Abstract
CC-4047, an immunomodulatory analog of thalidomide, inhibits multiple myeloma with unknown effects on the human osteoclast lineage. Early osteoclast progenitors are of hematopoietic origin and differentiate into mature bone resorbing multinucleated osteoclasts. We investigated the effects of CC-4047 and thalidomide on human osteoclastogenesis, using in vitro receptor activator of NFκ-B ligand/macrophage colony-stimulating factor–stimulated bone marrow cell cultures. Treating bone marrow cultures with CC-4047 for 3 weeks decreased osteoclast formation accompanied by complete inhibition of bone resorption. The inhibitory effect was similar when cultures were treated for 3 weeks or for only the first week (90% inhibition), indicating that CC-4047 inhibits early stages of osteoclast formation. Inhibition of osteoclastogenesis by CC-4047 was mediated by a shift of lineage commitment to granulocyte colony-forming units at the expense of granulocyte-macrophage colony-forming units. Further studies revealed that this shift in lineage commitment was mediated through down-regulation of PU.1. Treatment with thalidomide resulted in significantly less potent inhibition of osteoclast formation and bone resorption. These results provide evidence that CC-4047 blocks osteoclast differentiation during early phases of osteoclastogenesis. Therefore, CC-4047 might be a valuable drug for targeting both tumors and osteoclastic activity in patients with multiple myeloma and other diseases associated with osteolytic lesions.
Introduction
The major source of morbidity and possible mortality associated with multiple myeloma (MM) is osteolytic bone destruction throughout the axial skeleton. Lytic bone lesions occur in 70% to 80% of these patients, and are frequently associated with severe bone pain and pathologic fractures.1 Besides MM, osteolytic lesions also occur in connection with bone metastases from solid tumors. Although the exact incidence of bone metastases is unknown, it is estimated that 350 000 people die with bone metastases each year in the United States.1
Osteolytic lesions represent an extreme of a continuum of dysregulation of the normal bone remodeling process with excessive bone resorption mediated by osteoclasts (OCLs). OCLs are multinucleated cells that derive from monocyte/macrophage lineage cells.2-6 How commitment to a given lineage occurs among macrophages, OCLs, and dendritic cells has not been fully elucidated, but complex signaling mechanisms are known to control OCL differentiation.7 These signals include cytokines such as macrophage colony-stimulating factor (CSF-M), granulocyte-macrophage colony-stimulating factor (CSF-GM), and interleukin-3,8 which are macrophage-inducing cytokines, and the receptor activator of NFκ-B ligand (RANKL), which is a critical factor for late stages of OCL development.9 In addition to cytokines, several transcription factors are vital for OCL formation; the most important are PU.110 and c-fos.11,12 PU.1 (Spi-1) is essential for the development of myeloid and B-lymphoid cells.13-15 In myeloid cells, PU.1 is known to regulate transcription of both c-fms and CD11b/CD18 (Mac1), both of which are central to the OCL phenotype.16-18 PU.1-deficient mice show a failure in macrophage differentiation, and osteoclastogenesis is inhibited at a very early stage of differentiation.10,19 Mice deficient in c-fos exhibit osteopetrosis due to an OCL differentiation defect, while the number of macrophages increases, indicating that c-fos acts on a later stage of OCL development.10,11 It is clear that any disruption of hematopoiesis can profoundly affect OCL numbers.
Thalidomide was originally developed in the 1950s to treat pregnancy-induced morning sickness. Following the discovery of its teratogenic effects, it was subsequently withdrawn from the market in 1961.20 Since the discovery of thalidomide's antimyeloma activity in the late 1990s,21 new and more potent immunomodulatory derivatives (IMiDs) of thalidomide such as CC-4047 (Actimid) and CC-5013 (Revlimid) with fewer side effects have been developed. In previous studies, we showed that IMiDs inhibit the in vitro and in vivo growth of MM cells.22,23 In initial clinical phase 1 and 2 trials, single agent CC-4047 and CC-5013 in combination with dexamethasone achieved response rates of over 50% in relapsed and refractory MM.24-26 We have shown that CC-4047 induces a shift in lineage commitment, resulting in increased myeloid cell development at the expense of erythroid cell commitment from human hematopoietic progenitors.27 However, the effect of CC-4047 and thalidomide on human OCL differentiation is unknown.
Therefore, we studied the effects of CC-4047 and thalidomide on OCL formation in long-term human bone marrow cultures from healthy donors and patients with MM. We found that CC-4047 completely abrogated RANKL-induced OCL formation over 21 days. We also found that the inhibitory effects of CC-4047 on OCL formation were at the early stage of differentiation of hematopoietic progenitor cells, resulting from the down-regulation of the PU.1 transcription factor. We conclude that the IMiD analog CC-4047, in addition to its effects on MM cells, is a potent inhibitor of OCL formation.
Materials and methods
Chemicals
Thalidomide and CC-4047 were obtained from Celgene (Warren, NJ). Recombinant human (rh) RANKL was purchased from Roche (Nutley, NJ); CSF-M and CSF-GM were purchased from R&D Systems (Minneapolis, MN); α-minimal essential medium (α-MEM), fetal calf serum (FCS), l-glutamine, and other cell culture reagents were purchased from Invitrogen (Carlsbad, CA); and horse serum was purchased from Hyclone (Logan, UT). Hypaque-Ficoll and all other chemicals were obtained from Sigma-Aldrich Chemicals (St Louis, MO), unless otherwise stated.
Cells and cell culture
The institutional review board of the University of Pittsburgh approved these studies. Informed consent was provided according to the Declaration of Helsinki. Bone marrow cells were obtained from healthy volunteers or untreated patients with MM. Bone marrow mononuclear cells were then isolated by separation on Hypaque-Ficoll gradients as described previously.28
CD34+ cells were obtained from leukaphereses products from patients who were scheduled for autologous transplantation with enriched CD34+ cells. Leukaphereses products were subjected to positive selection with the Isolex 300 device (Baxter Healthcare, Irvine, CA).29 Subset analyses showed that CD34+ selected cells had a predominantly mature phenotype (mean ± standard deviation [SD]: CD34+/CD38+, 95.5% ± 5.3%; CD34+/CD33+, 79.9% ± 20.1%; CD34+/DR+,98.9% ± 1.2%). Cells in 10% dimethyl sulfoxide (DMSO) were stored in liquid nitrogen.
Osteoclast formation assay
Nonadherent mononuclear bone marrow cells from healthy donors or patients with MM as well as purified human peripheral blood CD34+ cells were seeded (105 cells/well) in 96-well multiplates at 100 μL/well in α-MEM containing 20% horse serum, 10 ng/mL CSF-M, and 50 ng/mL RANKL. CC-4047 (100 μM), thalidomide (100 μM), or DMSO (0.1% μM) was added into appropriate wells for different periods (either 1, 2, or 3 weeks). In one series, 16 μg/mL recombinant monoclonal anti–human neutralizing granulocyte colony-stimulating factor (CSF-G) antibody (R&D Systems) was added to the suitable wells to neutralize CSF-G. Half-media changes were carried out twice a week. The cultures were continued for a total of 3 weeks at 37°C with 5% CO2. Staining was performed with monoclonal antibody 23c6+ using a Vectastatin-ABC-AP kit (Vector Laboratories, Burlingame, CA). 23c6+ recognizes the CD51/61 dimer (the OCL vitronectin receptor) and was generously provided by Michael Horton, Rayne Institute, Bone and Mineral Center, London, United Kingdom. 23c6+ multinucleated OCLs containing 3 or more nuclei per OCL were scored using an inverted microscope. In addition, tartrate-resistant acid phosphatase (TRAP) staining was performed as described before.30,31 Briefly, after 3 weeks of culture, the cells were fixed with citrate-acetone-formaldehyde fixative, and cytochemical staining for TRAP was performed using a leukocyte acid phosphatase kit (Sigma-Aldrich) in the presence of 10 mM L(+)-tartaric acid as an inhibitor. Cells that contained 3 or more nuclei were counted with an inverted phase contrast microscope. Each osteoclast formation assay was performed at least 3 times with cells from different donors.
Osteoclastic bone resorption assays
Bone marrow cells from a healthy donor (2 × 105 cells/well) were seeded on whale dentin slices in 96-well multiplates in 100 μL/well α-MEM containing 20% horse serum. To stimulate OCL formation, 10 ng/mL CSF-M and 50 ng/mL RANKL were added to the cultures. Half-media changes were performed twice a week. CC-4047 (100 μM), thalidomide (100 μM), or DMSO (0.1%) was added to a final concentration of 100 μM. Cultures were maintained for 3 weeks and OCL formation on dentin slices was confirmed by TRAP staining. Resorption lacunae were stained with hematoxylin and the pit area was quantified by light microscopy. Each osteoclast formation assay was performed at least 3 times with cells from different donors.
Pit area was quantified by using the public domain NIH ImageJ program. Fixed small representative areas on dentin slices selected and the mean resorption areas were determined.
Flow cytometry analysis
Cells from OCL assays were detached using cell dissociation buffer (Sigma-Aldrich) and stained with anti–CD45-ECD, anti–CD33-PE, anti–CD34-PE (BD Biosciences, San Jose, CA), anti–CD114-PE, and anti–CD116-PE (Serotec, Oxford, United Kingdom). Data were acquired on a Dako-Cytomation MoFlo cytometer. Dyes were excited at 520 nM with mixed gas green laser to minimize autofluorescence of the cultured cells. Cell doublets and clusters were gated from the analysis using doublet discrimination (forward scatter pulse height by pulse width). Events with low forward scatter (dead cells and debris) were gated from the analysis.
Apoptosis was determined by annexin V–fluorescein isothiocyanate (FITC)/propidium iodide (PI) double staining according to the manufacturer's instructions (Bender MedSystems, Vienna, Austria) and analyzed by flow cytometry using a FACS Caliber flow cytometer and CELLQuest software (Becton Dickinson, Heidelberg, Germany).
Colony-forming assays
To generate CFU-GM colonies for further OCL assays, healthy donor and multiple myeloma bone marrow mononuclear cells were cultured at 5 × 105 cells/well in α-MEM containing 1.2% methylcellulose, 30% FCS, 1% bovine serum albumin (Sigma-Aldrich, San Jose, CA), and 100 pg/mL rh CSF-GM with CC-4047 (100 μM), thalidomide (100 μM), or DMSO (0.1%) and plated in a volume of 1 mL per plate in 35-mm culture dishes (Corning, New York, NY). The dishes were incubated at 37°C in 5% CO2 for 7 to 9 days. Colonies were collected, washed with phosphate-buffered saline 4 times, and used for OCL formation assays as described in “Osteoclast formation assay.”
Western blot analyses
Western blotting was performed as previously described.32 Briefly, cells were harvested, washed 3 times with phosphate-buffered saline, and lysed with lysis buffer (NP-40 1%, DTT 0.5 mM, sodium orthovanadat 1 mM, aprotinin 1 μg/mL, sodium fluoride 50 μM, phenylmethylsulfonyl fluoride 500 μM). Cell lysates were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to Hybond C super filters (Amersham, Arlington Heights, IL). The blots were probed with anti-PU.1 (Spi-1) antibody, anti–c-fos antibody, anti-GAPDH (all from Santa Cruz Biotechnology, Santa Cruz, CA), and β-actin antibody (Amersham). Immune complexes were detected using enhanced chemiluminescence (Amersham).
Osteoclast formation is inhibited dose dependently by 3 weeks of treatment with CC-4047 and thalidomide. (A, B) For osteoclast assays, nonadherent cells from HBM and MMBM were cultured with 10 ng/mL CSF-M and 50 ng/mL RANKL for 21 days; 0.1% DMSO (control), CC-4047, or thalidomide (Thal) was added to the culture twice a week to maintain a concentration of 100 μM. After 3 weeks of treatment, cells were fixed and stained for 23c6+ multinucleated OCLs. For all the micrographs, original magnification is ×20. Each value represents the mean plus or minus SD of multinucleated cells per well of at least 8 wells. All experiments were performed independently 4 times. (C) Nonadherent HBM cells were cultured with 10 ng/mL CSF-M and 50 ng/mL RANKL for 21 days and DMSO (control) at 0.1%, CC-4047 or thalidomide at 1, 25, 50, 75, 100 μM. Half-media change was performed twice a week. Following 3 weeks of treatment, cells were fixed and stained with 23c6 antibody to determine multinucleated OCLs. An asterisk indicates a significant difference from control (P < .05). MNC indicates multinucleated cells.
Osteoclast formation is inhibited dose dependently by 3 weeks of treatment with CC-4047 and thalidomide. (A, B) For osteoclast assays, nonadherent cells from HBM and MMBM were cultured with 10 ng/mL CSF-M and 50 ng/mL RANKL for 21 days; 0.1% DMSO (control), CC-4047, or thalidomide (Thal) was added to the culture twice a week to maintain a concentration of 100 μM. After 3 weeks of treatment, cells were fixed and stained for 23c6+ multinucleated OCLs. For all the micrographs, original magnification is ×20. Each value represents the mean plus or minus SD of multinucleated cells per well of at least 8 wells. All experiments were performed independently 4 times. (C) Nonadherent HBM cells were cultured with 10 ng/mL CSF-M and 50 ng/mL RANKL for 21 days and DMSO (control) at 0.1%, CC-4047 or thalidomide at 1, 25, 50, 75, 100 μM. Half-media change was performed twice a week. Following 3 weeks of treatment, cells were fixed and stained with 23c6 antibody to determine multinucleated OCLs. An asterisk indicates a significant difference from control (P < .05). MNC indicates multinucleated cells.
Statistical analyses
Each experiment was repeated at least 3 times, and all quantitative data are presented as mean plus or minus SD. Statistical differences were determined by Student t test. The results were considered statistically significant at P values below .05.
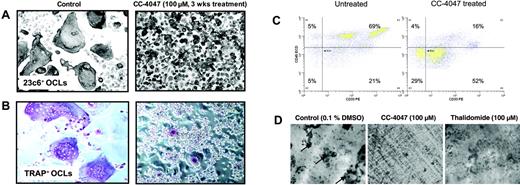
CC-4047 enhances development of granulocytic cells unable to perform bone resorption. (A, B) An OCL formation assay was performed in the presence of 100 μM CC-4047 or 0.1% DMSO for 3 weeks and cells were stained for CD51/61+ (23c6 antibody) and for TRAP+ multinucleated OCLs. Images were obtained using an Olympus IX70 microscope equipped with a 20 ×/0.40 numeric aperture objective lens (Olympus, Melville, NY), and were acquired through Magnafire 4.1 software (Optronics, Goleta, CA). For all micrographs, original magnification is ×20. (C) After 21 days of OCL culture, cells were detached using cell dissociation buffer (Sigma-Aldrich). Cells were stained with anti–CD45-ECD and anti–CD33-PE. Data were acquired on a Dako-Cytomation MoFlo cytometer. After an incubation period of 21 days cell survival was determined by annexin V–FITC/PI double staining. The percentage of viable cells, negative for both annexin V–FITC and PI, was determined. (D) Nonadherent healthy donor bone marrow cells (2 × 105 cells/well) were seeded on whale dentin slices in 96-well multiplates in 100 μL/well in α-MEM containing 20% horse serum and cultured as described in “Osteoclastic bone resorption assays.” OCL formation on dentin slices was confirmed by TRAP staining. Resorption lacunae (arrows) were stained with hematoxylin, and the pit area was quantified by light microscopy.
CC-4047 enhances development of granulocytic cells unable to perform bone resorption. (A, B) An OCL formation assay was performed in the presence of 100 μM CC-4047 or 0.1% DMSO for 3 weeks and cells were stained for CD51/61+ (23c6 antibody) and for TRAP+ multinucleated OCLs. Images were obtained using an Olympus IX70 microscope equipped with a 20 ×/0.40 numeric aperture objective lens (Olympus, Melville, NY), and were acquired through Magnafire 4.1 software (Optronics, Goleta, CA). For all micrographs, original magnification is ×20. (C) After 21 days of OCL culture, cells were detached using cell dissociation buffer (Sigma-Aldrich). Cells were stained with anti–CD45-ECD and anti–CD33-PE. Data were acquired on a Dako-Cytomation MoFlo cytometer. After an incubation period of 21 days cell survival was determined by annexin V–FITC/PI double staining. The percentage of viable cells, negative for both annexin V–FITC and PI, was determined. (D) Nonadherent healthy donor bone marrow cells (2 × 105 cells/well) were seeded on whale dentin slices in 96-well multiplates in 100 μL/well in α-MEM containing 20% horse serum and cultured as described in “Osteoclastic bone resorption assays.” OCL formation on dentin slices was confirmed by TRAP staining. Resorption lacunae (arrows) were stained with hematoxylin, and the pit area was quantified by light microscopy.
Results
CC-4047 inhibits early osteoclast formation in human bone marrow cultures
To investigate the effects of thalidomide and CC-4047 on OCL differentiation and function, we used human nonadherent mononuclear bone marrow cells from healthy donors and untreated patients with MM. In previous dose-finding studies, we showed that CC-4047 and thalidomide up to 100 μM are not toxic to human hematopoietic progenitors.27 Therefore, to achieve the most pronounced effects, we selected 100 μM as the standard dosage in all our experiments. In a 21-day culture system, addition of CC-4047 at a final concentration of 100 μM twice a week to either healthy donor bone marrow (HBM) or multiple myeloma bone marrow (MMBM) cultures almost completely inhibited OCL formations (mean ± SD, 0 ± 0 OCL per well and 2.8 ± 2.6 OCL per well, respectively) in comparison to control (mean ± SD, 59 ± 13 and 57 ± 35, respectively), and thalidomide (mean ± SD, 14 ± 1.9 and 10 ± 6.2, respectively) (Figure 1A-B).
In dose-escalation studies, we investigated the effects of different concentrations of CC-4047 and thalidomide on OCL formation. Both drugs showed significant inhibition of OCL formation at concentrations of 1 μM. OCL formation was almost completely inhibited by 100 μM CC-4047. In contrast, despite a 100-fold dose increase of thalidomide up to 100 μM, no further inhibition of OCL was observed (Figure 1C).
The inhibitory effect of CC-4047 on osteoclastogenesis was not caused by induction of cell death, but associated with the development of an overgrowing population of small cells, as can be seen in Figure 2A-B. CC-4047 increased the absolute cell number in median 2.5-fold from 0.66 × 104 cells/mL in the control up to 1.65 × 106 cells/mL under CC-4047 treatment. Annexin-PI staining showed no increase in the apoptotic or necrotic fraction (Figure S1A-B, available on the Blood website; see the Supplemental Figures link at the top of the online article). The overgrowing cells showed a high density, were not multinucleated, and lacked characteristics of mature OCLs. To analyze whether these small overgrowing cells were immature OCL precursors that did not fuse to form OCLs, we performed TRAP staining. TRAP is an enzyme universally expressed in both immature and mature OCLs and also by macrophages. As shown in Figure 2A-B, only a very low number of small cells were TRAP+, suggesting that CC-4047 induces a shift in lineage commitment prior to differentiation of hematopoietic progenitors into macrophages and OCL precursors. Flow cytometric analysis showed a decrease of CD45+ cells from 74% to 20% under treatment with CC-4047, and CD33 brightness was reduced dramatically (Figure 2C). Untreated and thalidomide-treated cultures formed significant numbers of multinucleated TRAP+ OCLs.
To confirm that the inhibition of OCL formation was accompanied by abrogation of bone resorption, we next performed bone resorption assays. After 21 days of culture in the presence of CC-4047, thalidomide, or DMSO as control, pit resorption areas were determined. Consistent with complete OCL inhibition, pit formation and bone resorption on dentin slices were also completely abrogated by CC-4047 (bone resorption area 0 mm2), but not by thalidomide (bone resorption area 3.5 mm2 vs control 8 mm2; Figure 2D). This result also confirms that the rare small TRAP+ cells identified in CC-4047–treated cultures do not have functional osteoclastogenic activity.
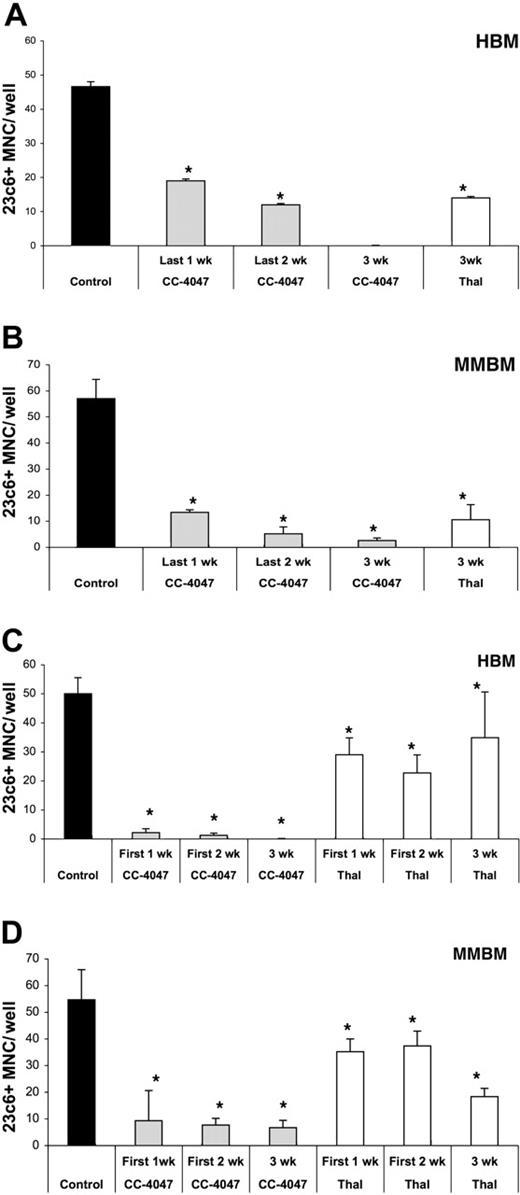
To investigate further if the inhibitory effect of CC-4047 is an early or late event in OCL development, we conducted time-course experiments. First, human bone marrow cells were treated with CC-4047 for all 3 weeks, the last 2 weeks, or only the last week of the culture. As shown in Figure 3A-B, inhibition of OCL formation of HBM and MMBM was significantly lower when the drug was added during only the last week (60% and 77% inhibition, respectively) in comparison to 3 weeks of treatment (100% and 95% inhibition, respectively). In contrast, when drug treatment was carried out during only the first week of HBM and MMBM cultures, the inhibitory effect was almost as potent (96% and 85%, respectively) as in the continuing presence of the drug (100% and 87%, respectively), indicating that CC-4047 interferes with the early stage of differentiation and proliferation of the OCL progenitors (Figure 3C-D).
After thalidomide treatment of healthy donor and MM bone marrow cultures for 3 weeks, OCL formation still occurred and the inhibitory effect was significantly less pronounced than the effects observed with CC-4047 (Figure 3). In contrast to CC-4047, treatment with thalidomide did not stimulate the overgrowth of a population of small cells. There was no effect on size and nuclei number in OCLs formed with thalidomide treatment when compared with control.
CC-4047 inhibits development of CFU-GMs
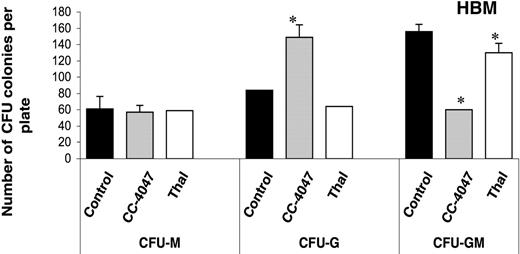
We next examined the effects of CC-4047 and thalidomide on the differentiation of granulocyte-macrophage colony-forming units (CFU-GMs), which are precursors for OCL. Both drugs were added to the colony cultures only once and the cultures were incubated for 9 days. Examination of colony phenotypes showed that CC-4047 significantly increased the number of granulocyte colony-forming units (149 ± 13 colonies per well) in comparison to control (84 ± 15) and thalidomide (64 ± 0). In contrast, numbers of CFU-GM, which are the precursors for OCLs, were significantly decreased with CC-4047 treatment (60 ± 14) in comparison to control (156 ± 11) and thalidomide (160 ± 14). Numbers of the CFU-M colonies formed with CC-4047 treatment were not different when compared with the control group (Figure 4). Thalidomide-treated cells formed similar numbers and types of colonies as control.
Inhibition of osteoclast formation is an early event. Nonadherent cells from HBM and MMBM were cultured with 10 ng/mL CSF-M and 50 ng/mL RANKL for 21 days and DMSO (control), CC-4047, or thalidomide (Thal) was added twice a week to maintain a concentration of 0.1% and 100 μM, respectively. Drugs were added to (A) HBM and (B) MMBM for either the last week, the last 2 weeks, or for 3 weeks. Drugs were also added to (C) HBM or (D) MMBM for either the first 1 week, the first 2 weeks, or for 3 weeks. Cells were fixed and stained with 23c6 antibody to detect the multinucleated mature OCLs. Data shown are the mean plus or minus SD of multinucleated cells per well of at least 8 wells. *Significant difference from control (P < .05). All experiments were performed independently 3 times.
Inhibition of osteoclast formation is an early event. Nonadherent cells from HBM and MMBM were cultured with 10 ng/mL CSF-M and 50 ng/mL RANKL for 21 days and DMSO (control), CC-4047, or thalidomide (Thal) was added twice a week to maintain a concentration of 0.1% and 100 μM, respectively. Drugs were added to (A) HBM and (B) MMBM for either the last week, the last 2 weeks, or for 3 weeks. Drugs were also added to (C) HBM or (D) MMBM for either the first 1 week, the first 2 weeks, or for 3 weeks. Cells were fixed and stained with 23c6 antibody to detect the multinucleated mature OCLs. Data shown are the mean plus or minus SD of multinucleated cells per well of at least 8 wells. *Significant difference from control (P < .05). All experiments were performed independently 3 times.
CC-4047 inhibits development of CFU-GM–derived osteoclasts
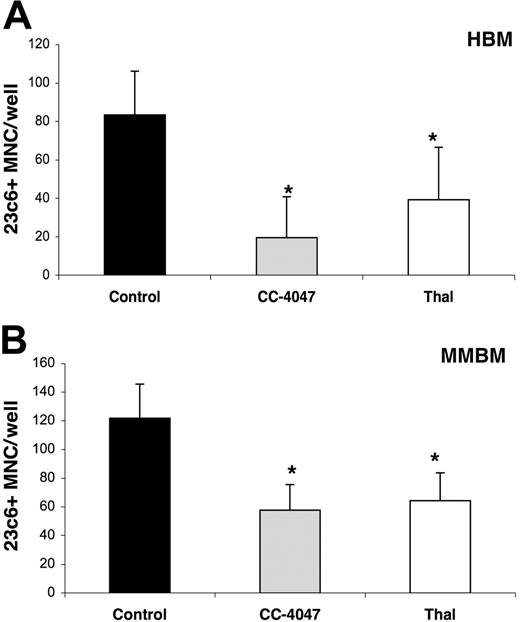
To confirm that an early stage of osteoclastogenesis is affected by CC-4047, CFU-GM colonies were isolated from colony CFU-GM assays treated with CC-4047, thalidomide, or control vehicle. OCL formation assays were set up with purified CFU-GM cells and cultured for 21 days without further drug treatment. One-time addition of CC-4047 and thalidomide at the beginning of the CFU-GM assays did not affect cell growth or reduce the total number of the colonies. But OCL assays with pretreated purified CFU-GM cells showed a significant inhibition of OCL formation (CC-4047 19 ± 21 OCL per well, and thalidomide 39 ± 27 for HBM; and CC-4047 57 ± 17 and thalidomide 64 ± 19 for MMBM) in comparison to control (83 ± 22 for HBM and 121 ± 23 for MMBM) (Figure 5A-B).
CC-4047 inhibits development of CFU-GM osteoclast precursors. Nonadherent bone marrow cells were cultured in methylcellulose culture containing 30% FCS and 100 pg/mL rh CSF-GM in the presence of 100 μM CC-4047, 100 μM thalidomide (Thal), or 0.1% DMSO. After 9 to 10 days of incubation, colony phenotypes and the numbers were determined by inverted microscope. Asterisks indicate a significant difference from control (P < .01). The experiment was performed independently 3 times. Each value is the mean plus or minus SD of CFU colonies per plate.
CC-4047 inhibits development of CFU-GM osteoclast precursors. Nonadherent bone marrow cells were cultured in methylcellulose culture containing 30% FCS and 100 pg/mL rh CSF-GM in the presence of 100 μM CC-4047, 100 μM thalidomide (Thal), or 0.1% DMSO. After 9 to 10 days of incubation, colony phenotypes and the numbers were determined by inverted microscope. Asterisks indicate a significant difference from control (P < .01). The experiment was performed independently 3 times. Each value is the mean plus or minus SD of CFU colonies per plate.
CSF-G is not involved in shift of lineage commitment of hematopoietic progenitors
OCL formation in the presence of CC-4047 led to the development of a population of small cells with high density and granulocytic features. We have shown in our previous studies that CC-4047 treatment of hematopoietic progenitors led to increased CSF-G production and an increase in myeloid colonies.27 It is well known that CSF-G can induce uncommitted hematopoietic cells to differentiate into granulocytes.33 Therefore, we determined if the inhibition of OCL development by CC-4047 was mediated by up-regulation of CSF-G, resulting in a shift of lineage commitment toward granulopoieses. OCL formation assays were performed with CC-4047 in the presence of recombinant monoclonal anti–human CSF-G neutralizing antibody. The concentration of neutralizing antibody (16 μg/mL) was chosen based on CSF-G cytokine levels achieved in bone marrow cultures treated with CC-4047 (up to 1514 μg/mL) in previous experiments.27 Despite treatment with high concentrations of CSF-G neutralizing antibody, the CC-4047–induced inhibition of OCL formation could not be abolished, and we still observed the development of an overgrowing population of small cells with CC-4047 (Figure 6). The neutralizing antibody was tested in standard colony assay and showed a significant down-regulation of granulocyte-CFU (CFU-G) at 16 μg/mL (Figure S1).
CC-4047 induces a loss of expression of the transcription factor PU.1
Transcription factors c-fos and PU.1 are essential for early OCL formation; therefore, we analyzed PU.1 and c-fos protein expression during early OCL development. Analysis of transcription factors important for osteoclastogenesis revealed that PU.1 protein expression was markedly down-regulated in a dose-dependent manner after 3 days of treatment of hematopoietic progenitors, with a complete inhibition observed at 100 μM. Thalidomide, which showed a weaker OCL inhibition, did not decrease PU.1 protein expression (Figure 7A). In contrast, c-fos protein expression, which is involved in later stages of OCL differentiation after macrophage commitment, was unaffected by CC-4047 treatment or thalidomide (Figure 7B).
Discussion
OCLs develop from CFU-GM–derived cells and are formed by the fusion of their precursors.2-6,34 Regardless of the precise origin of OCL progenitors, it is clear that any disruption of hematopoiesis can profoundly affect OCL numbers. In the current study we found that an IMiD of thalidomide (CC-4047) inhibits the generation and differentiation of OCLs by down-regulation of PU.1, resulting in a shift of lineage commitment of hematopoietic progenitors. In OCL formation assays, CC-4047 almost completely inhibited the formation of mature multinucleated OCLs and induced concomitantly an overgrowing population of small cells that lacked the features and activity of OCLs. Flow cytometry analysis showed under treatment with CC-4047 a 2.5-fold increase of cell number compared with untreated controls treatment. Interestingly, CD45+ cells decreased from 74% to 20%. This observation is in accordance with the literature. Anderson et al35 determined that PU.1 is critical for lineage-specific expression of CD45. Although CD45 is normally expressed in all leukocyte lineages, it is critically regulated by PU.1 only in myeloid cells. Myeloid cells from PU.1–/– mice failed to express CD45. We also observed a slight decrease of CD34+ in our treated group, but with a dramatically reduced brightness. This is in accordance with previous reports describing the surface marker profile of bone marrow progenitors with PU.1 deletion. PU.1 deficiency resulted in a dramatic loss of any readily identifiable lymphoid and myeloid progenitor or stem cell populations. Cells were c-kit+/Sca1–/IL-7R–/CD34–/Fc RII/IIIlow, a phenotype that is not defined by the existing progenitor scheme.36 Taken together, our findings reflect the effects of PU.1 loss resulting in increased cell population of immature granulocytes lacking adequate expression of CD45 and a decreased CD34 expression.
We also found that treatment with CC-4047 for only the first week of the 3-week OCL cultures was sufficient to significantly inhibit OCL formation. CC-4047 treatment of already committed CD14+ cells for only the last week had fewer effects on OCL formation. In addition, in CFU-GM assays we mixed only the initial cell assay with CC-4047 and used the cells from this assay to set up OCL assays without adding new CC-4047. This initial one-time treatment of the cells was sufficient to significantly inhibit the OCL formation in a 3-week culture. These data suggest that CC-4047 acts especially at the early stages of the OCL differentiation by induction of a shift in lineage commitment, resulting in inhibition of OCL formation.
CC-4047 blocks development of osteoclasts at a very early time point of hematopoietic lineage commitment. Nonadherent cells from (A) HBM and (B) MMBM were cultured in methylcellulose culture containing 30% FCS and 100 pg/mL rh CSF-GM in the presence of 100 μM CC-4047, 100 μM thalidomide (Thal), or 0.1% DMSO. After 9 to 10 days, hematopoietic precursors were purified and used to set up an OCL formation assay as described in “Materials and methods.” During the 21-day culturing time, none of the drug was added to the culture. Each value is the mean plus or minus SD of mononucleated cells per well of at least 8 wells. Asterisks indicate a significant difference from control (P < .01). All experiments were performed independently twice.
CC-4047 blocks development of osteoclasts at a very early time point of hematopoietic lineage commitment. Nonadherent cells from (A) HBM and (B) MMBM were cultured in methylcellulose culture containing 30% FCS and 100 pg/mL rh CSF-GM in the presence of 100 μM CC-4047, 100 μM thalidomide (Thal), or 0.1% DMSO. After 9 to 10 days, hematopoietic precursors were purified and used to set up an OCL formation assay as described in “Materials and methods.” During the 21-day culturing time, none of the drug was added to the culture. Each value is the mean plus or minus SD of mononucleated cells per well of at least 8 wells. Asterisks indicate a significant difference from control (P < .01). All experiments were performed independently twice.
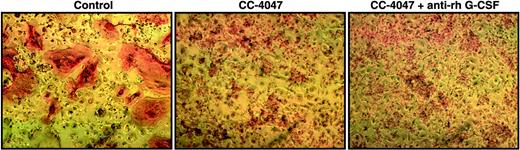
CC-4047–triggered shift of lineage commitment of hematopoietic progenitors is not induced by CSF-G. Nonadherent bone marrow cells from HBM were cultured with 10 ng/mL CSF-M and 50 ng/mL RANKL for 21 days. DMSO 0.1% (control), or CC-4047 (100 μM) alone or in combination with anti–rh CSF-G neutralizing antibody (16 μg/mL) were added to the culture twice a week. Following 3 weeks of treatment, cells were fixed and stained with 23c6 antibody to detect mature multinucleated OCLs. Images were obtained as described for Figure 2.
CC-4047–triggered shift of lineage commitment of hematopoietic progenitors is not induced by CSF-G. Nonadherent bone marrow cells from HBM were cultured with 10 ng/mL CSF-M and 50 ng/mL RANKL for 21 days. DMSO 0.1% (control), or CC-4047 (100 μM) alone or in combination with anti–rh CSF-G neutralizing antibody (16 μg/mL) were added to the culture twice a week. Following 3 weeks of treatment, cells were fixed and stained with 23c6 antibody to detect mature multinucleated OCLs. Images were obtained as described for Figure 2.
To confirm the lineage shift, we performed colony assays. In accordance with our previous results, CC-4047 had no toxic effects on hematopoietic progenitors since the total numbers of colonies were not decreased.27 However, CFU-G colonies were increased in CC-4047 cultures at the expense of CFU-GMs. Since CFU-GM–derived cells form OCLs at a high efficiency and are recognized as early precursors for OCLs,37,38 our data indicate that CC-4047 inhibits lineage commitment to CFU-GMs, resulting in impairment of OCL development. The concomitant up-regulation of CFU-G colonies is consistent with our observation that CC-4047 induced the development of high numbers of granulocytic cells.
Two models have been proposed to explain how hematopoietic stem cells commit to differentiation into various lineages. This occurs either through signaling by lineage-specific hematopoietic cytokines33 or is induced by lineage-specific transcription factors.39,40 In our previous work, we showed that CC-4047 induced an increase in CSF-G secretion accompanied by a significant up-regulation of myeloid-CFU colonies and a strong inhibition of erythroid-CFUs and erythroid burst-forming units.27 Metcalf has shown that lineage-specific cytokines such as CSF-G are able to instruct uncommitted cells to differentiate into a given cell type.33 Therefore, we determined if the inhibition of OCL development that resulted from a shift in lineage commitment toward granulopoieses was induced by up-regulation of CSF-G. Our experiments revealed that treatment with CSF-G neutralizing antibody did not abolish the CC-4047–induced shift of lineage commitment toward granulopoiesis, consistent with the possibility that CC-4047 specifies cell fate not by cytokines but by targeting essential lineage-specific transcription factors.
Numerous transcription factors have been shown to play a role in osteoclastogenesis. PU.1, NFATc1, and AP-1 (c-fos and Jun) are critical factors promoting OCL formation.12,41,42 NFATc1 is a calcium-regulated transcription factor that plays an integral role in the RANKL-induced transcriptional program during the terminal differentiation of OCLs. AP-1 transcription factors like c-fos are critical for the induction of NFATc1 and important transcriptional partners of NFATc1.43 Early c-fos expression is involved in the differentiation of OCLs and dendritic cells after macrophage commitment. Our experiments showed that during early osteoclastogenesis, NFATc1 was not expressed (data not shown) and c-fos protein expression was not affected by CC-4047 treatment, suggesting that CC-4047 acts on an earlier stage of osteoclastogenesis than both NFATc1 and c-fos. In contrast, PU.1 protein expression in hematopoietic progenitors was completely down-regulated in a dose-dependent manner after 3 days of treatment with CC-4047, suggesting that down-regulation of PU-1 results in a strong inhibition of OCL formation. Tondravi et al10 showed that development of both OCLs and macrophages is arrested in PU.l-deficient mice, reflecting the absence of OCLs, and PU.l–/– mice exhibit the classic hallmarks of osteopetrosis. The absence of both OCLs and macrophages in PU.1 mutant animals suggests that this transcription factor regulates the initial stages of myeloid differentiation. Besides its role in osteoclastogenesis, the inhibition of PU.1 leads also to an increase of granulopoiesis.19 Dahl et al19 have shown that granulocyte progenitors were increased at the expense of macrophage progenitors from PU.1+/– embryonic stem cell–derived bodies. They proposed that decreasing PU.1 hinders the ability of multipotential hematopoietic cells to differentiate into macrophage progenitors and directs the cells toward granulocytic differentiation.19,44 DeKoter et al45 showed that mutation in the PU.1 gene causes a severe reduction in granulocytic/macrophage progenitors, which is in accordance with our results. They propose that decreasing the expression of PU.1 hinders the ability of multipotential hematopoietic cells to make the requisite amount of PU.1 to be necessary for macrophage development. Dakic et al36 recently developed a conditional mutation that allows inactivation of PU.1 in adult hematopoietic cells. They found that PU.1 ablation resulted in dramatically perturbed hematopoiesis and greatly enhanced granulopoiesis, which we also observed in our analysis. Besides a marked expansion in granulocytic progenitors, the changes were accompanied by a loss of macrophages, which are the OCL progenitors. These results from Dakic et al36 suggest that PU.1 is essential for the normal transit through the common myeloid progenitors and common myeloid progenitors/granulocyte-macrophage progenitors stages of adult hematopoiesis, where it promotes lymphopoiesis, and conversely, restricts granulopoiesis. The data are consistent with our results showing that CC-4047 induces a complete down-regulation of the transcription factor PU.1 and shifts the early lineage commitment of hematopoietic progenitors toward granulopoiesis at the expense of CFU-GM46 and subsequently inhibits OCL formation. The CC-4047–triggered strong induction of granulopoiesis by transcription factors rather than by cytokines is confirmed by our observation that treatment of OCL cultures with CSF-G neutralizing antibodies could not abolish the CC-4047–induced shift toward granulopoiesis.
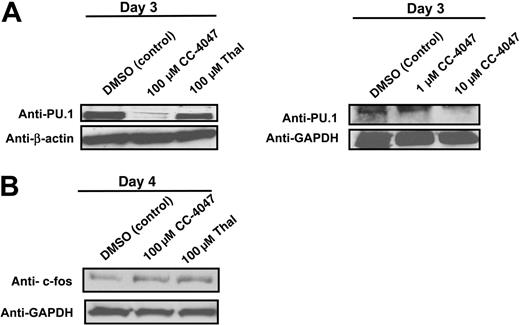
CC-4047 induces a loss of expression of the transcription factor PU.1, but not of c-fos. Nonadherent bone marrow cells from healthy donors were cultured in 10 ng/mL CSF-M and 50 ng/mL RANKL in the presence of CC-4047 (100, 10, and 1 μM), thalidomide (Thal; 100 μM), or DMSO (0.1%). After culturing for 3 and 4 days, total cell lysates were prepared for Western blotting to determine (A) PU.1 and (B) c-fos protein expression. GAPDH and β-actin antibody were used as loading control.
CC-4047 induces a loss of expression of the transcription factor PU.1, but not of c-fos. Nonadherent bone marrow cells from healthy donors were cultured in 10 ng/mL CSF-M and 50 ng/mL RANKL in the presence of CC-4047 (100, 10, and 1 μM), thalidomide (Thal; 100 μM), or DMSO (0.1%). After culturing for 3 and 4 days, total cell lysates were prepared for Western blotting to determine (A) PU.1 and (B) c-fos protein expression. GAPDH and β-actin antibody were used as loading control.
In all our experiments, thalidomide exhibited similar but less potent effects than CC-4047. This is consistent with previous preclinical and clinical data, showing that thalidomide is less potent than IMiDs.22-27
Pharmacokinetic studies from healthy volunteers and phase 1 trials of CC-4047 have shown that systemic levels between 1 μM and 5 μM are achievable (Dr David Stirling, Celgene, Warren, NJ; oral communication, October 5, 2005). In our study, significant inhibition of osteoclast formation was observed at concentrations of 1 μM CC-4047. Therefore, we think that treatment of patients with CC-4047 could lead to a significant inhibition of osteoclast formation.
Previous clinical trials have shown that IMiDs induce high response rates in patients with relapsed or even refractory MM.24-26 One of the most common side effects in these studies was neutropenia, which is in contrast to the enhanced granulopoiesis observed in our studies. This observation might be explained by the fact that PU.1-deficient hematopoietic progenitors show disturbed neutrophil maturation. Anderson et al47 verified that PU.1–/– mice develop neutrophils, which fail to attain functional competence as shown by the absence of messages for neutrophil secondary granule components and their inability to respond to stimuli that normally invokes neutrophil function. Dakic et al36 showed that PU.1–/– granulocytes, in contrast to wild-type controls, maintained c-kit expression and displayed some, but not complete, maturation as measured by morphologic criteria. Reverse transcriptase–polymerase chain reaction analysis revealed that the primary granule component, myeloperoxidase, as well as the secondary granule component, lactoferrin, were expressed normally, whereas the putative PU.1 target gene and component of nicotinamide adenine dinucleotide phosphate oxidase, gp91, was down-regulated, which might result in an aberrant differentiation.48 In their model, PU.1-deficient granulocytes were unable to compete with wild-type cells to repopulate blood and spleen; this effect was also observed in the peripheral blood of nonchimeric PU.1-deficient animals.
Taken together, these data indicate that PU.1 is not essential for specification of granulocytic precursors; however, it is required for further differentiation of these precursors into mature neutrophils.
Our results show that CC-4047 leads to dramatic inhibition of OCL formation induced by a shift in lineage commitment without showing any cell toxicity. Blocking bone resorption and concomitantly targeting the myeloma cell would be a major contribution to improve the treatment of MM. Therefore, CC-4047 might be a valuable drug for developing future therapies that target both the tumor and osteoclastic activity in patients with MM and, potentially, those with other diseases associated with the development of osteolytic lesions.
Prepublished online as Blood First Edition Paper, December 22, 2005; DOI 10.1182/blood-2005-08-3450.
D.S. is employed by Celgene Corporation, whose product was studied in the present work.
S.L. and D.R. designed the research; S.L., G.A., M.G., N.K., T.H., J.A., M.Y.M., and I.G. participated in performing the research; D.S. contributed vital new reagents; G.A., V.D., A.D., M.Y.M., and S.L. analyzed data; G.A. and S.L. wrote the paper; and all authors checked the final version of the manuscript.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge Rita Bhutta for excellent assistance in preparing this manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal