Abstract
Hematopoietic stem cells (HSCs) are the key elements responsible for maintaining blood-cell production throughout life and for lymphohematopoietic reconstitution following bone marrow (BM) transplantation. Enhancement of the engrafting potential and expansion capabilities of HSCs as well as hematopoietic progenitor cells (HPCs) has been a long-time desire as a means of reducing the risks and difficulties that accompany BM transplantation. The ability of HSCs/HPCs to reconstitute the hematopoietic system of irradiated hosts is negatively regulated by an intracellular adaptor protein, Lnk. Here we have identified the functional domains of Lnk and developed a dominant-negative (DN) Lnk mutant that inhibits the functions of Lnk endogenously expressed in the HSCs/HPCs and thereby potentiates the HSCs/HPCs for engraftment. Importantly, even transient expression of DN-Lnk in HSCs/HPCs facilitated their engraftment under nonmyeloablative conditions and fully reconstituted the lymphoid compartments of immunodeficient host animals. HPCs expressing DN-Lnk were efficiently trapped by immobilized vascular cell adhesion molecule-1 (VCAM-1) in a transwell migration assay, suggesting involvement of Lnk in the regulation of cell mobility or cellular interaction in microenvironments. Transient inhibition of Lnk or Lnk-mediated pathways could be a potent approach to augment engraftment of HSCs/HPCs without obvious side effects.
Introduction
Hematopoietic stem cells (HSCs) have self-renewal activity and give rise to all lineages of the blood system throughout the lifetime of an individual.1 The unique biologic properties of HSCs have been used extensively for therapeutic strategy to cure hematologic malignancies or genetic diseases by bone marrow (BM) transplantation. However, the relative inability to expand HSCs ex vivo imposes major limitations on the current use of purified adult HSCs or cord-blood HSCs for transplantation. In addition, although gene transfer into HSCs or hematopoietic progenitor cells (HPCs) may be a promising tool in the correction of a wide variety of hematopoietic and genetic disorders, a challenge to gene therapy protocols mediated through HSCs/HPCs is the low levels of engraftment by transduced progenitors.2 Enhanced efficacy of HSC/HPC engraftment could improve the outcome of clinical transplantations as well as gene therapy protocols and might be achieved by modulating the ability of HSCs/HPCs to home to and repopulate the recipient BM. Another major problem accompanied by the use of genetically modified HSCs/HPCs is an increasing risk of malignant transformation of progeny cells caused by deregulated expression of endogenous genes or the exogenous transgene following integration of virus-based vectors into the cellular genome.3-6
Among many steps involved in the reconstitution of hematopoietic cells after BM transplantation, transplanted HSCs/HPCs migrate to the BM and attach to the microenvironment (the so-called niche) through interaction of various adhesion molecules including integrins. The α4 integrins α4β1 (very-late antigen-4 [VLA-4]) and α4β7 mediate binding to fibronectin (FN), to vascular cell adhesion molecule-1 (VCAM-1), and to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and have been implicated in both HSC mobilization as well as in the rehoming of transplanted HSCs placed in the circulation.7-9 Analysis of α4-null mutant and chimeric mice has shown that terminal differentiation of blood cells is possible without α4 integrins, albeit inefficiently, and that these receptors are essential to maintain normal hematopoiesis in BM microenvironments, probably by regulating transmigration and proliferation of HPCs.10,11 For migration of HSCs/HPCs, interactions between the chemokine stromal-derived factor-1 (SDF-1, also referred to as CXCL12) and its receptor CXCR4 play an essential role in HSC seeding of the BM during murine embryonic development.12,13 Murine HSCs express CXCR4, CCR3, and CCR9 but migrated only in response to CXCL12.14 CXCR4 overexpression in human cord-blood HPCs resulted in improved definitive human stem-cell motility, retention, and multilineage repopulation in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice xenotransplantation models.15
Lnk is an intracellular adaptor protein mainly expressed in lymphocytes and HSCs/HPCs.16-18 Lnk, together with APS (adaptor molecule containing PH and SH2 domains) and SH2-B, forms an adaptor protein family whose members share an NH2-terminal homologous domain followed by a pleckstrin homology (PH) domain, an SH2 domain, and a highly conserved tyrosine phosphorylation site at the COOH terminus.19-25 Mutant mice lacking Lnk show overproduction of B-lineage cells24,26 that is partly due to enhanced signaling through c-Kit17 and thrombocytosis due to increased responsiveness of megakaryocytes to thrombopoietin (TPO).26,27 In addition, the numbers of HSCs/HPCs are increased in the absence of Lnk, and the repopulating ability is significantly enhanced.17,18 Importantly, to date, no malignancy, dysplasia, or dysfunction of blood cells is found to be caused by the Lnk deficiency. Based on those observations, we reasoned that Lnk inhibitors that block functions in HSCs/HPCs would be beneficial for reconstituting a blood system after severe myelosuppression induced by irradiation or chemotherapy and for engrafting blood cells into immunodeficient hosts without risk of malignant transformation. In this paper, we investigated the functional domains of Lnk for its negative regulatory roles in lymphohematopoiesis and developed a dominant-negative (DN) Lnk mutant that inhibits the functions of Lnk endogenously expressed in the HSCs/HPCs. We also revealed that impairment of Lnk functions resulted in augmented interaction or trapping of HPCs on cell-adhesion molecules, such as VCAM-1. Our results unveiled an unrecognized regulatory pathway in HSCs/HPCs and showed that inhibition of Lnk or Lnk-mediated pathways could be a potent approach to augment HSC/HPC engraftment.
Materials and methods
Cells, reagents, and mice
MC9 cells were cultured in RPMI 1640 medium supplemented with 8% fetal bovine serum (FBS), antibiotics, and 10 U/mL IL-3. Plat-E cells were maintained as described previously.28 COS7 cells were maintained in RPMI 1640 supplemented with 8% FBS and antibiotics. Purified mouse IL-3, stem-cell factor (SCF), and TPO were purchased from Peprotech (London, United Kingdom). C57BL/6 mice (Ly5.2+) and C.B-17 scid/scid (SCID) mice were purchased from CLEA Japan (Tokyo, Japan). Mice congenic for the Ly5 locus (Ly5.1+), GFP-transgenic mice,29 and Lnk-/- mice17,24,26 were bred and maintained under specific pathogen-free conditions at the animal facility of the Institute of Medical Science, the University of Tokyo.
Lnk mutants and expression constructs
DNA fragments encoding R364E, dPH, dN, and Y536F Lnk mutations were generated by polymerase chain reaction (PCR)–based, site-directed mutagenesis and confirmed by DNA sequencing. Mutant cDNA encoding Lnk lacking the C-terminal tail (dC) was constructed by digesting the Lnk cDNA with BglII, blunting with the Klenow fragment, followed by self-ligation. Other Lnk cDNAs carrying the mutations in various combinations were constructed by replacing the appropriate cDNA fragments with mutated fragments using restriction enzymatic digestion followed by ligation. The resulting cDNA fragments were subcloned into a pcDNA3 mammalian expression vector (Invitrogen, Carlsbad, CA) or a pMY retroviral vector containing an internal ribosomal entry site (IRES) between the multicloning site and the eGFP-encoding cDNA (a generous gift from Dr Toshio Kitamura, the Institute of Medical Science, the University of Tokyo). The Lnk cDNA cassette lacking the stop codon was generated by PCR and inserted into pcDNA-DEST47 (Invitrogen) to generate expression vector for the Lnk-GFP fusion protein.
Retroviral transduction and BM transplantation
The pMY vectors were transfected into Plat-E cells by lipofection using FuGENE 6 (Roche Diagnostics, Basel, Switzerland). Supernatant containing the packaged retroviral particles was harvested and condensed by centrifugation in a microcentrifuge for 16 hours at 4°C. For transduction, MC9 cells were incubated in fresh medium containing 8 μg/mL polybrene (Sigma, St Louis, MO) and condensed retroviral supernatant for 3 hours. Cells were washed and further cultured in fresh medium containing 10 ng/mL SCF. For HSC/HPC transduction, BM cells negative for lineage markers (B220, CD3, Mac-1, Gr-1, and TER-119) were purified using a MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany) as described previously.17 The lineage marker–negative (Lin-) cells (4 × 105) were incubated with the retroviral supernatants for 3 hours and plated on Retronectin-coated dishes (Takara Bio, Shiga, Japan) with RPMI 1640 medium containing 8% FBS, 50 μM 2-mercaptoethanol, 1 × nonessential amino acids (Gibco Invitrogen, Grand Island, NY), 10 ng/mL SCF, 100 ng/mL TPO, and 5 ng/mL IL-3. The next day, the transduction was repeated with fresh retroviral supernatants, and the cells were cultured for an additional 24 hours. The transduced Lin- cells (2.0 × 105) were washed and intravenously injected into lethally irradiated (9.5 Gy) recipient mice.
Flow cytometry
MC9 cells expressing eGFP following retroviral transduction were analyzed on a FACSCalibur instrument (BD Biosciences, San Jose, CA). Peripheral-blood nucleated cells from recipient mice were stained using predetermined optimal concentrations of the respective antibodies and were then analyzed on a FACSCalibur. The following monoclonal antibodies were used: FITC-conjugated anti-B220 (anti-CD45R, RA3-6B2), anti–Mac-1 (anti-CD11b, M1/70), and anti–Sca-1 (E13-161.7); PE-conjugated anti-CD3ϵ (145-2C11), anti–Gr-1 (RB6-8C5), anti–TER-119, anti–Sca-1, and anti–c-Kit (anti-CD117, 2B8); biotin-conjugated anti-Ly5.1 (anti-CD45.1, A20) and anti-Ly5.2 (anti-CD45.2, 104); APC-conjugated anti-B220 and anti–c-Kit (all purchased from BD Biosciences or eBioscience, San Diego, CA). Anti-CD41 (MWReg30; BD Biosciences) was biotinylated using NHS-biotin (Pierce Biotechnology, Rockford, IL). Binding of biotin-conjugated antibodies was visualized with APC- or PerCP-conjugated streptavidin (BD Biosciences). Dead cells were gated out by their bright intensity after 7-amino actinomycin D (Sigma) staining. Platelet counts were calculated from the number of red blood cells and the ratio of GFP+CD41+ platelets to TER-119+ red blood cells.
Chemical crosslinking, immunoprecipitation, and immunoblotting
COS7 cells were transfected with expression vectors encoding wild-type, mutant Lnk proteins or Lnk-GFP fusion protein using Superfect (Qiagen, Hilden, Germany) and were harvested 48 hours after transfection. Cells were lysed in lysis buffer17 and subjected to chemical cross-linking using various concentrations of BS3 (Pierce Biotechnology) or immunoprecipitated using anti-GFP–conjugated agarose (MBL, Nagoya, Japan). Proteins were separated, transferred on membranes, and then immunoblotted using anti-Lnk antibodies.24,26 For stimulation experiments, transfectants were serum-starved for 12 hours and stimulated with 100 ng/mL SCF for 5 minutes. Cells were then lysed, and lysates were subjected to immunoprecipitation and immunoblotting as previously described.17
Plasmid transfection to primary BM cells
Whole BM cells (5.0 × 106) obtained from Ly5.1+ mice, GFP-transgenic mice,29 or C.B-17+/+ mice were suspended in Human CD34 Nucleofector solution (Amaxa, Cologne, Germany) containing 5 μg plasmid DNA and pulsed using program U-3 on a Nucleofector Device (Amaxa). Cells were cultured for 24 hours in medium containing 10 ng/mL SCF, 100 ng/mL TPO, and 5 ng/mL IL-3. For competitive repopulation assays, transfected Ly5.1+ cells (0.6 × 106 to 2.4 × 106) were mixed with fresh Ly5.2+ BM cells in a ratio of 3:1 and were intravenously injected into lethally irradiated (9.5 Gy) Ly5.2+ mice. For platelet reconstitution experiments, transfected BM cells from GFP-transgenic mice (1.2 × 106) were intravenously injected into lethally irradiated mice together with nontransgenic BM cells (0.2 × 106) from C57BL/6 mice. For BM transplantation studies in SCID mice, transfected C.B-17+/+ cells (2.0 × 105) were washed and intravenously injected into SCID mice that had been treated with 1.0 Gy nonmyeloablative irradiation.
Genomic PCR
Peripheral-blood nuclear cells obtained from mice that received transplants were lysed in lysis buffer (100 mM Tris-HCl, pH 8.5; 5 mM EDTA; 0.2% SDS; 200 mM NaCl; and 100 μg/mL proteinase K) and the genomic DNA purified by isopropanol precipitation. The extracted genomic DNA was subjected to PCR amplification with the following primer pairs: for the Rag2 gene (forward 5′-TTAATTCAACCAGGCTTCTCACTT-3′, reverse 5′-GCCTGCTTATTGTCTCCTGGTATG-3′) or for the neomycin-resistant gene (forward 5′-CAGGATCTCCTGTCATCTCACCTT-3′, reverse 5′-TCAGAAGAACTCGTCAAGAAGGCG-3′).
Migration assay
Lin- BM cells purified from the Ly5.2+ wild-type or Ly5.2+Lnk-/- mice were suspended in RPMI 1640 containing 8% FBS at a density of 5 × 105/mL, and a 100 μL per transwell insert was pipetted in duplicate into the upper chamber of 5 μm pore size transwells (Corning Costar, Cambridge, MA). In some experiments, 1 × 106 unpurified total BM cells from Ly5.1+ wild-type mice were applied to the upper chamber, or the transwell membrane was precoated with mouse VCAM-1/Fc chimera (R&D Systems, Minneapolis, MN) solution at a concentration of 5 μg/mL in PBS for 1 hour at room temperature. Anti-CD49d, the α4 chain of the VLA-4 (PS/2; Southern Biotech, Birmingham, AL), or its class-matched control antibody was added at the concentration of 10 μg/mL. The lower chamber contained medium with or without CXCL12 (SDF-1, 100 ng/mL; Peprotech) to test CXCL12-induced chemotactic migration or chemokinetic motility. After 3 hours of incubation, cells migrated into the lower chamber were collected and divided into 2 groups. One was stained with labeled antibodies, and the other was supplied with a known concentration of Flow-Count Fluorospheres (Beckman Coulter, Fullerton, CA), and the number of Ly5.2+c-Kit+Sca-1+ cells was determined by flow cytometry. Lin- cells from C57BL/6 mice were retrovirally transduced, washed, and subjected to transmigration assay through noncoated or VCAM-1–coated membranes. Cells migrated into the lower chamber in the presence of CXCL12 were harvested, stained, and analyzed by flow cytometry to measure GFP-positive transfectants in Lin-c-Kit+Sca-1+ cells.
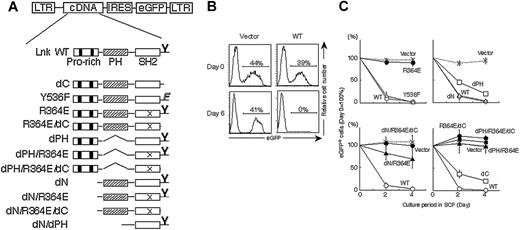
Functional domains of Lnk critical for cell-growth regulation. (A) Schematic representations of Lnk mutants and the pMY vector used for coexpressing Lnk mutants and eGFP from a single mRNA carrying an IRES. The N-terminal domain with its proline-rich stretches (▪), the PH domain ( ), the SH2 domain (□), and a tyrosine phosphorylation site at the C terminus (Y) are illustrated. Deletions of the N-terminal domain (dN), the PH domain (dPH), and the C-terminal tail (dC) as well as substitutions for Y536 by phenylalanine (Y536F) and R364 by glutamic acid (R364E; depicted as “X” in the SH2 domain) are indicated. (B) MC9 cells transduced with a control vector (left column, “vector”) or a Lnk-expressing vector (right column, “WT”) were cultured in the presence of SCF, and the percentages of live eGFP-positive cells were determined by flow cytometry at the indicated time points. (C) SCF-induced growth rates of MC9 cells expressing the indicated Lnk mutants were compared with that of nontransduced cells by dividing the percentage of eGFP-positive cells at each indicated time point by that at the start of culture (day 0). Data represent means ± SD of at least 3 experiments.
), the SH2 domain (□), and a tyrosine phosphorylation site at the C terminus (Y) are illustrated. Deletions of the N-terminal domain (dN), the PH domain (dPH), and the C-terminal tail (dC) as well as substitutions for Y536 by phenylalanine (Y536F) and R364 by glutamic acid (R364E; depicted as “X” in the SH2 domain) are indicated. (B) MC9 cells transduced with a control vector (left column, “vector”) or a Lnk-expressing vector (right column, “WT”) were cultured in the presence of SCF, and the percentages of live eGFP-positive cells were determined by flow cytometry at the indicated time points. (C) SCF-induced growth rates of MC9 cells expressing the indicated Lnk mutants were compared with that of nontransduced cells by dividing the percentage of eGFP-positive cells at each indicated time point by that at the start of culture (day 0). Data represent means ± SD of at least 3 experiments.
Functional domains of Lnk critical for cell-growth regulation. (A) Schematic representations of Lnk mutants and the pMY vector used for coexpressing Lnk mutants and eGFP from a single mRNA carrying an IRES. The N-terminal domain with its proline-rich stretches (▪), the PH domain ( ), the SH2 domain (□), and a tyrosine phosphorylation site at the C terminus (Y) are illustrated. Deletions of the N-terminal domain (dN), the PH domain (dPH), and the C-terminal tail (dC) as well as substitutions for Y536 by phenylalanine (Y536F) and R364 by glutamic acid (R364E; depicted as “X” in the SH2 domain) are indicated. (B) MC9 cells transduced with a control vector (left column, “vector”) or a Lnk-expressing vector (right column, “WT”) were cultured in the presence of SCF, and the percentages of live eGFP-positive cells were determined by flow cytometry at the indicated time points. (C) SCF-induced growth rates of MC9 cells expressing the indicated Lnk mutants were compared with that of nontransduced cells by dividing the percentage of eGFP-positive cells at each indicated time point by that at the start of culture (day 0). Data represent means ± SD of at least 3 experiments.
), the SH2 domain (□), and a tyrosine phosphorylation site at the C terminus (Y) are illustrated. Deletions of the N-terminal domain (dN), the PH domain (dPH), and the C-terminal tail (dC) as well as substitutions for Y536 by phenylalanine (Y536F) and R364 by glutamic acid (R364E; depicted as “X” in the SH2 domain) are indicated. (B) MC9 cells transduced with a control vector (left column, “vector”) or a Lnk-expressing vector (right column, “WT”) were cultured in the presence of SCF, and the percentages of live eGFP-positive cells were determined by flow cytometry at the indicated time points. (C) SCF-induced growth rates of MC9 cells expressing the indicated Lnk mutants were compared with that of nontransduced cells by dividing the percentage of eGFP-positive cells at each indicated time point by that at the start of culture (day 0). Data represent means ± SD of at least 3 experiments.
Results
Identification of the functional domains of Lnk
The precise functional domain of Lnk that is involved in the negative regulation of growth signaling is still undetermined. Various Lnk mutants were constructed, and their influence on c-Kit–mediated cell growth was studied by expressing them in c-Kit+ MC9 mast cells using a retroviral vector that bicistronically drives expression of Lnk mutants and eGFP (Figure 1A). c-Kit–mediated growth of transduced cells was compared with that of nontransduced cells by monitoring the percentage of eGFP-positive cells using flow cytometry. The expression of mutant proteins was confirmed in sorted eGFP-positive cells by immunoblotting (data not shown). MC9 cells expressing eGFP alone proliferated as well as the nontransduced MC9 cells upon stimulation with SCF, and the percentage of eGFP-positive cells was well maintained. In contrast, MC9 cells expressing the wild-type Lnk did not proliferate and rapidly disappeared during culture (Figure 1B). Neither deletion of the N-terminal domain (dN) nor mutation of a conserved tyrosine phosphorylation site (Y536F) affected the inhibitory effect of Lnk, while deletion of either the PH domain (dPH) or the C-terminal tail (dC) partly compromised the inhibitory effect. On the other hand, a point mutation in the SH2 domain (R364E) completely abolished the growth inhibition (Figure 1C). Thus, the SH2 domain of Lnk has an essential role in inhibiting c-Kit–mediated cell growth. The PH domain and C-terminal tail of Lnk, but not the Y536 residue within the region, partly contributed to the efficient inhibition.
Generation of DN-Lnk mutants
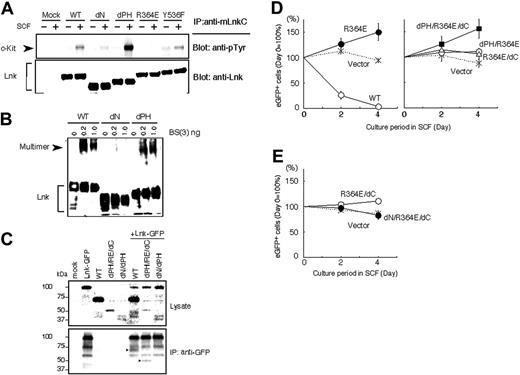
The SH2 domain of Lnk was critical for its association with c-Kit tyrosine kinase receptor as demonstrated by coimmunoprecipitation of Lnk mutants with c-Kit in COS7 transfectants. R364E mutation completely abolished the ability to interact with phosphorylated c-Kit (Figure 2A). We also found that the Lnk PH domain was indispensable for localization to plasma membrane (data not shown). SH2-B, one of the Lnk-family adaptor proteins, forms a pentameric complex via its N-terminal domain.30 Because the N-terminal domains of Lnk-family adaptor proteins are well conserved, we examined whether Lnk also forms a multimer via its N-terminal region. Multimer complexes stabilized by the presence of a chemical cross-linker were detected in cells expressing the wild-type Lnk as well as in those expressing the dPH mutant but not in cells expressing the dN mutant (Figure 2B). Multimer formation of Lnk was further demonstrated by coimmunoprecipitation of Lnk with Lnk-GFP fusion using anti-GFP antibody that does not recognize Lnk (Figure 2C).
Lnk SH2 mutants dominant-negatively inhibit the negative regulatory function of Lnk in cell growth. (A) Lnk associates with c-Kit via the SH2 domain. COS7 transfectants coexpressing the indicated Lnk mutants and c-Kit were stimulated with SCF and lysed. Proteins immunoprecipitated with an anti-Lnk antibody were separated and immunoblotted with an antiphosphotyrosine antibody to detect c-Kit (top panel) or with an anti-Lnk antibody (bottom panel). The association of c-Kit with Lnk was abolished by the R364E SH2 mutation. (B) Multimer formation of Lnk via the N-terminal region. Total cell lysates of COS7 transfectants expressing the indicated Lnk mutants were treated with various concentrations of the chemical cross-linker BS3 and then subjected to immunoblotting using anti-Lnk antibodies. Lnk formed multimer complexes with slower mobility (arrowhead). Multimerized complexes were not detected with the dN Lnk mutants. (C) Wild-type Lnk or dPH/RE/dC mutant associated and coimmunoprecipitated with Lnk-GFP by anti-GFP (arrowheads in bottom panel). In contrast, dN/dPH mutant did not coimmunoprecipitate with Lnk-GFP. (D-E) Lnk SH2 mutants were retrovirally transduced into MC9-Lnk cells expressing wild-type Lnk. The transduced cells were cultured in the presence of SCF, and their growth was compared with that of nontransduced MC9-Lnk cells by dividing the percentage of eGFP-positive cells at the indicated time points by that at the start of culture (day 0). Data shown are means ± SD of 3 experiments.
Lnk SH2 mutants dominant-negatively inhibit the negative regulatory function of Lnk in cell growth. (A) Lnk associates with c-Kit via the SH2 domain. COS7 transfectants coexpressing the indicated Lnk mutants and c-Kit were stimulated with SCF and lysed. Proteins immunoprecipitated with an anti-Lnk antibody were separated and immunoblotted with an antiphosphotyrosine antibody to detect c-Kit (top panel) or with an anti-Lnk antibody (bottom panel). The association of c-Kit with Lnk was abolished by the R364E SH2 mutation. (B) Multimer formation of Lnk via the N-terminal region. Total cell lysates of COS7 transfectants expressing the indicated Lnk mutants were treated with various concentrations of the chemical cross-linker BS3 and then subjected to immunoblotting using anti-Lnk antibodies. Lnk formed multimer complexes with slower mobility (arrowhead). Multimerized complexes were not detected with the dN Lnk mutants. (C) Wild-type Lnk or dPH/RE/dC mutant associated and coimmunoprecipitated with Lnk-GFP by anti-GFP (arrowheads in bottom panel). In contrast, dN/dPH mutant did not coimmunoprecipitate with Lnk-GFP. (D-E) Lnk SH2 mutants were retrovirally transduced into MC9-Lnk cells expressing wild-type Lnk. The transduced cells were cultured in the presence of SCF, and their growth was compared with that of nontransduced MC9-Lnk cells by dividing the percentage of eGFP-positive cells at the indicated time points by that at the start of culture (day 0). Data shown are means ± SD of 3 experiments.
These observations prompted us to test whether noninhibitory Lnk mutants could act as DN mutants by forming a multimer complex with the wild-type Lnk by virtue of homophilic interaction of the N-terminal domain, thereby segregating Lnk from its target receptor tyrosine kinases and downstream signaling molecules. Next, noninhibitory Lnk mutants were expressed in MC9 transfectants overexpressing the wild-type Lnk (MC9-Lnk),17 which were maintained in the presence of IL-3 but did not proliferate well upon stimulation with SCF. MC9-Lnk cells expressing R364E outgrew nontransduced cells, indicating that the R364E mutant acts as a DN mutant (Figure 2D). Of the generated mutations, the R364E mutant with combined deletions in the PH domain and the C-terminal tail (dPH/R364E/dC) cancelled the growth inhibition by the wild-type Lnk most effectively. Deletion of the N-terminal domain of the R364E/dC mutant (dN/R364E/dC) completely abolished the DN effect (Figure 2E). These observations are consistent with Lnk multimerization through its N-terminal domain. The dPH/R364E/dC mutant formed complex and coimmunoprecipitated with Lnk-GFP. In contrast, Lnk mutant lacking N-terminal and PH domains (dNdPH) did not coimmunoprecipitate with Lnk-GFP (Figure 2C).
Enhanced hematopoietic reconstitution by HSCs/HPCs expressing DN-Lnk
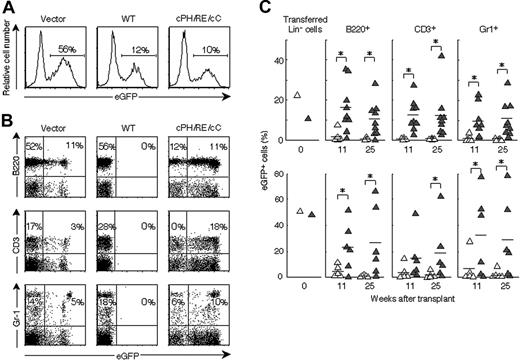
We next assessed whether identified DN-Lnk mutants inhibit Lnk endogenously expressed in primary HSCs/HPCs and facilitate the repopulating ability in the BM transplantation model. Lin- BM cells containing HSCs/HPCs were purified and retrovirally transduced with Lnk or dPH/R364E/dC mutant expression constructs during a 48-hour culture in the presence of IL-3, SCF, and TPO. Retrovirus titers were adjusted so that the percentage of eGFP-positive cells expressing Lnk or dPH/R364E/dC was comparable and did not exceed that of cells transduced with the control vector (Figure 3A). Cells containing transduced eGFP-positive cells were then transferred into lethally irradiated recipient mice, and peripheral blood cells of recipients were monitored several weeks after transplantation (Figure 3B). In mice that received transplants with cells transduced with Lnk, few eGFP-positive cells were detected in any lineages of peripheral-blood cells, whereas cells expressing vector alone contributed to the generation of cells with a B220+ B lineage or a CD3+ T lineage as well as to Gr-1+ myeloid cells. In contrast, in the recipient mice undergoing transplantation with Lin- cells expressing dPH/R364E/dC, more contribution by eGFP-positive cells to all 3 lineages was observed with significant differences (Figure 3B-C). The percentage of eGFP-positive cells in spleen and BM obtained from mice that received transplants was similar to that in peripheral blood (data not shown). Mice that received transplants with dPH/R364E/dC-expressing cells displayed a significantly higher percentage of eGFP-positive cells in each lineage than those that received transplants with vector-treated cells during the 25 weeks of observation (Figure 3C). Interestingly, R364E mutant did not show any enhancing effects in the same experimental protocols (data not shown). These results indicate that the dPH/R364E/dC mutant inhibits functions of Lnk endogenously expressed in HSCs/HPCs and enhances the repopulating ability in irradiated host animals.
Transient inhibition of Lnk in HSCs/HPCs
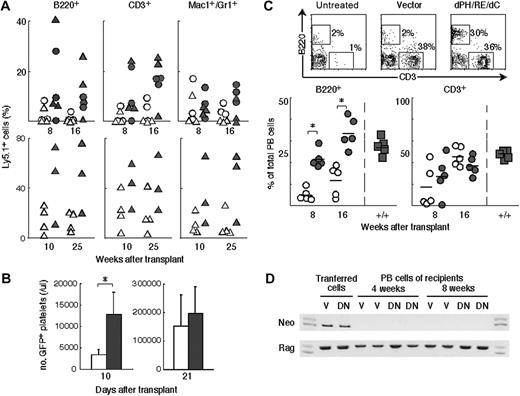
Gene transfer by viral vectors that are integrated into the cellular genome is now facing an ethical problem regarding the increased risk of malignant transformation.3-6 To avoid using retroviral transduction and to gain further insight into the mechanism of Lnk inhibition in enhanced repopulation, we examined the effectiveness of short-term transient expression of dPH/R364E/dC by plasmid transfection. Whole BM cells from Ly5.1+ mice were transfected by electroporation with a control plasmid vector or one encoding dPH/R364E/dC and were cultured in the presence of IL-3, SCF, and TPO for 24 hours. The transfection efficiency in the Lin-c-Kit+ population was approximately 50% using an eGFP-expressing plasmid in the employed conditions (data not shown). Transfected BM cells were transplanted into lethally irradiated recipient mice (Ly5.2+) together with nontransfected Ly5.2+ competitor BM cells. Ly5.1+ cells were measured in the peripheral blood of recipient mice 8 to 10 and 16 to 25 weeks later. Mice that received transplants with cells carrying the dPH/R364E/dC-expressing plasmid reconstituted a much higher percentage of the Ly5.1+ B-, T-, and myeloid-lineage cells, whereas only small portions of Ly5.1+ cells were detected in mice that received transplants with cells treated with the control vector (Figure 4A). We also examined the effect of transient expression of dPH/R364E/dC in the recovery of platelet production at early time points. BM cells from GFP-transgenic mice were transfected and injected into lethally irradiated mice. Platelet counts in recovering animals were significantly increased at 10 days after transfer (Figure 4B). Thus the short-term, transient inhibition of Lnk in HSCs/HPCs was also effective in augmenting the engraftment of HSCs/HPCs and early recovery of platelet production.
Expression of dPH/R364E/dC in HSCs/HPCs facilitates the repopulation of blood cells in irradiated hosts. (A) Transduction efficiencies of retroviral vectors into Lin- cells. The percentage of eGFP-positive cells in Lin- populations transduced with control vector (left), vectors encoding Lnk (middle), or dPH/RE/dC (right) are indicated. (B) Lethally irradiated mice received transplants with transduced Lin- cells. Peripheral-blood cells harvested from mice 11 weeks after transplantation were stained with anti-B220, anti-CD3, or anti–Gr-1, and the contribution of eGFP-positive cells to B-, T-, or myeloid-lineage cells was analyzed. Numbers represent the percentage of cells that fall into the indicated boxes. Typical results of multiple experiments are shown. (C) Long-term repopulating ability of dPH/R364E/dC-transduced cells. The contribution of transduced cells to B-, T-, and myeloid-lineage cells was analyzed at indicated time points. ▵ indicate the control vector-transduced group; ▴, the dPH/R364E/dC-transduced group. Two representative results of 5 experiments are shown. *P < .05: significant differences between dPH/R364E/dC-transduced and control groups.
Expression of dPH/R364E/dC in HSCs/HPCs facilitates the repopulation of blood cells in irradiated hosts. (A) Transduction efficiencies of retroviral vectors into Lin- cells. The percentage of eGFP-positive cells in Lin- populations transduced with control vector (left), vectors encoding Lnk (middle), or dPH/RE/dC (right) are indicated. (B) Lethally irradiated mice received transplants with transduced Lin- cells. Peripheral-blood cells harvested from mice 11 weeks after transplantation were stained with anti-B220, anti-CD3, or anti–Gr-1, and the contribution of eGFP-positive cells to B-, T-, or myeloid-lineage cells was analyzed. Numbers represent the percentage of cells that fall into the indicated boxes. Typical results of multiple experiments are shown. (C) Long-term repopulating ability of dPH/R364E/dC-transduced cells. The contribution of transduced cells to B-, T-, and myeloid-lineage cells was analyzed at indicated time points. ▵ indicate the control vector-transduced group; ▴, the dPH/R364E/dC-transduced group. Two representative results of 5 experiments are shown. *P < .05: significant differences between dPH/R364E/dC-transduced and control groups.
Efficient reconstitution of lymphoid compartments in SCID mice using DN-Lnk
We sought to test the efficacy of the transient inhibition of Lnk for treatment of SCID mice under nonmyeloablative conditions. BM cells from wild-type mice were transfected with plasmid DNAs by electroporation and then transplanted into SCID mice treated with a low dose of irradiation (1.0 Gy). Only few control vector-transfected cells engrafted into the BM of the recipients and produced some B lymphocytes. In contrast, the dPH/R364E/dC-transfected cells successfully repopulated and fully reconstituted the lymphoid compartments of SCID mice (Figure 4C). In mice that received transplants with dPH/R364E/dC-transfected cells, lymphocyte production was observed from early time points. Especially, B-lineage cells produced in BM were rapidly and robustly reconstituted from dPH/R364E/dC-transfected cells. Plasmid DNAs scarcely integrate into the host genome without linearization and are lost as the transfected cells proliferate. Consistently, a neomycin-resistant gene on the employed plasmid was not detected at all in genomic DNA isolated from donor cell–derived lymphocytes examined 4 or 8 weeks after transplantation (Figure 4D). The transient expression of dPH/R364E/dC by plasmid DNA potentiated HSCs/HPCs for engraftment even in nonmyeloablative conditions without any notable integration of plasmid DNA into the cellular genome.
Increased interaction of HPCs to VCAM-1 in the absence or by inhibition of Lnk
Transplanted HSCs/HPCs migrate and attach to the microenvironment (the so-called niche) through interaction of adhesion molecules, proliferate, and differentiate to various hematopoietic cells. The forced expression of dPH/R364E/dC facilitated the multilineage reconstitution of HSCs/HPCs in vivo, leading to an increased contribution of transduced cells in lymphoid and myeloid lineages. The transient inhibition of endogenous Lnk at an early phase following transplantation successfully increased the engraftment capacity of HSCs/HPCs. These imply that blocking Lnk-dependent signaling pathway(s) may also affect the migration or engraftment of HSCs/HPCs in addition to influencing their expansion in recipient mice undergoing transplantation. To examine these possibilities, we performed transmigration assay and investigated whether Lnk played a role in controlling mobility of progenitor cells. First we compared Lin-c-Kit+Sca-1+ BM cells from Lnk-/- mice with wild-type cells and found that CXCL12-induced migration through membrane was comparable. However, in the presence of BM cells other than Lin- precursors, Lin-c-Kit+Sca-1+ cells from Lnk-/- mice did not migrate as efficiently as normal cells (Figure 5A). We thought Lnk-/- cells might more efficiently interact with and be trapped by BM cells such as stroma cells expressing various adhesion molecules. VCAM-1 is one of the adhesion molecules involved in HSC/HPC homing to BM.8,31-33 We examined transwell migration through VCAM-1–coated membrane and found that migration of Lnk-/- precursor cells was not as efficient as that of normal precursors (Figure 5A), indicating that the mobility of HSCs/HPCs on VCAM-1 was regulated by Lnk-mediated pathway(s). The decrease in the number of migrated Lnk-/- cells through VCAM-coated membrane was abolished by antibody against the α4 subunit of VLA-4 (Figure 5A). Anti-α4 treatment only partially inhibited the impaired migration of Lnk-/- cells in the presence of other BM cells, indicating that VCAM is not the only adhesion molecule involved in the process in this experimental condition.
Transient expression of dPH/R364E/dC facilitated HSC/HPC engraftment under myeloablative or nonmyeloablative conditions. (A) Enhanced engraftment under myeloablative conditions demonstrated by a competitive repopulation assay. Ly5.1+ cells were transfected with control or dPH/R364E/dC-expressing vector by electroporation and injected into lethally irradiated Ly5.2+ mice together with Ly5.2+ competitor cells. Peripheral-blood cells of recipient mice that underwent transplantation with cells (○ and •, 0.6 × 106; ▵ and ▴, 2.4 × 106 cells) transfected with control vector (○ and ▵) or with dPH/R364E/dC-expressing vector (• and ▴) were stained and analyzed at indicated time points. Results of 2 experiments are shown. (B) Early platelet production from progenitors transiently expressing dPH/R364E/dC mutant. BM cells from GFP-transgenic mice were transfected and injected into lethally irradiated mice. GFP-positive platelet counts in animals that received cells transfected with control vector (□) or dPH/R364E/dC expression vector (▪) were measured 10 or 21 days after transfer. Shown are means ± SD of results obtained from 2 transfer experiments. *P < .01. (C) Enhanced engraftment under nonmyeloablative conditions and reconstitution of the lymphoid compartment of SCID mice. Transfected wild-type cells were washed and intravenously injected into SCID mice treated with 1.0 Gy nonmyeloablative irradiation. Peripheral-blood cells obtained from untreated SCID mice (top left) or from mice that received transplants with cells treated with control vector (top middle) or dPH/R364E/dC-expressing vector (top right) were stained and analyzed 8 weeks after transplantation. The percentage of B220+ B-lineage or CD3+ T-lineage cells derived from donor cells transfected with control vector (bottom panels, ○) or with dPH/R364E/dC plasmid (•), harvested at the indicated time points, was compared with that of normal wild-type cells (▪). Results obtained from 2 transfer experiments are shown. *P < .03. (D) Lack of plasmid DNA integration into genomic DNA of reconstituted lymphocytes. Genomic DNA isolated from peripheral-blood nucleated cells of recipients at the indicated time points was subjected to PCR amplification for the neomycin-resistant gene derived from the plasmid DNA or for the Rag2 gene for control. V indicates mice that received transplants with vector-transfected cells; DN, mice that received transplants with dPH/R364E/dC-transfected cells. Representative results of multiple experiments are shown.
Transient expression of dPH/R364E/dC facilitated HSC/HPC engraftment under myeloablative or nonmyeloablative conditions. (A) Enhanced engraftment under myeloablative conditions demonstrated by a competitive repopulation assay. Ly5.1+ cells were transfected with control or dPH/R364E/dC-expressing vector by electroporation and injected into lethally irradiated Ly5.2+ mice together with Ly5.2+ competitor cells. Peripheral-blood cells of recipient mice that underwent transplantation with cells (○ and •, 0.6 × 106; ▵ and ▴, 2.4 × 106 cells) transfected with control vector (○ and ▵) or with dPH/R364E/dC-expressing vector (• and ▴) were stained and analyzed at indicated time points. Results of 2 experiments are shown. (B) Early platelet production from progenitors transiently expressing dPH/R364E/dC mutant. BM cells from GFP-transgenic mice were transfected and injected into lethally irradiated mice. GFP-positive platelet counts in animals that received cells transfected with control vector (□) or dPH/R364E/dC expression vector (▪) were measured 10 or 21 days after transfer. Shown are means ± SD of results obtained from 2 transfer experiments. *P < .01. (C) Enhanced engraftment under nonmyeloablative conditions and reconstitution of the lymphoid compartment of SCID mice. Transfected wild-type cells were washed and intravenously injected into SCID mice treated with 1.0 Gy nonmyeloablative irradiation. Peripheral-blood cells obtained from untreated SCID mice (top left) or from mice that received transplants with cells treated with control vector (top middle) or dPH/R364E/dC-expressing vector (top right) were stained and analyzed 8 weeks after transplantation. The percentage of B220+ B-lineage or CD3+ T-lineage cells derived from donor cells transfected with control vector (bottom panels, ○) or with dPH/R364E/dC plasmid (•), harvested at the indicated time points, was compared with that of normal wild-type cells (▪). Results obtained from 2 transfer experiments are shown. *P < .03. (D) Lack of plasmid DNA integration into genomic DNA of reconstituted lymphocytes. Genomic DNA isolated from peripheral-blood nucleated cells of recipients at the indicated time points was subjected to PCR amplification for the neomycin-resistant gene derived from the plasmid DNA or for the Rag2 gene for control. V indicates mice that received transplants with vector-transfected cells; DN, mice that received transplants with dPH/R364E/dC-transfected cells. Representative results of multiple experiments are shown.
Expression of dPH/R364E/dC in Lin-c-Kit+Sca-1+ cells from wild-type mice also changed their migration behavior on VCAM-1. Although Lin-c-Kit+Sca-1+ cells expressing dPH/R364E/dC by retrovirus transduction migrated as well as control cells through noncoat membrane, their migration through VCAM-1–coated membrane was impaired, with a significant difference when compared with control-vector–transduced cells (Figure 5B; Table 1). These results support the idea that impairment of Lnk functions resulted in augmented interaction or trapping of HSCs/HPCs on cell-adhesion molecules and may reflect one of the molecular mechanisms for the enhanced engraftment of HSCs/HPCs by transient expression of dPH/R364E/dC.
Increased trapping of HSCs/HPCs expressing dPH/R364E/dC by VCAM-1
. | Transwell migration ratio in the presence of VCAM-1 . | . | |
|---|---|---|---|
| Cell populations . | Vector . | dPH/RE/dC . | |
| Lin+ | 0.947 ± 0.021 | 0.969 ± 0.015 | |
| Lin–Kit+Sca1– | 1.052 ± 0.007 | 1.045 ± 0.021 | |
| Lin–Kit+Sca1+ | 1.002 ± 0.021 | 0.865 ± 0.015* | |
. | Transwell migration ratio in the presence of VCAM-1 . | . | |
|---|---|---|---|
| Cell populations . | Vector . | dPH/RE/dC . | |
| Lin+ | 0.947 ± 0.021 | 0.969 ± 0.015 | |
| Lin–Kit+Sca1– | 1.052 ± 0.007 | 1.045 ± 0.021 | |
| Lin–Kit+Sca1+ | 1.002 ± 0.021 | 0.865 ± 0.015* | |
Lin– BM precursor cells were retrovirally transduced by control vector or dPH/R364E/dC expression vector. After transduction, cell migration induced by CXCL12 was analyzed by transwell migration assay, and migration of GFP-positive transfectants through the noncoated membrane or through the VCAM-1-coated membrane was compared (see Figure 5). Migration ratios were calculated by dividing the percentage of GFP-positive cells that migrated through the VCAM-1-coated membrane by the percentage of those migrating through the noncoated membrane in indicated cell populations. Data are presented as mean ± SD of results from 3 experiments.
P < .001 compared with vector-transduced cells
Discussion
We developed a new approach to enhance the engrafting capability of HSCs/HPCs for reconstituting the blood system after severe myelosuppression and for reconstituting the immune system of immunodeficient hosts. A Lnk mutant protein identified in this study inhibits the functions of Lnk endogenously expressed in the HSCs/HPCs and thereby potentiates the HSCs/HPCs for engraftment. We showed that even transient inhibition of Lnk is a useful approach for enhancing the ability of HSCs/HPCs to engraft without integration of transgenes to the cellular genome. The genetic manipulation of HSCs by viral vectors that permanently integrate into the cellular genome is accompanied by considerable risk of side effects, including malignant transformation in both humans and mice.3-6 Our approach using a plasmid vector has an advantage in reducing the side effects associated with genomic integration and long-term transgene expression.
The R364E mutant inhibits the negative regulatory function of Lnk for cell growth in MC9 mast cells. Early hematopoiesis in the aorta-gonad-mesonephros (AGM) region is also inhibited by Lnk overexpression.34 However, the R364E mutant did not show any effects and did not augment early hematopoiesis in an AGM culture system.34 This discrepancy implies additional deletions of the PH and C-terminal tail are important to efficiently block Lnk functions. Indeed, the dPH/R364E/dC mutant worked more efficiently as a DN mutant than the R364E mutant when evaluated by the effect on growth of MC9-Lnk transfectants. It is highly likely that Lnk mutants form multimer through the N-terminal domain and sequester the wild-type Lnk from their target receptors and effector molecules. Cellular sublocalization of Lnk multimers is probably altered by deletions of the PH domain that is known to bind phospholipids in cellular membranes. Alternatively, deletion of the PH domains as well as the C-terminal tail may further compromise the Lnk functions that are dependent on these domains through protein-protein interactions. For example, these regions in addition to the SH2 domain may have critical functions to control cell adhesion or cell movement. The growth of MC9 mast cells is dependent on the stimulation through c-Kit but not on cell adhesion or cellular interactions in culture. Engraftment of HSCs/HPCs as well as early hematopoiesis in the AGM region is presumably highly dependent on cell adhesion or cellular interactions of progenitor cells with cells forming the microenvironments. To inhibit Lnk-mediated negative regulation, simultaneous disruption of the SH2, PH, and C-terminal domains seems important.
Change in the mobility of HSCs/HPCs on VCAM-1 by Lnk deficiency or by Lnk inhibition with dPH/R364E/dC. (A) Increased trapping of Lnk-deficient Lin-c-Kit+Sca-1+ cells by BM nucleated cells or by VCAM-1 in transmigration assay. Lin- cells were purified from the Ly5.2+ wild-type (□) or Ly5.2+Lnk-/- mice (▪), and their migration induced by CXCL12 was examined through noncoat membrane (membrane: noncoat; input cells: Lin-), through noncoat membrane in the presence of Ly5.1+ total BM nucleated cells (noncoat, Lin- plus total BM), or through VCAM-1–coated membrane (VCAM-1, Lin-). Anti-α4 chain of the VLA-4 (anti-α4 Ab) or control antibody (control Ab) was added at the concentration of 10 μg/mL. Ly5.2+ Lin-c-Kit+Sca-1+ cells in the bottom wells were counted, and the migration was determined as a percentage of total Ly5.2+ Lin-c-Kit+Sca-1+ cells input into upper wells. Migration induced by CXCL12 through noncoat membrane was comparable between Lnk+/+ (21% ± 7.6%) and Lnk-/- cells (23% ± 3.4%). Results are represented as relative migration compared with that induced by CXCL12 through noncoat membrane, and mean values ± SD of data obtained from 3 experiments are shown. (B) Trapping of the wild-type Lin-c-Kit+Sca-1+ cells by VCAM-1 was augmented by dPH/R364E/dC expression. Lin- cells expressing eGFP alone (vector) or coexpressing dPH/R364E/dC with eGFP (dPH/R364E/dC) were subjected to transwell migration assay through noncoated membrane (noncoat: top panels) or through VCAM-1–coated membrane (VCAM-1: bottom panels). CXCL12-induced migration of Lin-c-Kit+Sca-1+ cells (within boxes in the insets) was analyzed, and the percentage of GFP-positive transfectants was compared. Representative results of 3 experiments are shown.
Change in the mobility of HSCs/HPCs on VCAM-1 by Lnk deficiency or by Lnk inhibition with dPH/R364E/dC. (A) Increased trapping of Lnk-deficient Lin-c-Kit+Sca-1+ cells by BM nucleated cells or by VCAM-1 in transmigration assay. Lin- cells were purified from the Ly5.2+ wild-type (□) or Ly5.2+Lnk-/- mice (▪), and their migration induced by CXCL12 was examined through noncoat membrane (membrane: noncoat; input cells: Lin-), through noncoat membrane in the presence of Ly5.1+ total BM nucleated cells (noncoat, Lin- plus total BM), or through VCAM-1–coated membrane (VCAM-1, Lin-). Anti-α4 chain of the VLA-4 (anti-α4 Ab) or control antibody (control Ab) was added at the concentration of 10 μg/mL. Ly5.2+ Lin-c-Kit+Sca-1+ cells in the bottom wells were counted, and the migration was determined as a percentage of total Ly5.2+ Lin-c-Kit+Sca-1+ cells input into upper wells. Migration induced by CXCL12 through noncoat membrane was comparable between Lnk+/+ (21% ± 7.6%) and Lnk-/- cells (23% ± 3.4%). Results are represented as relative migration compared with that induced by CXCL12 through noncoat membrane, and mean values ± SD of data obtained from 3 experiments are shown. (B) Trapping of the wild-type Lin-c-Kit+Sca-1+ cells by VCAM-1 was augmented by dPH/R364E/dC expression. Lin- cells expressing eGFP alone (vector) or coexpressing dPH/R364E/dC with eGFP (dPH/R364E/dC) were subjected to transwell migration assay through noncoated membrane (noncoat: top panels) or through VCAM-1–coated membrane (VCAM-1: bottom panels). CXCL12-induced migration of Lin-c-Kit+Sca-1+ cells (within boxes in the insets) was analyzed, and the percentage of GFP-positive transfectants was compared. Representative results of 3 experiments are shown.
Lnk deficiency causes the increased signaling through cytokine receptors that are critical for growth of HSCs/HPCs such as c-Kit and TPO receptor, c-mpl.17,24,27 It is likely that HSCs/HPCs expressing DN-Lnk may proliferate better in recipients, especially in the case of continuous Lnk inhibition by retroviral transduction. The effect of DN-Lnk to enhance engraftment of HSCs/HPCs was obvious even by its transient expression. It may result from prolonged survival of HSCs/HPCs due to augmented cytokine receptor signaling, leading to their increased chances to find correct microenvironments. Enhanced proliferation and self-renewal of HSCs/HPCs in short times after homing could still be likely events accounting for the enhanced engraftment by transiently blocking Lnk. Our results from transmigration assays, however, provide a new point of view to the possible mechanisms. Enhanced interaction with cell-adhesion molecules may enhance proper and prompt lodging of transferred HSCs/HPCs to niche-forming cells in the BM or spleen. The enhanced interaction in the microenvironment may also support facilitated expansion or self-renewal of HSCs/HPCs.
SH2-B and APS, members of the Lnk adaptor family, are recently shown to be involved in the regulation of the actin cytoskeleton.35-38 SH2-B interacts with Rac and enhances GH-induced actin reorganization and cell migration.35,36 A potential binding partner of APS has been identified as Vav3,39 a member of the Vav family proteins that function as guanine nucleotide exchange factors (GEFs) for Rho/Rac.40 Rac plays an essential role in regulating the actin cytoskeleton of HSCs/HPCs, which affects their migration, adhesion, and cell-cycle progression.41 Whether Lnk may affect Rac-mediated actin cytoskeletal regulation and cell movement will be an important issue that should be addressed. It is also intriguing that Lnk associates with filamin A (actin binding protein 280, ABP-280) through the PH domain.42 Increased filamin binding to the β-integrin subunit cytoplasmic tail affects cell migration on VCAM-1.43 Lnk might participate in the regulation of integrin-mediated cell adhesion or migration through its interaction with filamin.
Thus, our results shed light on an unrecognized critical regulatory pathway in HSC/HPC and showed transient inhibition of Lnk or Lnk-mediated pathways could be a potent approach to augment HSC/HPC engraftment without obvious side effects. Further generation of more effective Lnk inhibitors and the elucidation of the target receptors and signaling pathways of Lnk will be important.
Prepublished online as Blood First Edition Paper, December 6, 2005; DOI 10.1182/blood-2005-05-2138.
Supported by Special Coordination Funds for Promoting Science and Technology and Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology; the Japanese government; and a grant from the Uehara Memorial Foundation.
H.T. and S.T. designed research, planned and performed experiments, and prepared the manuscript; I.N. and S.-M.K. contributed vital new reagents; M.I. and C.K.-A. performed experiments; and T.T. and K.T. planned experiments and reviewed the manuscript.
We thank Dr Toshio Kitamura for providing a retroviral vector and a packaging cell line. We are also grateful to our colleagues for helpful discussions, technical advice, and critical reading of this manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal