast to the plasmin-induced monocyte chemotaxis, the antibodies directed against annexin A2 or S100A10 did not impair the migration of monocytes to 10 nM fMLP (Figure 3B). These data suggest that both annexin A2 and S100A10 play a specific functional role in the plasmin-induced chemotactic signaling of monocytes.
Inhibition of the annexin A2 and S100A10 expression affects the plasmin-induced monocyte activation
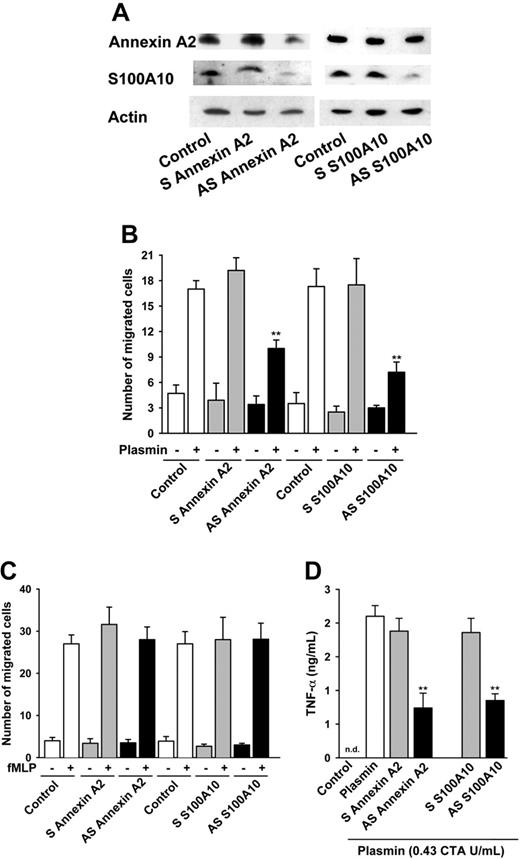
To gain further evidence for a role of the annexin A2 heterotetramer in the plasmin-induced signaling in monocytes, we abrogated expression of annexin A2 and S100A10 by antisense ODNs. A resting period of 12 hours allowed recovery from the cellular stress caused by the ODN exposure. Western blot analysis of annexin A2 and S100A10 demonstrated that 60 hours after antisense ODN pretreatment the expression levels of annexin A2 and S100A10 were significantly reduced (Figure 4A). Consistent with the notion that annexin A2 stabilizes S100A10,17,39 down-regulation of annexin A2 concomitantly decreased the expression of S100A10. By contrast, reduction of the S100A10 expression did not affect the expression of annexin A2 (Figure 4A).
We analyzed the effects of down-regulation of annexin A2 or S100A10 expression on the plasmin-induced chemotaxis. Neither sense nor antisense ODN pretreatment significantly affected the nondirectional cell migration, that is, chemokinesis (Figure 4B). Also pretreatment with annexin A2 or S100A10 sense ODNs had no effect on the plasmin-induced monocyte migration (Figure 4B). However, cells pretreated with antisense ODN against annexin A2 exhibited a significant reduction of the chemotaxis toward 0.43 CTA U/mL plasmin compared with cells pretreated with sense ODN (Figure 4B), whereas no significant change was observed when cells were stimulated with 10 nM fMLP (Figure 4C). Similarly, monocytes pretreated with antisense ODN against S100A10 exhibited reduction of chemotaxis toward plasmin compared to cells pretreated with sense ODN (Figure 4B), and again the chemotaxis induced by 10 nM fMLP remained unaffected (Figures 4C).
In human monocytes plasmin stimulates multiple signaling pathways,8,9,11 including activation of the transcription factor NF-κB leading to TNF-α production.10 Therefore, we tested the effects of down-regulation of the expression of annexin A2 or S100A10 on the plasmin-induced expression of TNF-α. Pretreatment with ODN did not elicit any detectable TNF-α release by monocytes (data not shown). As expected, 0.43 CTA U/mL plasmin triggered TNF-α release, which was not significantly affected by pretreatment of the cells with sense ODN (Figure 4D). The release of TNF-α was, however, significantly impaired by pretreatment with antisense ODN to either annexin A2 or S100A10 (Figure 4D). By contrast, the TNF-α release induced by LPS (100 ng/mL) remained unaffected (data not shown).
The mAbs directed against annexin A2 and S100A10 inhibit the plasmin-induced monocyte chemotaxis. (A) Effects of mAbs on the plasmin-induced monocyte chemotaxis. (B) Effects of mAbs on the fMLP-induced monocyte chemotaxis. Monocytes, nontreated (control, plasmin, and fMLP) or pretreated for 20 minutes with either mouse IgG or anti-annexin A2, anti-S100A10 (each at 10 μg/mL), or anti-PAR1 (WEDE15, 50 μg/mL) antibodies. Monocytes were allowed to migrate across a polycarbonate membrane (pore size, 5 μm) toward 0.43 CTA U/mL plasmin or 10 nM fMLP for 90 minutes. Cells on the membranes were fixed, stained, and counted microscopically. Data are presented as number of migrated cells per high-power oil immersion field (× 1000). **P < .01 versus IgG control. Results are the mean ± SEM of 5 independent experiments each.
The mAbs directed against annexin A2 and S100A10 inhibit the plasmin-induced monocyte chemotaxis. (A) Effects of mAbs on the plasmin-induced monocyte chemotaxis. (B) Effects of mAbs on the fMLP-induced monocyte chemotaxis. Monocytes, nontreated (control, plasmin, and fMLP) or pretreated for 20 minutes with either mouse IgG or anti-annexin A2, anti-S100A10 (each at 10 μg/mL), or anti-PAR1 (WEDE15, 50 μg/mL) antibodies. Monocytes were allowed to migrate across a polycarbonate membrane (pore size, 5 μm) toward 0.43 CTA U/mL plasmin or 10 nM fMLP for 90 minutes. Cells on the membranes were fixed, stained, and counted microscopically. Data are presented as number of migrated cells per high-power oil immersion field (× 1000). **P < .01 versus IgG control. Results are the mean ± SEM of 5 independent experiments each.
Down-regulation of the annexin A2 and S100A10 expression inhibits the plasmin-induced monocyte chemotaxis and TNF-α release. (A) Antisense ODNs down-regulate the expression of annexin A2 and S100A10. After 48 hours of pretreatment with either sense (S) or antisense (AS) ODNs followed by a 12-hour recovery period monocyte cell lysates were resolved on 15% Tricine-SDS-PAGE and immunoblotted with mAbs against annexin A2 and S100A10. Actin served as loading control. (B) Effects of the ODN pretreatment on the plasmin-induced monocyte chemotaxis. Monocytes were pretreated for 48 hours with sense or antisense ODNs directed against either annexin A2 or S100A10, followed by a 12-hour recovery period. Monocytes were allowed to migrate across polycarbonate membranes (pore size, 5 μm) toward 0.43 CTA U/mL plasmin for 90 minutes. **P < .01 versus sense controls. (C) Effects of the ODN pretreatment on the fMLP-induced monocyte chemotaxis. Monocytes were allowed to migrate across polycarbonate membranes (pore size, 5 μm) toward 10 nM fMLP for 90 minutes. Chemotaxis data are presented as number of migrated cells per high-power oil immersion field (× 1000). Results are mean ± SEM of 4 independent experiments each. (D) Effects of the ODN treatment on the plasmin-induced TNF-α release by monocytes as analyzed by ELISA. Monocytes were incubated for 4 hours with 0.43 CTA U/mL plasmin; nd indicates not detectable. **P < .01, antisense versus sense. Results are the mean ± SEM of 5 independent experiments.
Down-regulation of the annexin A2 and S100A10 expression inhibits the plasmin-induced monocyte chemotaxis and TNF-α release. (A) Antisense ODNs down-regulate the expression of annexin A2 and S100A10. After 48 hours of pretreatment with either sense (S) or antisense (AS) ODNs followed by a 12-hour recovery period monocyte cell lysates were resolved on 15% Tricine-SDS-PAGE and immunoblotted with mAbs against annexin A2 and S100A10. Actin served as loading control. (B) Effects of the ODN pretreatment on the plasmin-induced monocyte chemotaxis. Monocytes were pretreated for 48 hours with sense or antisense ODNs directed against either annexin A2 or S100A10, followed by a 12-hour recovery period. Monocytes were allowed to migrate across polycarbonate membranes (pore size, 5 μm) toward 0.43 CTA U/mL plasmin for 90 minutes. **P < .01 versus sense controls. (C) Effects of the ODN pretreatment on the fMLP-induced monocyte chemotaxis. Monocytes were allowed to migrate across polycarbonate membranes (pore size, 5 μm) toward 10 nM fMLP for 90 minutes. Chemotaxis data are presented as number of migrated cells per high-power oil immersion field (× 1000). Results are mean ± SEM of 4 independent experiments each. (D) Effects of the ODN treatment on the plasmin-induced TNF-α release by monocytes as analyzed by ELISA. Monocytes were incubated for 4 hours with 0.43 CTA U/mL plasmin; nd indicates not detectable. **P < .01, antisense versus sense. Results are the mean ± SEM of 5 independent experiments.
These data demonstrate that the plasmin-induced signaling, leading either to chemotaxis or to TNF-α expression, is critically dependent on the expression of annexin A2 and S100A10.
Plasmin induces cleavage of annexin A2
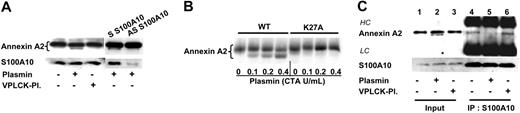
We have previously demonstrated that plasmin-induced signaling requires an intact catalytic center and that inactivated plasmin abrogates the plasmin-induced monocyte activation.8-10 In addition, annexin A2 is a substrate for calpain40,41 and can be cleaved in vitro by plasmin.42 We therefore investigated whether plasmin is able to cleave annexin A2 on the monocyte membrane. Indeed, incubation of monocytes with 0.43 CTA U/mL plasmin led to generation of an annexin A2 fragment of about 33 to 34 kDa (Figure 5A). As expected, catalytically blocked plasmin was unable to generate the cleaved annexin A2 fragment. S100A10 targets annexin A2 to the plasma membrane27,54 ; consequently, its down-regulation by antisense ODN also leads to significant reduction of the annexin A2 fragment (Figure 5A). Thus, treatment of monocytes with plasmin leads to cleavage of annexin A2.
Annexin A2 is targeted by calpain and plasmin through lysine in position 27.40-42 To identify the possible cleavage site, we created a mutant form of annexin A2 (annexin A2 K27A) by substitution of lysine in position 27 by alanine. Radiolabeled annexin A2 wild-type (WT) or mutated (K27A) proteins were incubated with increasing concentrations of plasmin. Analysis by SDS-PAGE revealed that, whereas plasmin at a concentration as low as 0.1 CTA U/mL cleaved WT annexin A2, no cleavage of annexin A2 K27A was detected, not even with the highest concentration of 0.43 CTA U/mL plasmin (Figure 5B). Thus, plasmin cleaves annexin A2 at a single site at lysine 27 leading to a loss of the first 27 amino acids of the N-terminus of annexin A2.
Proteolytic cleavage of annexin A2 by plasmin induces dissociation of the annexin A2 heterotetramer
Protein-protein interaction studies have identified the first 14 amino acids of the N-terminal domain of annexin A2 as binding domain for S100A10.43,44 The proteolysis of annexin A2 at position 27 might therefore lead to dissociation of the heterotetramer complex. To address this question, we investigated whether plasmin modulates the physical interaction between annexin A2 and S100A10. The monocyte cell lysates were subjected to immunoprecipitation using S100A10 mAbs and the coimmunoprecipitated proteins were identified by immunostaining. As expected, mAbs against S100A10 coimmunoprecipitated annexin A2 confirming that both proteins form the heterotetramer (Figure 5C, lane 4). However, when monocytes were pretreated for 30 minutes with plasmin, the coimmunoprecipitation of annexin A2 with anti-S100A10 antibodies was significantly inhibited indicating dissociation of the complex (Figure 5C, lane 5). In contrast, no dissociation of the annexin A2 heterotetramer complex was observed with equivalent amounts of catalytically blocked plasmin (Figure 5C, lane 6). Thus, treatment of monocytes with plasmin triggers proteolysis of annexin A2 and subsequent dissociation of the annexin A2 heterotetramer.
Discussion
Most data concerning plasmin binding to the membrane of various cells have been interpreted in terms of pericellular fibrinolysis.4-7,17,45,46 However, recent data demonstrating activation of kinase cascades and a number of transcription factors pointed to plasmin-mediated activation in various cell types that could only be explained on the basis of a plasmin-induced receptor signaling.1,2,10,11,22-24 Yet, despite considerable efforts, little progress has been made in the identification of a signaling plasmin receptor.
Plasmin cleaves annexin A2 and disrupts the annexin A2 heterotetramer. (A) Plasmin cleaves annexin A2 on the monocyte membrane. For the in vivo studies, 2 × 106 monocytes were incubated for 30 minutes at 37°C in RPMI 1640 without lysine in absence or presence of 0.43 CTA U/mL plasmin or equivalent amounts of plasmin catalytically inactivated by pretreatment with 25 μM VPLCK. The reaction was stopped by the addition of aprotinin (6000 KI/mL), and proteins were separated on 15% SDS-PAGE and visualized by immunoblotting using mAbs against annexin A2 and S100A10. Antisense ODN treatment was performed as described for Figure 4A. (B) Plasmin cleaves annexin A2 at lysine 27. Wild-type (WT) and K27A annexin A2 (Lys27Ala) proteins were translated in vitro in the presence of 35S-methionine and were subsequently incubated for 10 minutes at 37°C in the absence or presence of 0.43 CTA U/mL plasmin. After addition of protease inhibitor, the proteins were separated on 15% SDS-PAGE and the bands were visualized by PhosphoImager. (C) Plasmin induces dissociation of the annexin A2 heterotetramer complex. Monocytes, either unstimulated (lanes 1 and 4) or stimulated for 30 minutes either with 0.43 CTA U/mL plasmin (lanes 2 and 5) or equivalent amounts of catalytically inactivated plasmin (lanes 3 and 6) were lysed and S100A10 was immunoprecipitated (IP). Proteins coimmunoprecipitating with S100A10 were resolved by Tricine-SDS-PAGE and analyzed using mAbs against annexin A2 and S100A10. All data are representative of at least 3 independent experiments.
Plasmin cleaves annexin A2 and disrupts the annexin A2 heterotetramer. (A) Plasmin cleaves annexin A2 on the monocyte membrane. For the in vivo studies, 2 × 106 monocytes were incubated for 30 minutes at 37°C in RPMI 1640 without lysine in absence or presence of 0.43 CTA U/mL plasmin or equivalent amounts of plasmin catalytically inactivated by pretreatment with 25 μM VPLCK. The reaction was stopped by the addition of aprotinin (6000 KI/mL), and proteins were separated on 15% SDS-PAGE and visualized by immunoblotting using mAbs against annexin A2 and S100A10. Antisense ODN treatment was performed as described for Figure 4A. (B) Plasmin cleaves annexin A2 at lysine 27. Wild-type (WT) and K27A annexin A2 (Lys27Ala) proteins were translated in vitro in the presence of 35S-methionine and were subsequently incubated for 10 minutes at 37°C in the absence or presence of 0.43 CTA U/mL plasmin. After addition of protease inhibitor, the proteins were separated on 15% SDS-PAGE and the bands were visualized by PhosphoImager. (C) Plasmin induces dissociation of the annexin A2 heterotetramer complex. Monocytes, either unstimulated (lanes 1 and 4) or stimulated for 30 minutes either with 0.43 CTA U/mL plasmin (lanes 2 and 5) or equivalent amounts of catalytically inactivated plasmin (lanes 3 and 6) were lysed and S100A10 was immunoprecipitated (IP). Proteins coimmunoprecipitating with S100A10 were resolved by Tricine-SDS-PAGE and analyzed using mAbs against annexin A2 and S100A10. All data are representative of at least 3 independent experiments.
A common theme in plasmin-induced signaling is the necessity of an intact catalytic center of plasmin and, thus, uncompromised proteolytic activity.8-10 For this reason a family of proteolytically activated receptors, the PARs, have been repeatedly discussed as putative plasmin receptors. In fibroblasts the plasmin-induced CYR61 gene expression seems to proceed via cleavage of PAR1 and rapid and transient activation of ERK1/2.22 Surprisingly and in contrast to thrombin, which triggers an increase of intracellular Ca2+ levels by activation of PAR1, no changes in cytosolic Ca2+ concentrations have been detected in plasmin-stimulated fibroblasts, although it ought to act through the same receptor22 ; the authors could not explain this apparent paradox. Previous studies from our laboratory revealed that human monocytes express PAR1 and PAR3, which as in other cells elicit a cytosolic Ca2+ response upon stimulation with either thrombin or PAR1- or PAR3-activating peptides.2,8,26 By contrast, plasmin does not induce a cytosolic Ca2+ response in human monocytes. In addition, we have shown that plasmin stimulates the p38 MAPK, but not the ERK1/2, signaling pathway in human monocytes.11 Because it has also been reported that PAR1 might be important for the plasmin-induced CHO cell migration,24 we performed additional experiments using WEDE15, a mAb blocking PAR1 signaling,47 in a concentration that effectively inhibits the PAR1-mediated MCP-1 induction in human peripheral monocytes.26 Here we demonstrate that the plasmin-mediated monocyte chemotaxis is not impaired by the mAbs against PAR1, confirming that PAR1 is not the functional plasmin receptor in human monocytes.
Other authors concluded that plasmin actually desensitizes PAR1 by cleavage at 3 arginine and lysine residues located at the C-terminal end of the tethered region and N-terminal to the ligand binding sites.25 Further, using recombinant extracellular segments of PARs as well as analysis by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry and surface plasmon resonance, it was similarly concluded that plasmin would disable PAR1 and PAR2.48
However, cross-talk between thrombin- and plasmin-signaling cascades cannot be excluded. Thus, in endothelial cells thrombin treatment induces a rapid cell surface exposure of annexin A2,49 identified here as a component of the plasmin receptor on monocytes. One might hypothesize that plasmin might conversely also indirectly affect the PAR1-mediated signaling. In platelets plasmin supposedly induces a moderate Ca2+ signaling through activation of PAR4, but not PAR1.23 In our attempt to identify the plasmin receptor on monocytes, we have carefully analyzed whether plasmin might exert its effects on monocytes, at least in part, via activation of PAR4. However, monocytes do not express PAR4 and, thus, cannot signal via this receptor.26
One of our first indications that the annexin A2 heterotetramer may be more than a passive binding site for plasmin came with the observations that plasmin induces chemotaxis9 and lipid-mediator release8 in monocytes, but not in neutrophils. Western blot analysis demonstrated that monocytes express both components of the annexin A2 heterotetramer complex, whereas neutrophils express only extremely low amounts of S100A10 and no annexin A2. As to the expression of the annexin A2 protein, a recent report confirmed our observation.50
The annexin A2 heterotetramer was originally described as an intracellular submembranous cytoskeleton-associated protein complex.27 Meanwhile, the presence of annexin A2 and annexin A2 heterotetramer has been demonstrated at the surface of various cell types.16,34,50,51 Our flow cytometric data confirm the expression of annexin A2 and for the first time show the expression of S100A10 on the surface of human peripheral monocytes. In addition, we demonstrate the surface expression of the annexin A2 heterotetramer by 3 independent techniques including immunofluorescence microscopy, coimmunoprecipitation of S100A10 together with annexin A2, and the combination of the latter approach with surface biotinylation.
To show that plasmin indeed binds annexin A2 and S100A10 in vivo, we synthesized a plasmin cross-linker and demonstrated transfer of the biotin tag from plasmin to annexin A2 and S100A10, indicating binding of plasmin to the annexin A2 heterotetramer. Due to its configuration, the plasmin cross-linker can react only at a maximum distance of approximately 2.0 nm (20 Å) around the ligand thereby confining cross-linking and finally biotin tagging to directly interacting molecules. Consistent with the interaction between plasmin and the annexin A2 heterotetramer,18,50 recent studies by surface plasmon resonance demonstrated that plasmin binds to the annexin A2 heterotetramer with the highest affinity, whereas the individual subunits annexin A2 and S100A10 bound plasmin with Kd values approximately one order of magnitude lower.17
In addition, we demonstrate that plasmin interacts with the annexin A2 heterotetramer via lysine-binding sites, because the binding was abolished by the lysine analog t-AMCA. This observation is rather important, because as shown earlier, the plasmin-induced monocyte activation can also be antagonized by lysine analogs including t-AMCA.8-10
Unexpectedly, in Western blot a conjugated-annexin A2 protein migrated more slowly than the unconjugated annexin A2. This could be due to posttranslational modification of annexin A2, in particular phosphorylation at serine and tyrosine residues that have been shown to generate such slowly migrating proteins.52 By contrast, no phosphorylation has been reported for S100A10 and indeed no change in the apparent size of S100A10 was observed in our experiments. Annexin A2 can be phosphorylated at its N-terminus at serine 25 and probably also at serine 11 by protein kinase C (PKC) as well as at tyrosine 23 by protein tyrosine kinases of the src family.7,30,31 Indeed, plasmin triggers activation of PKC, which is indispensable for the plasmin-induced chemotaxis.9 It is plausible to hypothesize that plasmin binding might trigger a phosphorylation of annexin A2.
To gain final evidence for the hypothesis that the annexin A2 heterotetramer is indeed a signaling plasmin receptor on monocytes, we down-regulated the expression of annexin A2 and S100A10 by specific phosphorothioate antisense ODNs. This approach allowed us to analyze the role of the annexin A2 heterotetramer directly in primary monocytes without the need for a model cell line.53 A similar approach has already been used to investigate the role of annexin A2 for the binding of plasminogen to human endothelial cells.18 Our ODNs were specifically designed to target only annexin A2 or S100A10 and the sequences did not overlap with homologous proteins. The expression level of both proteins was extensively reduced. In line with our hypothesis, the plasmin-induced monocyte activation in terms of chemotaxis and TNF-α release was impaired by pretreatment with the antisense ODNs against annexin A2 and S100A10.
Due to stabilization of S100A10 by annexin A2,39 and targeting of annexin A2 by S100A10 to the membrane,27,54 it is impossible to completely dissect the effect of either of the 2 proteins on the plasmin-induced signaling. Similarly, steric hindrance due to the antibody binding to one component of the heterotetramer complex might impair the accessibility of the second component. This might be supported by the fact that we could not achieve any better inhibitory effects on plasmin-induced monocyte chemotaxis using a combination of annexin A2 and S100A10 antibodies (T. Syrovets and T. Simmet, unpublished results, November 2002). However, our data provide unambiguous evidence for the role of the annexin A2 heterotetramer as a signaling plasmin receptor on human peripheral monocytes.
The plasmin-mediated activation of human monocytes requires an intact catalytic center of the plasmin molecule.8-10 On the other hand, cleavage of annexin A2 by plasmin42 and calpain40,41 has been reported to occur at lysine 27, and this cleavage is known to abolish annexin A2 binding to lipid rafts.41 Our data demonstrate that annexin A2 located at the cell surface is a substrate for plasmin. Treatment of annexin A2 either in vitro or in vivo on the monocyte membrane generated truncated annexin A2. To confirm the position of the cleavage, we created an annexin A2 mutant with substitution of lysine 27 by alanine. As expected, this annexin A2 mutant was resistant to plasmin cleavage. These data show that plasmin, similar to calpain, cleaves annexin A2 at a single site at the N-terminus. The truncated annexin A2 cannot bind S100A10, because it lacks the first 14 amino acids implicated in the S100A10 binding.43,44 As a consequence of this cleavage, we expected and indeed observed dissociation of the annexin A2 heterotetramer after stimulation of the monocytes with plasmin.
Together our data clearly identify the annexin A2 heterotetramer as a signaling plasmin receptor on human peripheral monocytes. Binding of plasmin to the annexin A2 heterotetramer triggers cleavage of annexin A2 at lysine 27 followed by dissociation of the heterotetramer complex, which initiates downstream signaling and finally functional responses such as chemotaxis and TNF-α release. It is possible that either S100A10 or truncated annexin A2 or both are involved in further downstream signaling. Identification of annexin A2 heterotetramer as a signaling receptor is an important step in our understanding of the plasmin-mediated proinflammatory activation of human peripheral monocytes; this mechanism might be particularly relevant in a variety of chronic inflammatory processes, including atherogenesis.
Prepublished online as Blood First Edition Paper, December 22, 2005; DOI 10.1182/blood-2005-07-2840.
Supported by the Deutsche Forschungsgemeinschaft SFB 451.
Y.L. and T. Syrovets contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr M. Cathcart and Dr V. Gerke for valuable advice and thank J. Marinaci, M. Löhr, and I. Forstreuter for expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal