Abstract
Plasmacytoid dendritic cells (PDCs), which produce IFN-α in response to autoimmune complexes containing nuclear antigens, are thought to be critically involved in the pathogenesis of systemic lupus erythematosus (SLE). One of the immunostimulatory components of SLE immune complexes (SLE-ICs) is self DNA, which is recognized through Tlr9 in PDCs and B cells. Small nuclear ribonucleoproteins (snRNPs) are another major component of SLE-ICs in 30% to 40% of patients. In this study, we show that murine PDCs are activated by purified U1snRNP/anti-Sm ICs to produce IFN-α and proinflammatory cytokines and to up-regulate costimulatory molecules. The induction of IFN-α and IL-6 by U1snRNPs in murine bone marrow–derived PDCs required the presence of intact U1RNA and was largely dependent on Tlr7 but independent of Tlr3. Intracellularly delivered isolated U1snRNA and oligoribonucleotides derived from the stem loop regions and the Sm-binding site of U1snRNA efficiently induced IFN-α and IL-6 in Flt3L-cultured DCs in a Tlr7-dependent manner. The U1snRNA component of U1snRNP immune complexes, found in patients with SLE, acts as an endogenous “self” ligand for Tlr7 and triggers IFN-α and IL-6 production in PDCs.
Introduction
Systemic lupus erythematosus (SLE) is a prototypic autoimmune disorder affecting approximately 1 in 2000 people. The etiology of the disease is unknown. Genetic susceptibility and environmental factors play a role. Defects in tolerance, apoptosis, clearance of immune complexes (ICs), or generation of regulatory cells lead to uncontrolled hyperactivity of self-reactive T- and B-lymphocytes and to the sustained production of tissue-damaging autoantibodies and ICs. The presence of high levels of circulating autoantibodies against nuclear antigens is the immunologic hallmark of SLE. Anti-dsDNA antibodies are found in 70% of patients, and high titers correlate with disease severity. Thirty percent of patients with SLE also have circulating anti–Smith (Sm) antibodies recognizing the 7 Sm proteins (B, D1, D2, D3, E, F, G), which are common to all small nuclear ribonucleoproteins (snRNPs) and which associate with U snRNA (U1, 2, 4, 5). In addition, 40% of SLE patients have anti-RNP antibodies, which specifically bind to the A and C and the 70-kDa proteins within U1snRNPs.1 It has been demonstrated that ICs containing nucleic acids can directly activate murine B lymphocytes and conventional DCs as well as human plasmacytoid DCs, thus contributing to the development of full-blown SLE.2-4 Type I interferon (IFN-α/β), which is induced by SLE ICs in vitro, is found at high levels in the sera of most patients with active SLE. Microarray analysis in peripheral blood mononuclear cells of patients with SLE revealed the typical “IFN signature” of gene expression, which correlates with disease activity.5,6 There is strong evidence for a central role of IFN-α/β in SLE pathogenesis from studies in the New Zealand Black (NZB)/New Zealand White (NZW) mouse model of lupus,7,8 whereas conflicting results were obtained in the Faslpr mouse model of lupus-like disease.9,10 The SLE-promoting role of IFN-α/β may be attributed to the support of B-cell differentiation and antibody production11,12 and to the induction of dendritic cell (DC) differentiation and maturation.13 Plasmacytoid DCs (PDCs), which have been identified as the major source of IFN-α/β in SLE,14 are thought to be important players in this systemic autoimmune disorder.15,16 Apart from IFN-α, IL-6, which is produced by PDCs and other cell types, has also been implicated in the pathogenesis of SLE because it induces plasma cell and effector T-cell differentiation and blocks the activity of CD4+CD25+ regulatory T cells.17,18
Together, these data lead to the question of which receptors are involved in the recognition of SLE ICs. In recent studies, DNA-containing ICs isolated from sera of SLE patients or generated experimentally have been shown to trigger Toll-like receptor 9 (Tlr9), the receptor for bacterial CpG DNA, in B lymphocytes and DCs.4,19,20 Activation of Tlr9 by “self” DNA requires endosomal translocation. This is achieved by Fc receptor or B cell receptor–mediated endocytosis of DNA-containing ICs and can be mimicked by cationic lipid-mediated transport.4,20,21 Interestingly, Tlr9-independent activation of myeloid DCs by antinucleosome ICs has also been observed.19 This may be attributed to an as yet unidentified DNA recognition mechanism or to additional stimulatory components within nucleosomes. A recent study found that IFN-α induction in PDCs by complexes generated from necrotic cell material and SLE-IgG was sensitive to RNase pretreatment.3 Given that snRNPs (specifically U1snRNPs) are the major SLE autoantigens in addition to dsDNA, we investigated their stimulatory potential for murine PDCs. In this study, we show that purified U1snRNP complexed with cationic lipid or anti-Sm autoantibody and intracellularly delivered isolated U1snRNA induced robust type I IFN and inflammatory cytokine responses in murine bone marrow (BM)–derived DCs and PDCs. Thus, in murine DCs, the U1snRNA within U1snRNP autoimmune complexes acts as an endogenous ligand for Tlr7, which was originally identified as the receptor for viral ssRNA.22-24
Materials and methods
Mice
C57BL/6 mice were purchased from Harlan Winkelmann (Borchen, Germany). Tlr7–/– mice25 (4 generations C57BL/6) and Tlr3–/– mice26 (5 generations C57BL/6) have been described. Mice were held in accordance with German guidelines for animal care. Experiments were approved by the regional governmental commission for animal protection (Regierung von Oberbayern).
Media and reagents
RPMI 1640 (PAA Laboratories, Pasching, Austria) supplemented with 10% FCS (Biochrom, Berlin, Germany), 1% glutamax, 1% penicillin/streptomycin, 1% nonessential amino acids, 1% sodium pyruvate (all from Invitrogen, Karlsruhe, Germany) and 50 μM β-mercaptoethanol (Sigma, Deisenhofen, Germany) was used as complete medium. U1snRNP (which is conserved between species) was purified from HeLa cell nuclear extracts, as described.27 U1snRNA was isolated from U1snRNP by phenol/chloroform/isoamyl alcohol extraction and ethanol precipitation. The anti-Sm (B/D) antibody clone Y12, mouse IgG3 κ isotype28 was purified from Y12 hybridoma supernatant CpG 2216 oligonucleotide (MWG Biotech, Munich, Germany). Poly-I:C RNA, poly-U RNA (Amersham Biosciences, Freiburg, Germany), and oligoribonucleotides (Curevac, Tübingen, Germany) were used as indicated. RNA digestion was performed with 20 μg/mL RNase A (Sigma, Deisenhofen, Germany) for 1 hour at 37°C.
Preparation, culture, and stimulation of cells
BM cells from wild-type and knockout mice were cultured with 20 ng/mL human recombinant Flt3L (R&D Systems, Wiesbaden, Germany) in complete medium for 7 days, as described,29 to generate greater than 90% CD11c+ FL-DCs with 40% to 50% CD11blow/CD86low/B220high PDCs and 40% to 50% CD11bhigh/B220low DCs. For some experiments, 1000 U/mL murine recombinant IFN-β (PBL Laboratories, Piscataway, NJ) was added to the culture on day 6. On day 7, cells were harvested, resuspended in fresh medium, and seeded at 4 × 105 cells per 100 μL/well in 96-well plates. For data shown in Table 1, PDCs and conventional DCs were isolated from FL-DCs by sorting in a MoFlo cytometer (greater than 97% purity). PDCs were sorted as CD11c+/CD11blow/B220high or CD11c+/120G8+/B220 high cells, and conventional DCs were sorted as CD11c+/CD11bhigh/B220low or CD11c+/120G8–/B220low DCs. RNAs and the isolated U1snRNP were preincubated with DOTAP cationic liposomes (Carl Roth, Karlsruhe, Germany), as described.24 Y12 antibody was incubated with U1snRNP in PBS for 15 minutes on ice plus 5 minutes at 37°C. Stimuli were added in 100 μL volume per well (concentrations indicated) for 36 to 40 hours.
Cytokine production by sorted plasmacytoid DCs and conventional DCs
Cytokines and experiment no. . | IFN-α, U/mL . | IL-6, pg/mL . |
|---|---|---|
| IFN-α (U/mL) | ||
| 1 | 1023 | < d.l. |
| 2 | 230 | < d.l. |
| 3 | 215 | < d.l. |
| IL-6 (pg/mL) | ||
| 1 | 377 | 1900 |
| 2 | 152 | 1121 |
| 3 | 629 | 6134 |
Cytokines and experiment no. . | IFN-α, U/mL . | IL-6, pg/mL . |
|---|---|---|
| IFN-α (U/mL) | ||
| 1 | 1023 | < d.l. |
| 2 | 230 | < d.l. |
| 3 | 215 | < d.l. |
| IL-6 (pg/mL) | ||
| 1 | 377 | 1900 |
| 2 | 152 | 1121 |
| 3 | 629 | 6134 |
FL-DCs were sorted into PDCs and conventional DCs (greater than 97% purity) as described in “Materials and methods” and were stimulated with ICs formed with U1snRNP (20 μg/mL) and anti-Sm antibody (100 μg/mL) for 36 hours. IFN-α and IL-6 were measured in the supernatants by ELISA. The mIFN-α ELISA kit from PBL Laboratories was used in these experiments. Results of 3 independent experiments are shown. d.l. indicates detection limit.
Flow cytometry
Cells were stained with anti–CD11b-FITC, anti–B220-APC, and anti–CD86-PE (BD Biosciences, Heidelberg, Germany) and were analyzed on a FACSCalibur flow cytometer (BD Biosciences). Dead cells were excluded from analysis by gating on propidium iodide–negative (PI–) cells.
ELISA
IFN-α was measured in the culture supernatant by enzyme-linked immunosorbent assay (ELISA) with rat anti–mIFN-α (clone RMMA-1) as capture and rabbit anti–mIFN-α as detection antibody (PBL Laboratories), followed by peroxidase-labeled donkey antirabbit antiserum (Jackson ImmunoResearch, Cambridgeshire, United Kingdom). Concentrations were calculated from a standard curve of recombinant mIFN-α (Hycult, Uden, Netherlands; lower detection limit, 1 U/mL). Alternatively, the mIFN-α ELISA kit from PBL Laboratories was used (Table 1). Murine IL-6 ELISA was performed using matched antibody pairs (clones MP5-20F3 and MP5-32C11; BD Biosciences) and streptavidin-HRP (Amersham). ABTS (2,2′-azino-di-3-ethylbenzthiazoline sulfonate; Roche Diagnostics, Mannheim, Germany) was used as substrate.
Results
Activation of murine DCs by U1snRNP ICs
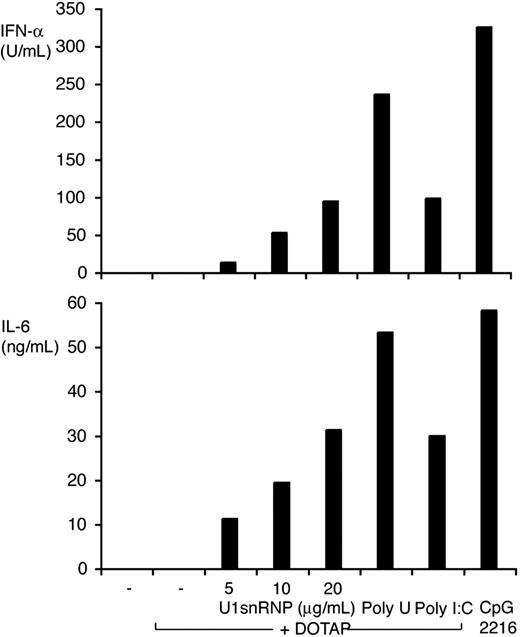
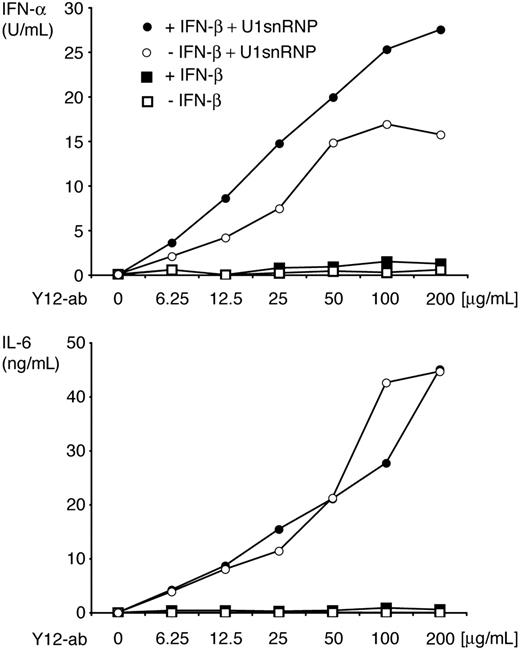
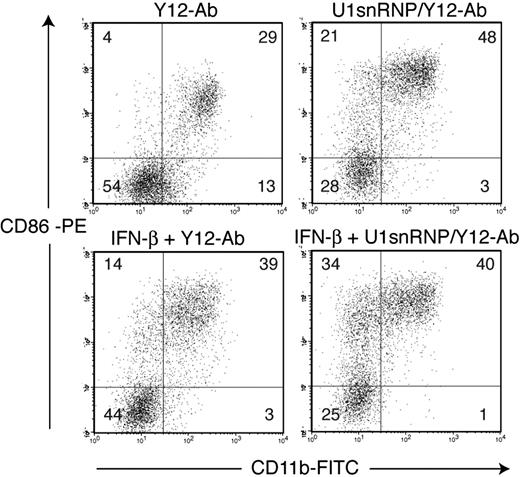
DCs derived from murine BM cells by culture with Flt3L (FL-DCs) contained the IFN-α–producing CD11blow/B220high PDCs and conventional CD11bhigh/B220low DCs. FL-DCs were incubated with increasing doses of purified U1snRNP complexed with DOTAP cationic liposomes or with control stimuli poly-U ssRNA/DOTAP (as Tlr7 ligand), poly-I:C dsRNA/DOTAP (as ligand for Tlr3 and other dsRNA recognition receptors), and CpG 2216 oligonucleotide (as Tlr9 ligand). U1snRNP induced a robust and dose-dependent secretion of IFN-α and IL-6 in FL-DC (Figure 1). Cytokine induction by U1snRNP required an enhanced intracellular delivery because U1snRNP alone had very low immunostimulatory activity (Figure 2). We tested whether complexation with cationic liposomes could be replaced by the formation of ICs, which are internalized by DCs through Fc receptors. FL-DCs, which had been cultured in regular growth medium or with IFN-β for the last 18 hours as an enhancer of IFN-α production,30 were incubated with U1snRNP alone or in complex with increasing doses of purified anti-Sm monoclonal antibody Y12 or Y12 antibody alone. As shown in Figure 2, cytokine responses increased dose dependently with the concentrations of Y12 antibody added to U1snRNP but not with Y12 antibody alone. Preincubation of FL-DCs with IFN-β significantly enhanced IFN-α production in response to U1snRNP/Y12 antibody ICs. As shown in Figure 3, U1snRNP/Y12 antibody ICs induced high-level expression of CD86 in most CD11bhigh DCs and in a subpopulation of CD11blow PDCs that was further enhanced by IFN-β preincubation. PDCs and conventional DCs purified to 97% by cell sorting were incubated with U1snRNP/Y12 antibody ICs. Isolated PDCs produced IFN-α and IL-6 in response to U1snRNP ICs, whereas isolated conventional DCs produced high amounts of IL-6 but IFN-α was below the detection limit (Table 1). Therefore, PDCs and conventional DCs within FL-DCs are directly activated by U1snRNP ICs, but PDCs are the major source of IFN-α in response to U1snRNP ICs.
IFN-α and IL-6 induction by U1snRNP/DOTAP complexes in FL-DCs. FL-DCs were incubated with U1snRNP/DOTAP complexes (5, 10, and 20 μg/mL U1snRNP and 12.5 μg/mL DOTAP) or control stimuli (DOTAP/poly-U 20 μg/mL, DOTAP/poly-I:C 20 μg/mL, CpG 2216 0.5 μM) for 40 hours. Concentrations of IFN-α and IL-6 were measured in the supernatants by ELISA. Results of 1 of 3 representative independent experiments are shown.
IFN-α and IL-6 induction by U1snRNP/DOTAP complexes in FL-DCs. FL-DCs were incubated with U1snRNP/DOTAP complexes (5, 10, and 20 μg/mL U1snRNP and 12.5 μg/mL DOTAP) or control stimuli (DOTAP/poly-U 20 μg/mL, DOTAP/poly-I:C 20 μg/mL, CpG 2216 0.5 μM) for 40 hours. Concentrations of IFN-α and IL-6 were measured in the supernatants by ELISA. Results of 1 of 3 representative independent experiments are shown.
U1snRNP triggers Tlr7-dependent and -independent activation pathways
We hypothesized that U1snRNA could be responsible for the immunostimulatory effect of the U1snRNP particle. This assumption was tested by comparing the capacity of RNase A–treated and –untreated U1snRNP complexed with DOTAP or Y12 antibody to stimulate cytokine production and CD86 expression in FL-DCs (Figure 4). The ability of U1snRNP to induce IFN-α and IL-6 secretion and to up-regulate CD86 expression in FL-DCs was significantly reduced, though not completely abrogated, by prior digestion of the RNA component with RNase. Overall, U1snRNP was less sensitive to RNase digestion than poly-U RNA, possibly because of nuclease protection of U1snRNA within the U1snRNP complex.
IFN-α and IL-6 production in response to U1snRNP/anti–Sm complexes. ICs formed with U1snRNP (20 μg/mL final concentration) and Y12-antibody or Y12-antibody alone (final concentration, 6.25-200 μg/mL) were added to FL-DCs, which had been cultured in regular medium or pretreated with IFN-β for 18 hours. After 36 hours of incubation, IFN-α and IL-6 were measured in the supernatant by ELISA. Results of 1 of 3 representative independent experiments are shown.
IFN-α and IL-6 production in response to U1snRNP/anti–Sm complexes. ICs formed with U1snRNP (20 μg/mL final concentration) and Y12-antibody or Y12-antibody alone (final concentration, 6.25-200 μg/mL) were added to FL-DCs, which had been cultured in regular medium or pretreated with IFN-β for 18 hours. After 36 hours of incubation, IFN-α and IL-6 were measured in the supernatant by ELISA. Results of 1 of 3 representative independent experiments are shown.
CD86 up-regulation by U1snRNP/anti–Sm complexes in FL-DCs. FL-DCs (untreated or pretreated with IFN-β) were incubated with Y12 antibody (50 μg/mL) alone or in complex with U1snRNP (20 μg/mL) for 40 hours. CD11b and CD86 expression were measured by flow cytometry. Low CD11b expression correlated with high B220 expression (not shown). Percentages are indicated in the quadrants. Results of 1 of 4 representative independent experiments are shown.
CD86 up-regulation by U1snRNP/anti–Sm complexes in FL-DCs. FL-DCs (untreated or pretreated with IFN-β) were incubated with Y12 antibody (50 μg/mL) alone or in complex with U1snRNP (20 μg/mL) for 40 hours. CD11b and CD86 expression were measured by flow cytometry. Low CD11b expression correlated with high B220 expression (not shown). Percentages are indicated in the quadrants. Results of 1 of 4 representative independent experiments are shown.
RNase sensitivity of U1snRNP induced FL-DC activation. FL-DCs (untreated or pretreated with IFN-β) were incubated with untreated or RNAse-treated poly-U and U1snRNP (20 μg/mL) in complex with DOTAP (12.5 μg/mL) or Y12 antibody (50 μg/mL). Cytokine production was measured by ELISA, and CD86 expression (expressed as mean fluorescence intensity) was determined by flow cytometry. Mean values and standard deviations (error bars) are shown (n = 3). Asterisks indicate significant differences between conditions with and without RNAse treatment (P < .05; 2-tailed paired Student t test).
RNase sensitivity of U1snRNP induced FL-DC activation. FL-DCs (untreated or pretreated with IFN-β) were incubated with untreated or RNAse-treated poly-U and U1snRNP (20 μg/mL) in complex with DOTAP (12.5 μg/mL) or Y12 antibody (50 μg/mL). Cytokine production was measured by ELISA, and CD86 expression (expressed as mean fluorescence intensity) was determined by flow cytometry. Mean values and standard deviations (error bars) are shown (n = 3). Asterisks indicate significant differences between conditions with and without RNAse treatment (P < .05; 2-tailed paired Student t test).
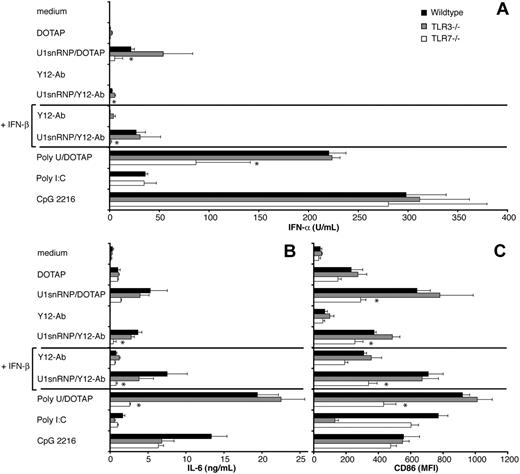
Influence of Tlr7 and Tlr3 deficiency on FL-DC activation of by U1snRNP. FL-DCs generated from wild-type, Tlr3–/–, and Tlr7–/– mice were incubated with the indicated stimuli (10 μg/mL U1snRNP and poly-U with 12.5 μg/mL DOTAP; 20 μg/mL U1snRNP with 50 μg/mL Y12-antibody; CpG 2216 0.1 μM, poly-I:C 20 μg/mL) for 36 hours. (A) IFN-α and (B) IL-6 were measured in the supernatants by ELISA, and (C) CD86 expression was determined by flow cytometry. Mean values and standard deviations are shown (n = 3). Asterisks indicate significant differences between wild-type and Tlr7–/– cells (P < .05; 2-tailed paired Student t test).
Influence of Tlr7 and Tlr3 deficiency on FL-DC activation of by U1snRNP. FL-DCs generated from wild-type, Tlr3–/–, and Tlr7–/– mice were incubated with the indicated stimuli (10 μg/mL U1snRNP and poly-U with 12.5 μg/mL DOTAP; 20 μg/mL U1snRNP with 50 μg/mL Y12-antibody; CpG 2216 0.1 μM, poly-I:C 20 μg/mL) for 36 hours. (A) IFN-α and (B) IL-6 were measured in the supernatants by ELISA, and (C) CD86 expression was determined by flow cytometry. Mean values and standard deviations are shown (n = 3). Asterisks indicate significant differences between wild-type and Tlr7–/– cells (P < .05; 2-tailed paired Student t test).
The 165-nucleotide U1snRNA forms a characteristic secondary structure consisting of stem loops 1 to 4 and a 5′-3′ connecting helix.31 The double helices of stems 1, 2, and 3 and the connecting helix are stacked to form a 4-way junction. Single-stranded RNA stretches are found in the loops, the conserved Sm-binding site, and the 5′terminal sequence. Tlr3 and Tlr7 have been implicated in the recognition of viral double-stranded and single-stranded RNA and could, therefore, be involved in the DC response to U1snRNP. FL-DCs contained 40% to 50% PDCs, which have been shown to express Tlr7 but not Tlr3, and 40% to 50% conventional DCs, which express Tlr3 and in part also Tlr7.32 The role of Tlr3 and Tlr7 for recognition of U1snRNP was investigated by comparing cytokine production and CD86 up-regulation of FL-DCs derived from bone marrow cells of C57BL/6 wild-type, Tlr3–/–, and Tlr7–/– mice in response to U1snRNP/DOTAP and U1snRNP/Y12 antibody IC (Figure 5). Activation of FL-DC by U1snRNP complexes was independent of Tlr3 by all parameters measured, whereas the response to poly-I:C was mediated mainly by Tlr3. IFN-α and IL-6 production by FL-DCs in response to U1snRNP complexes were significantly reduced in the absence of Tlr7, similar to the response to poly-U RNA, suggesting that recognition of U1RNA by Tlr7 is mainly responsible for the induction of IFN-α and IL-6 by U1snRNP/Y12 antibody IC (Figure 5A-B). However, a small residual IFN-α and IL-6 response to U1snRNP and poly-U ssRNA was observed in Tlr7-deficient FL-DCs, suggesting the presence of an additional recognition mechanism for ssRNA in FL-DCs. This was in contrast to the response to imidazoquinolin, which was entirely Tlr7 mediated (data not shown). In contrast to IFN-α and IL-6 release, RANTES and IL-12p40 production induced by U1snRNP and poly-U RNA were lower but not significantly reduced in the absence of Tlr7 (data not shown). Up-regulation of CD86 expression by U1snRNP was significantly lower but still present in Tlr7-deficient cells compared with wild-type and Tlr3–/– cells (Figure 5C).
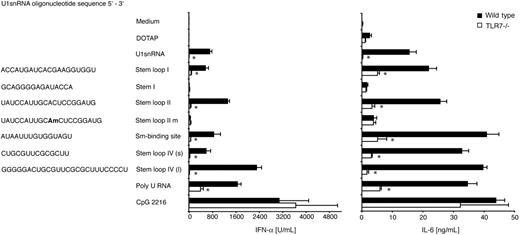
To verify that the U1snRNA within U1snRNP does indeed act as an endogenous ligand for Tlr7, we tested the stimulatory capacity of U1snRNA isolated from U1snRNP and synthetic oligoribonucleotides derived from the U1snRNA stem loop 1, 2, and 4 regions and the Sm-binding site (Figure 6). The RNAs were transfected to wild-type and Tlr7–/– FL-DCs with cationic liposome DOTAP. Total U1snRNA stimulated IFN-α and IL-6 production by FL-DCs in a Tlr7-dependent manner (1636 ± 753 U/mL IFN-α in wild-type vs 1.8 ± 0.4 U/mL IFN-α in Tlr7–/– FL-DCs). Oligoribonucleotides derived from stem loop 1, 2, and 4 (short and long) and from the Sm-binding site were also effective stimulators for wild-type, but much less for Tlr7–/–, FL-DCs. The stem 1 oligoribonucleotide, which contained only one uridine in the sequence, induced very low levels of cytokines. Incorporation of the naturally occurring 2′O-methyl-adenosine instead of the unmodified adenosine in the stem loop 2 oligonucleotide greatly reduced its stimulatory capacity. This 2′O-methylation is the only modification of U1snRNA outside the heavily modified 5′ end. Therefore, modifications within the U1snRNA sequence may reduce, but do not inhibit, the immunostimulatory activity of intracellularly delivered total U1snRNA.
Discussion
In this study, we show that ICs containing U1snRNP, a major autoantigen in human and murine SLE, stimulate murine BM-derived PDCs and conventional DCs, leading to the production of IFN-α and IL-6 and to the up-regulation of costimulatory molecule expression. The induction of IFN-α and IL-6 by U1snRNP in FL-DCs required the presence of intact U1snRNA and was largely dependent on Tlr7 but independent of Tlr3. U1snRNA isolated from U1snRNP and synthetic RNA oligonucleotides derived from the U1snRNA sequence directly triggered Tlr7-mediated IFN-α and IL-6 release when delivered to FL-DCs with cationic liposomes. This demonstrates that the U1snRNA within the U1snRNP particle can function as an endogenous ligand for Tlr7 but not for Tlr3.
Tlr7-dependent cytokine induction in FL-DCs by total U1snRNA and oligoribonucleotides derived from U1snRNA. Wild-type and Tlr7–/– FL-DCs were incubated with 10 μg/mL (0.2 μM) total U1snRNA isolated from U1snRNP, 2 μM oligoribonucleotides derived from the U1snRNA sequence, or 10 μg/mL poly-U RNA in complex with 12.5 μg/mL DOTAP, with DOTAP alone or with 0.5 μM CpG 2216 as positive control stimulus. After 36 hours of incubation, IFN-α and IL-6 were measured in the supernatant by ELISA. Mean values and standard deviations are shown (n = 3). Asterisks indicate significant differences between wild-type and Tlr7–/– cells (P < .05; 2-tailed paired Student t test).
Tlr7-dependent cytokine induction in FL-DCs by total U1snRNA and oligoribonucleotides derived from U1snRNA. Wild-type and Tlr7–/– FL-DCs were incubated with 10 μg/mL (0.2 μM) total U1snRNA isolated from U1snRNP, 2 μM oligoribonucleotides derived from the U1snRNA sequence, or 10 μg/mL poly-U RNA in complex with 12.5 μg/mL DOTAP, with DOTAP alone or with 0.5 μM CpG 2216 as positive control stimulus. After 36 hours of incubation, IFN-α and IL-6 were measured in the supernatant by ELISA. Mean values and standard deviations are shown (n = 3). Asterisks indicate significant differences between wild-type and Tlr7–/– cells (P < .05; 2-tailed paired Student t test).
U1snRNP was only active in complex with autoantibody, which binds to Fc receptors and thus facilitates internalization into the endosomal compartment, as has been shown for human SLE ICs containing DNA.20,33 Requirements for IC formation could be bypassed by the complexation of U1snRNP with cationic liposome, thus ruling out a major contribution of Fc receptor signaling for stimulation of PDCs by U1snRNP/Y12 antibody complexes. The need for enhanced intracellular delivery of autoantigens for immunostimulation, however, may depend on the cell type and the form of autoantigen. The induction of splenocyte proliferation by isolated U1snRNA34 and Fc receptor-independent, MyD88-independent activation of myeloid DC by nucleosomes35 have also been reported. The fact that U1snRNP or Y12 antibody alone showed little effect in FL-DCs and that intracellular delivery was required for full stimulatory capacity of U1snRNP also makes it unlikely that the cytokine responses and costimulatory molecule up-regulation induced by U1snRNP-IC in FL-DCs are caused by endotoxin contamination of the U1snRNP or the Y12 antibody preparation. In human PDCs, the internalization of SLE-ICs was mediated by FcγRIIa, which is expressed in a subpopulation of PDCs (CD32).20,33 This receptor is not expressed in mice, but another study has shown that murine myeloid DCs required FcγRIII expression for activation by chromatin-IC.19 FcγRI (CD64), which is also expressed in murine DCs36 and has been shown to bind murine IgG3-ICs,37 could be responsible for the uptake of U1snRNP/Y12 antibody ICs in our study.
Pretreatment of FL-DCs with IFN-β enhanced IFN-α production in response to U1snRNP complexes (Figures 2, 5) and the synthetic Tlr7-ligand imidazoquinolin (data not shown), as has been previously described for human PDCs stimulated with SLE-IgG plus apoptotic cells.30 Despite constitutive expression of IRF-7 in PDCs, efficient and sustained IFN-α production in PDCs also strongly depends on the positive feedback loop of type I IFN, which leads to IRF-7 up-regulation, as shown by Honda et al.38 In addition to IRF-7 induction, the up-regulation of Tlr7 by type I IFN stimulation, which has been demonstrated for human B lymphocytes,39 may contribute to the observed enhancing effect of IFN-β.
Despite the important role of Tlr7 for triggering the IFN-α and IL-6 response to U1snRNP in FL-DCs, we observed a small residual production of these cytokines in the absence of Tlr7. Additionally, CD86 up-regulation induced by U1snRNP was only partially Tlr7 dependent (Figure 5), and the release of RANTES and IL-12p40 in response to U1snRNP complexes was only slightly, but not significantly, reduced in the absence of Tlr7 (data not shown). Although it has been suggested that U1snRNA induces splenocyte proliferation through Tlr3,34 the activation of FL-DCs by U1snRNP and U1snRNA was Tlr3 independent. However, other intracellular dsRNA-binding molecules, such as RIG-I and MDA5,40 which trigger type I IFN and inflammatory cytokine production, could play a role in U1snRNP recognition. In addition, the response to poly-U RNA and synthetic RNA oligonucleotides derived from the U1snRNA sequence was not entirely abrogated in Tlr7-deficient cells, suggesting that ssRNA can trigger cytokine responses through an additional unidentified mechanism.
Interestingly, a single 2′O-methylation in the U1snRNA stem loop 2 oligoribonucleotide, which naturally occurs in U1snRNA, abrogated its immunostimulatory activity in murine FL-DCs, similar to what has recently been reported for modified synthetic oligoribonucleotides in human DCs.41 However, intracellularly delivered U1snRNA efficiently triggered cytokine release in murine DCs despite the presence of modifications, such as methylations or pseudouridines within the sequence, that suppress the immunostimulatory activity of ssRNA.41 Therefore, although protective mechanisms such as RNA modifications are in place, some self-RNAs, such as U1snRNA, which is carried to the Tlr7-containing endosomal compartment by internalization of U1snRNP autoimmune complexes, can be sensed as a danger signal by immune cells and may promote autoimmunity.
Overall, this study shows that Tlr7 plays an essential role for IFN-α and IL-6 production by murine PDCs and conventional DCs in response to U1snRNP autoantigen. We have thus identified a second mechanism of immunostimulation by nuclear self-antigens in addition to the recently described triggering of Tlr9 by chromatin-containing ICs.4,19,20 Given the important roles of type I IFN and IL-6 in human SLE,16 the recognition of ICs containing nuclear autoantigens by the endosomally localized Tlr7 and Tlr9, leading to the induction of these cytokines through a common signaling pathway, are attractive targets for therapeutic intervention. Pharmacologic agents interfering with the internalization of ICs, endosomal maturation, and binding of endogenous ligands to Tlr7 and Tlr9 or with the Tlr7- and Tlr9-specific signal tranduction pathway downstream of MyD88 should be promising candidates for immunotherapy of SLE. The study by Barrat et al,42 which was published during revision of this manuscript, shows that specific Tlr7 antagonists inhibit IFN-α production in human PDCs induced by ICs formed with anti-RNP antibodies from SLE serum and apoptotic cell material, thus supporting our results obtained with purified U1snRNP/anti-Sm ICs in murine PDCs.
Prepublished online as Blood First Edition Paper, December 20, 2005; DOI 10.1182/blood-2005-07-2650.
A.K. and E.S. are supported by DFG grants KR2199 and SFB455.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Note added in proof: While this paper was in press, a study by Vollmer et al43 supporting our data was published.
We thank I. Mattaij for providing the Y12 hybridoma, M. Schiemann for excellent cell sorting, A. Heit and F. Schmitz for critical reading of the manuscript, and B. Grohs and W. Ballhorn for excellent technical assistance. This work is part of the thesis of E.S.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal