Abstract
We report the generation of a tetracycline-regulated (Tet ON) transgenic mouse model for acute and chronic expression of the iron regulatory peptide hepcidin in the liver. We demonstrate that short-term and long-term tetracycline-dependent activation of hepcidin in adult mice leads to hypoferremia and iron-limited erythropoiesis, respectively. This clearly establishes the key role of hepcidin in regulating the extracellular iron concentration. We previously demonstrated that, when expressed early in fetal development, constitutive transgenic hepcidin expression prevented iron accumulation in an Hfe-/- mouse model of hemochromatosis. We now explore the effect of chronic hepcidin expression in adult Hfe-/- mice that have already developed liver iron overload. We demonstrate that induction of chronic hepcidin expression in 2-month-old Hfe-/- mice alters their pattern of cellular iron accumulation, leading to increased iron in tissue macrophages and duodenal cells but less iron in hepatocytes. These hepcidin-induced changes in the pattern of cellular iron accumulation are associated with decreased expression of the iron exporter ferroportin in macrophages but no detectable alteration of ferroportin expression in the hepatocytes. We speculate that this change in iron homeostasis could offer a therapeutic advantage by protecting against damage to parenchymal cells.
Introduction
Although iron is essential for many cellular processes, particularly for mammalian erythropoiesis, iron in excess can be deleterious as it triggers production of free radicals. To maintain iron homeostasis, there is tight regulation of iron absorption by the duodenum and iron recycling from senescent erythrocytes by tissue macrophages. The recently identified hepatic peptide hepcidin has been proposed to be central to this regulation, acting as the principal iron-regulatory hormone to maintain iron homeostasis. Hepcidin is a 25-amino acid peptide synthesized by the liver, secreted into the bloodstream, and excreted by the kidney.1,2 Hepcidin expression is increased in iron overload3 and decreased in iron deficiency.4,5 Hepcidin-deficient mice accumulate iron in parenchymal cells with sparing of the reticuloendothelial system6 and, conversely, transgenic mice constitutively expressing hepcidin have markedly lower iron stores resulting in severe anemia.7 In humans, the important physiologic role of hepcidin was demonstrated by the identification of homozygous mutations of the hepcidin gene in patients with juvenile hemochromatosis,8 a severe iron overload disorder characterized by increased intestinal iron absorption, increased serum iron, and deposition of excess iron in parenchymal cells. Together, these observations are all consistent with that idea that hepcidin is a key regulator of iron homeostasis that acts by limiting both intestinal iron absorption and macrophage iron release. Recent studies indicate that, to achieve this function, hepcidin binds to ferroportin,9 a transmembrane iron transporter necessary for iron export out of intestinal epithelial cells and macrophages.10 Hepcidin binding induces internalization of ferroportin and its subsequent degradation.9
In humans, hepcidin dysregulation was demonstrated in 2 phenotypically opposite iron disorders, hereditary hemochromatosis (HH) and the anemia of inflammation (also referred to as the anemia of chronic disease). Hepcidin production was reported to be deficient in patients with HH attributable to mutations in HFE, TFR2, or HFE2,11-13 thus explaining their persistently increased iron absorption in the face of iron overload. In contrast, excessive hepcidin production was reported in patients with inflammatory and infectious disorders associated with the anemia of inflammation.14,15 Increased hepcidin expression is expected to rapidly sequester iron in the macrophages and inhibit duodenal iron absorption, 2 hallmarks of the anemia of inflammation.
Recently, transgenic and mutant mouse models with hepcidin deficiency have provided significant insights into the important role of the hepcidin-ferroportin axis in iron metabolism.16-19 In these models, hepcidin deficiency correlated with up-regulated ferroportin protein in enterocytes, leading to increased intestinal iron absorption and increased plasma iron levels. In contrast, there have been few models developed to study the effects of hepcidin overexpression. We previously reported a transgenic mouse model in which the transthyretin (TTR) promoter was used to drive constitutive hepcidin synthesis.20 Due to the very early activity of the TTR promoter in the fetal liver, the majority of the transgenic pups died within a few hours after birth from systemic iron deficiency associated with severe microcytic, hypochromic anemia. This phenotype suggested a hepcidin-dependent blockade of placental iron transport resulting from transgenic fetal hepcidin production late in gestation, when the endogenous hepcidin gene is not detectably expressed. To circumvent the early effects of hepcidin, we developed a conditional transgenic mouse model using a tetracycline-dependent inducible system (Tet ON).21 This model allows overexpression of hepcidin in the liver in response to doxycycline (a tetracycline analog) treatment, allowing investigation of the effects of hepcidin on iron absorption and recycling in adult animals. Here, we report the effects of induced transgenic hepcidin expression on iron homeostasis in normal mice and mice with genetic hemochromatosis (Hfe-/- mice).
Materials and methods
Animals
All mice used in the experiments were cared for according to criteria outlined by the European Convention for the Protection of Laboratory Animals. Animals were maintained in a temperature- and light-controlled environment and were given free access to tap water and standard laboratory mouse food (AO3, iron content 280 mg/kg, UAR, Epinay-sur-Orge, France). Only male mice were used in this study.
Generation of transgenic mice
Null Hfe-deficient mice (Hfe-/- mice) have been described previously.22 Full-length murine Hepc1 cDNA was amplified using the forward primer 5′-GGGGGATATCAGGCCTCTGCACAGCAGAACAGAAGG-3′ and the reverse primer 5′-GGGGGATATCAGGCCTCTATGTTTTGCAACAGATACC-3′. The Hepc1 polymerase chain reaction (PCR) fragment was subcloned into the Ptetbi-1 plasmid (Clontech, Palo Alto, CA) containing a minimal CMV promoter regulated by a tet operator sequence, tetO, which is responsive to the regulatory rTA transactivator. The construct was confirmed by DNA sequence analysis. The 3.4-kbp tetO-Hepc1 transgene was separated from the plasmid sequence by digestion with AseI and used for pronuclear microinjection. rTALAP-1 mice, in which rTA is driven by the liver activator protein (LAP) promoter, were described previously.23 Both transgenic lines (tetO-Hepc1 and rTALAP-1) had mixed genetic backgrounds that included contributions from C57BL/6, 129/Sv, and DBA/2 strains.
Mice harboring only one of the 2 transgenes (either tetO-Hepc1 or rTALAP-1) were used as controls because they do not express transgenic hepcidin. Mice harboring both transgenes (tetO-Hepc1/rTALAP-1 mice), referred to as “inducible mice,” expressed transgenic hepcidin after doxycycline treatment.
Inducible mice were crossed with Hfe-/- mice, to generate inducible/Hfe+/- mice (ie, tetO-Hepc1/rTALAP-1/Hfe+/- mice). Inducible/Hfe+/- mice were then intercrossed to generate inducible/Hfe-/- mice (ie, tetO-Hepc1/rTALAP-1/Hfe-/- mice). In this set of experiments littermates with tetO-Hepc1/Hfe+/-, rTALAP-1/Hfe+/-, tetO-Hepc1/Hfe+/+, or rTALAP-1/Hfe+/+ genotypes were considered controls and animals with tetO-Hepc1/Hfe-/- and rTALAP-1/Hfe-/- genotypes were considered Hfe-/- mice.
Genotyping of transgenic mice by PCR analysis and Southern blotting
Southern blot genotyping of tetO-Hepc1 mice was done as described.7 AseI-digested DNA was electrophoresed, transferred to a nylon membrane (Hybond-N, Amersham Life Sciences, Saclay, France), and hybridized with a probe corresponding to the 3.4-kb AseI fragment of the tetO plasmid. The probe was labeled with [32P]dCTP by random priming with a commercially available kit (Megaprime DNA labeling systems, Amersham Life Sciences).
gDNA (0.5-1 μg) was used in 50-μL PCRs. The tetO-Hepc1 transgene was amplified using forward primer 5′-CGTGCTAGCGCGGCCTCGAC-3′ (annealing to a sequence in multicloning site I of the Ptetbi-1 plasmid) and reverse primer 5′-AACAGATACCACACTGGGAA-3′ (annealing in Hepc1 cDNA). The PCR was performed for 34 cycles (40 seconds at 94°C, 40 seconds at 60°C, and 40 seconds at 72°C), after initial denaturation at 94°C for 4 minutes, concluding with a final elongation step at 72°C for 5 minutes. A 297-bp-specific product corresponded to the tetO-Hepc1 transgene. The rTALAP-1 transgene was amplified using forward primer 5′-CCATGTCTAGACTGGACAAGA-3′ and reverse primer 5′-CTCCAGGCCACATATGATTAG-3′. The PCR was performed for 37 cycles (30 seconds at 94°C, 60 seconds at 57°C, and 30 seconds at 72°C) after initial denaturation at 94°C for 4 minutes, concluding with a final elongation step at 72°C for 5 minutes. PCRs were carried out in 20 mM Tris HCl (pH 8.4), 50 mM KCl, 0.05% W-1, 2 mM MgCl2, 0.2 mM each dNTP, 0.2 μM of each primer, and 4 U Taq polymerase (Invitrogen, Karlsruhe, Germany). The reaction mixtures were analyzed on 1.5% agarose gels containing ethidium bromide. Genotyping results from Southern blot and PCR analyses were consistent. Finally, Hfe genotyping was performed using primers specific for the wild-type Hfe allele (forward 5′-TTCTTTAGATAGCCTCTCAC-3′ and reverse 5′-GTGGCGAGTCACTTTCACCA-3′) and the targeted Hfe allele (forward 5′-AGTTGGGAGTGGTGTCCGA-3′ and reverse 5′-CTAGCTTCGGCCGTGACG-3′), resulting in 502-bp and 190-bp products, respectively. The PCR was performed for 40 cycles (30 seconds at 94°C, 56 seconds at 56°C, and 30 seconds at 72°C) after initial denaturation at 94°C for 4 minutes, concluding with a final elongation step at 72°C for 5 minutes.
Doxycycline administration
Doxycycline, a derivative of tetracycline, is the substance of choice for the induction of the Tet ON system. Mice of all genotypes were treated with doxycycline. For short-term induction, doxycycline hydrochloride (Sigma-Aldrich, Lyon, France) was freshly dissolved in 0.9% NaCl at a final concentration of 4 mg/mL and filter-sterilized prior to intraperitoneal injection. A dose of 2 mg doxycycline was given once to each mouse.
For long-term induction, doxycycline hydrochloride (2 mg/mL final concentration) and sucrose (3% final concentration) were dissolved in distilled sterile water and administered ad libitum from foil-covered water bottles. The doxycycline-sucrose solution was prepared fresh every 3 to 4 days.
Hematologic analysis
Blood was sampled by retro-orbital phlebotomy into heparinized tubes (Capiject T-MLH, Laboratories Terumo, Guyancourt, France) before the mice were humanely killed. Blood cell counts and erythrocyte parameters were determined using a MaxM Coulter automatic analyzer (Coulter, Hialeah, FL).
Northern blotting
Total RNA from mouse tissues was isolated using RNAplus (QBiogene, Illkirch, France). Liver RNA (20 μg) was denatured in formaldehyde-containing buffer and electrophoresed through 1% agarose, 2.2 M formaldehyde gels. Northern blot analysis was performed as previously described.6,24 Briefly, after electrophoresis, RNA was transferred to a nylon membrane (GeneScreen Plus, Perkin Elmer Life Sciences, Courtaboeuf, France) in 20 × SSC buffer and probed simultaneously with cDNA probes specific for hepcidin and ribosomal 18S mRNAs. The probe used to detect hepcidin was prepared from a plasmid isolated by suppressive subtractive hybridization, pT-Adv/Hepc1.6
Iron measurements
Quantification of serum or liver iron content was performed as previously described by Torrance and Bothwell25 using the IL test (Instrumentation Laboratory, Lexington, MA) on an Olympus AU400 automat. For histology, tissues were fixed in 4% formaldehyde, embedded in paraffin, mounted onto slides, and stained with Prussian blue and nuclear red counterstain using standard procedures. For Perls 3,3-diaminobenzidine tetrahydrochloride (DAB) staining, tissue sections were incubated for 20 minutes in 1% H2O2 in 0.1 M PBS, immersed in Perls solution (1:1, 2% HCl and 2% potassium ferrocyanide) at room temperature for 30 minutes, and then incubated for 10 minutes in 0.015% H2O2 in DAB.
Immunoblotting
Microsomal and cytosolic fractions from frozen tissues of 3-month-old mice were prepared as previously described.16 Cytosolic protein (20 μg) solubilized in 1 × Laemmli buffer and incubated for 30 minutes at room temperature (RT), or 20 to 50 μg microsomal protein solubilized in 1 × Laemmli buffer and incubated for 5 minutes at 100°C was electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF filters. Ponceau red staining confirmed similar gel loading and similar transfer of proteins to the membranes. Immunoblots were preincubated with blocking solution (7% skim milk in 0.15% Tween 20 in Tris-buffered saline [TBST]) prior to incubation with primary antibodies. Rabbit polyclonal anti-mouse ferroportin antibody was previously described16,18 and rabbit polyclonal anti-mouse L-ferritin antibody was a kind gift from P. Santambrogio and S. Levi.26 Antibodies were diluted in blocking solution as follows: anti-mouse ferroportin, 1:600, and anti-mouse L-ferritin, 1:1000. After incubation with antibodies, membranes were washed in TBST and then incubated with peroxidase-labeled anti-rabbit immunoglobulin (1:8000, Amersham Life Sciences). Signals were visualized by enhanced chemiluminescence (Supersignal West Pico; Perbio Science France, Brebières, France).
Immunohistochemistry
Tissues from 3-month-old mice were fixed in 4% formaldehyde. Paraffin sections were subjected to microwave treatment and then processed for immunohistochemistry using the EnVision+ System-HRP (DAB) kit (Dako, Trappes, France) according to the manufacturer's recommendations. Primary antibody against ferroportin was diluted 1:75 before use. As a negative control, immunohistochemistry with no primary antibody was performed (not shown).
Results
Generation of a mouse line controlling hepcidin expression
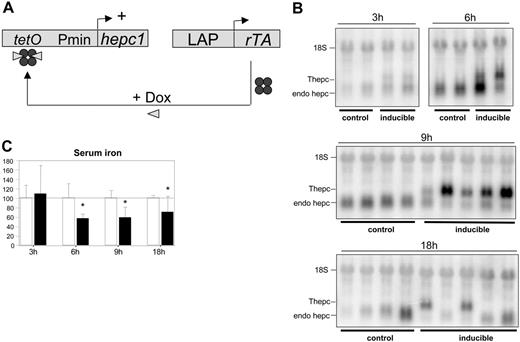
To inducibly express transgenic hepcidin we chose a tetracycline-dependent Tet ON system that permits quantitative and deliberately regulated control of gene activity in the liver (Figure 1A). To develop transgenic mice, Hepc1 cDNA controlled by the tet operator (tetO-Hepc1 transgene) was transferred into mice by pronucleus injection and 11 potential founder animals carrying the transgene were generated. To select for the best trans-responder line, the tetO-Hepc1 mice were crossed with rTALAP-1 mice (previously described, see Schonig et al23 ), harboring the coding sequence of the tetracycline-dependent transactivator under the control of a liver-specific promoter (LAP). The mothers were given doxycycline (2 mg/mL in 3% sucrose) in their drinking water from day 7 after birth until weaning to induce transgenic hepcidin expression in the breastfeeding pups. Expression of the Hepc1 transgene was analyzed in offspring carrying both transgenes by assessing liver transgenic hepcidin mRNA on Northern blots. Of the 11 lines examined, only one showed high levels of transgenic Hepc1 mRNA expression. This line was selected for further study.
Short-term induction of transgenic hepcidin causes hypoferremia
Short-term expression of transgenic hepcidin was induced in 2-month-old inducible mice (ie, double transgenic mice harboring both tetO-Hepc1 and rTALAP-1 transgenes) by a single intraperitoneal injection of doxycycline. Mice were humanely killed 3, 6, 9, or 18 hours after injection. Liver hepcidin mRNA levels were assessed by Northern blot and serum iron was determined. As shown in Figure 1B, transgenic hepcidin mRNA (Thepc, distinguished from endogenous hepcidin mRNA by its longer β-globin polyA) was detected as early as 3 hours after doxycycline injection. It reached a maximum level at 9 hours and then declined by 18 hours after injection. Doxycycline induction of transgenic hepcidin was associated with decreased serum iron level beginning after 6 hours and lasting up to 18 hours (Figure 1C). Of interest, expression of endogenous hepcidin was repressed 3 hours after serum iron level decreased (ie, 9 hours after injection of doxycycline).
Development of mice expressing an inducible hepcidin transgene. (A) Schematic outline of the Tet ON regulatory system. The rTA protein acts as a strong transcriptional activator in the presence of doxycycline by binding to its cognate operator sequence (tetO). (B) Hepcidin mRNA levels in the livers of control mice and inducible mice (ie, double transgenic mice harboring both tetO-Hepc1 and rTALAP-1 transgenes), 3, 6, 9, or 18 hours after a single intraperitoneal injection of doxycycline. Total liver RNA (20 μg) was fractionated by electrophoresis, blotted, and hybridized with hepcidin and 18S-labeled probes. A typical experiment is shown. (C) Serum iron levels (μM) in animals injected with doxycycline 3, 6, 9, or 18 hours after treatment. Results are expressed relative to control serum iron (arbitrarily 100 ± standard deviation). Control animals, □; inducible mice, ▪. Statistical analysis was performed using Student t test (unpaired, 2 tailed): *P < .05, at least 3 animals. Thepc indicates transgenic hepcidin mRNA; endo hepc, endogenous hepcidin mRNA.
Development of mice expressing an inducible hepcidin transgene. (A) Schematic outline of the Tet ON regulatory system. The rTA protein acts as a strong transcriptional activator in the presence of doxycycline by binding to its cognate operator sequence (tetO). (B) Hepcidin mRNA levels in the livers of control mice and inducible mice (ie, double transgenic mice harboring both tetO-Hepc1 and rTALAP-1 transgenes), 3, 6, 9, or 18 hours after a single intraperitoneal injection of doxycycline. Total liver RNA (20 μg) was fractionated by electrophoresis, blotted, and hybridized with hepcidin and 18S-labeled probes. A typical experiment is shown. (C) Serum iron levels (μM) in animals injected with doxycycline 3, 6, 9, or 18 hours after treatment. Results are expressed relative to control serum iron (arbitrarily 100 ± standard deviation). Control animals, □; inducible mice, ▪. Statistical analysis was performed using Student t test (unpaired, 2 tailed): *P < .05, at least 3 animals. Thepc indicates transgenic hepcidin mRNA; endo hepc, endogenous hepcidin mRNA.
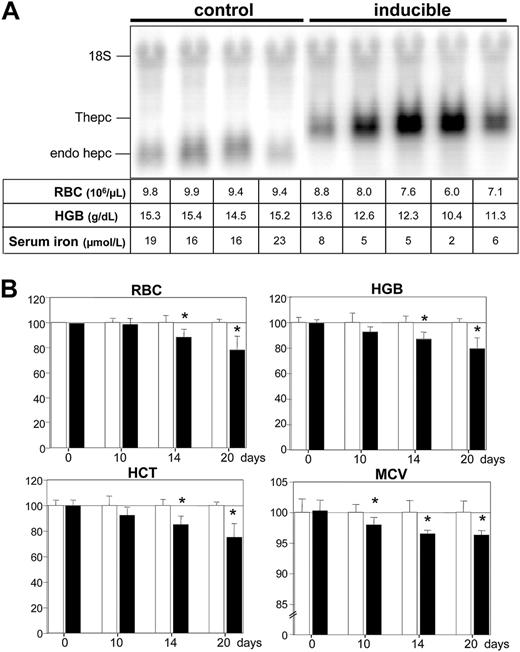
Long-term induction of transgenic hepcidin. (A) Liver hepcidin mRNA levels. Control mice and inducible mice (ie, double transgenic mice harboring both tetO-Hepc1 and rTALAP-1 transgenes) were given doxycycline in their drinking water for 3 weeks. Total liver RNA (20 μg) was fractionated by electrophoresis, blotted, and hybridized with hepcidin and 18S-labeled probes. Values for RBC count, hemoglobin (HGB) level, and serum iron level are shown for each animal. Thepc indicates transgenic hepcidin mRNA; endo hepc, endogenous hepcidin mRNA. (B) Hematologic indices of mice treated for 10, 14, or 20 days with doxycycline. Results are expressed relative to control serum parameters (arbitrarily 100 ± standard deviation). Control animals, □; inducible mice, ▪. Statistical analysis was performed using Student t test (unpaired, 2 tailed): *P < .05, n = at least 5 animals. RBC indicates red blood cell count (106/μL); HGB, hemoglobin (g/dL); HCT, hematocrit (%); MCV, mean cell volume (fl).
Long-term induction of transgenic hepcidin. (A) Liver hepcidin mRNA levels. Control mice and inducible mice (ie, double transgenic mice harboring both tetO-Hepc1 and rTALAP-1 transgenes) were given doxycycline in their drinking water for 3 weeks. Total liver RNA (20 μg) was fractionated by electrophoresis, blotted, and hybridized with hepcidin and 18S-labeled probes. Values for RBC count, hemoglobin (HGB) level, and serum iron level are shown for each animal. Thepc indicates transgenic hepcidin mRNA; endo hepc, endogenous hepcidin mRNA. (B) Hematologic indices of mice treated for 10, 14, or 20 days with doxycycline. Results are expressed relative to control serum parameters (arbitrarily 100 ± standard deviation). Control animals, □; inducible mice, ▪. Statistical analysis was performed using Student t test (unpaired, 2 tailed): *P < .05, n = at least 5 animals. RBC indicates red blood cell count (106/μL); HGB, hemoglobin (g/dL); HCT, hematocrit (%); MCV, mean cell volume (fl).
Long-term induction of transgenic hepcidin results in iron-restricted erythropoiesis
Long-term induction of transgenic hepcidin was achieved by administering doxycycline to inducible mice in their drinking water. After 3 weeks of treatment, high levels of transgenic hepcidin mRNA were detected in the livers of the inducible mice (Figure 2A). As a consequence, these mice developed iron-restricted erythropoiesis and anemia as demonstrated by decreased levels of serum iron and hemoglobin and red blood cell (RBC) numbers (Figure 2A). The sustained expression of transgenic hepcidin resulted in decreased levels of the endogenous hepcidin mRNA (Figure 2A), as previously reported in mice constitutively expressing transgenic hepcidin.7 To assess the time course of the development of anemia in inducible mice, blood was sampled and analyzed at different time points during the 3-week treatment (Figure 2B). Hematologic indices tended to change 10 days after induction. Decreased values became significant for all hematologic parameters after 2 weeks of doxycycline treatment and lasted for at least 3 weeks. The effect of a 2-week doxycycline course was reversible, with RBC and hemoglobin levels returning to control values 10 days after doxycycline withdrawal (not shown).
Transgenic hepcidin induces an altered pattern of iron distribution in Hfe-/- mice
We next asked whether hepcidin-induced iron depletion might alter the pattern of cellular iron accumulation seen in hereditary hemochromatosis. To this end, inducible mice were crossed with Hfe-/- mice. Inducible/Hfe+/- offspring were intercrossed to yield inducible/Hfe-/- mice (ie, mice harboring both tetO-Hepc1 and rTALAP-1 transgenes and with Hfe-/- genotype). Two-month-old animals (control, Hfe-/- and inducible/Hfe-/- mice) were given doxycycline in their drinking water for 3 weeks.
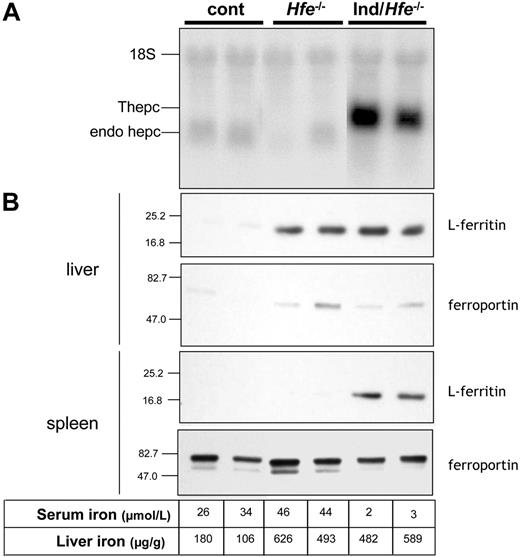
As compared to control mice, Hfe-/- mice had increased serum iron levels (43 ± 3 μM in Hfe-/- mice, n = 3 versus 30 ± 9 in control mice, n = 5; P = .05), increased liver iron content (471 ± 166 μg iron/g wet tissue in Hfe-/- mice, n = 3 versus 127 ± 33 in control mice, n = 6; P = .0012), and concomitant accumulation of liver L-ferritin proteins (as assessed by immunoblot; Figure 3B). Ferritin levels in the spleens of Hfe-/- mice were, as in control mice, at the limit of detection. Finally, as previously reported,11,20,27,28 hepatic endogenous hepcidin mRNA levels were inappropriately low relative to the iron burden in Hfe-/- mice (Figure 3A).
Characterization of mice carrying the inducible hepcidin transgenes crossed with Hfe-/- mice after treatment for 3 weeks with doxycycline. In this figure, each column represents the same animal. (A) Hepcidin mRNA levels in the livers of control (cont), Hfe-/- and inducible/Hfe-/- mice (ind/Hfe-/, ie, mice harboring both tetO-Hepc1 and rTALAP-1 transgenes and with Hfe-/- genotype) treated for 3 weeks with doxycycline. Total liver RNA (20 μg) was fractionated by electrophoresis, blotted, and hybridized with hepcidin and 18S-labeled probes. (B) Ferritin and ferroportin levels as assessed by immunoblot analysis of livers and spleens of control, Hfe-/- and ind/Hfe-/- mice treated for 3 weeks with doxycycline. Anti-mouse L-ferritin antibody was used to probe 20 μg hepatic (top panel) or splenic (bottom panel) cytosolic extract. Anti-mouse ferroportin antibody was used to probe 50 μg hepatic (top panel) or 20 μg splenic (bottom panel) microsomal extract. Molecular weight markers (kDa) are indicated on the left. The levels of serum iron and liver iron are shown for each animal.
Characterization of mice carrying the inducible hepcidin transgenes crossed with Hfe-/- mice after treatment for 3 weeks with doxycycline. In this figure, each column represents the same animal. (A) Hepcidin mRNA levels in the livers of control (cont), Hfe-/- and inducible/Hfe-/- mice (ind/Hfe-/, ie, mice harboring both tetO-Hepc1 and rTALAP-1 transgenes and with Hfe-/- genotype) treated for 3 weeks with doxycycline. Total liver RNA (20 μg) was fractionated by electrophoresis, blotted, and hybridized with hepcidin and 18S-labeled probes. (B) Ferritin and ferroportin levels as assessed by immunoblot analysis of livers and spleens of control, Hfe-/- and ind/Hfe-/- mice treated for 3 weeks with doxycycline. Anti-mouse L-ferritin antibody was used to probe 20 μg hepatic (top panel) or splenic (bottom panel) cytosolic extract. Anti-mouse ferroportin antibody was used to probe 50 μg hepatic (top panel) or 20 μg splenic (bottom panel) microsomal extract. Molecular weight markers (kDa) are indicated on the left. The levels of serum iron and liver iron are shown for each animal.
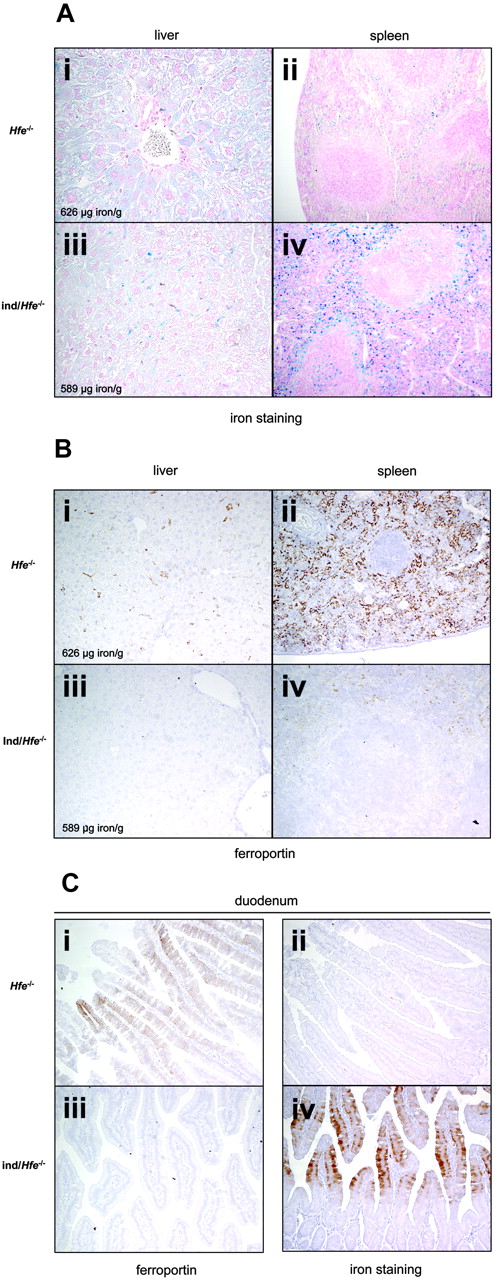
High levels of transgenic hepcidin mRNA were detected in inducible/Hfe-/- mice; endogenous hepcidin mRNA was undetectable (Figure 3A). Similar to inducible transgenic mice with wild-type Hfe alleles, inducible/Hfe-/- mice had severe hypoferremia (5 ± 2.5 μM in inducible/Hfe-/- mice, n = 5 versus 43 ± 3 μM in Hfe-/- mice, n = 5; P < .001) and developed signs of iron-restricted erythropoiesis. Surprisingly, total liver nonheme iron content and liver L-ferritin protein levels were similar in mice of both genotypes (472 ± 166 μg iron/g wet tissue in inducible/Hfe-/- mice, n = 5 versus 485 ± 169 in Hfe-/- mice, n = 5; Figure 3B). However, Perls staining of liver sections indicated that, although total liver iron content was unchanged, induction of hepcidin in inducible/Hfe-/- mice resulted in an altered pattern of cellular iron accumulation. Indeed, whereas iron was primarily detected in hepatocytes of Hfe-/- mice, iron was predominantly found in tissue macrophages (Kupffer cells) in inducible/Hfe-/- mice (Figure 4A, panel i versus panel iii). Similarly, splenic macrophage iron content was increased in inducible/Hfe-/- mice as shown by increased splenic L-ferritin levels (Figure 3B) and markedly enhanced Perls staining of splenic red pulp macrophages (Figure 4A, panel iv versus panel ii).
Altered pattern of iron distribution is associated with changes in ferroportin expression
Because ferroportin is a major target of hepcidin,9 we examined ferroportin expression in inducible/Hfe-/- mice using immunoblotting and immunohistochemistry. In the livers of control mice, ferroportin is normally expressed at low levels (Figure 3B). Its expression was increased in the livers of Hfe-/- mice, due to enhanced expression of ferroportin in Kupffer cells (Figure 4Bi). The sustained expression of hepcidin in the inducible/Hfe-/- mice leads to a decrease in ferroportin in Kupffer cells as observed by both immunoblot analysis and immunohistochemistry (Figures 3B and 4B, panel iii versus panel i). Ferroportin expression varied in a similar fashion in splenic macrophages, with increased levels in Hfe-/- mice and decreased levels in inducible/Hfe-/- mice (Figures 3B and 4B, panel iv versus panel ii).
To assess the effect of transgenic hepcidin expression on intestinal iron absorption, Perls DAB staining and ferroportin immunodetection were used to evaluate the duodenum of Hfe-/- and inducible/Hfe-/- mice.
Whereas ferroportin was clearly detected at the basolateral membrane of the absorptive enterocytes lining the intestinal villi of the Hfe-/- mice, a marked decrease in the amount of ferroportin was observed in inducible/Hfe-/- mice (Figure 4C, panel i versus panel iii). Concomitantly, there was a strong increase in the amount of stainable iron present in duodenal enterocytes in inducible/Hfe-/- mice as compared to Hfe-/- mice (Figure 4C, panel iv versus panel ii).
Perls staining and tissue ferroportin protein levels of liver, spleen, and duodenum sections from mice carrying the inducible hepcidin transgenes crossed with Hfe knockout (Hfe-/-) mice and treated for 3 weeks with doxycycline. (A-B) Typical Perls staining (A) and ferroportin immunodetection (B) using anti-mouse ferroportin antibody of liver (i,iii; original magnification × 40 in panel A and × 20 in panel B) and spleen (ii,iv; original magnification × 10) sections from Hfe-/- (i-ii) and ind/Hfe-/- mice (ie, mice harboring both tetO-Hepc1 and rTALAP-1 transgenes and with Hfe-/- genotype; iii-iv). Nonheme iron stains blue. The liver iron content for each animal is shown. (C) Duodenum (original magnification × 20) was stained for iron (ii,iv, Perls DAB staining, nonheme iron stains brown) and ferroportin (i,iii) immunodetection in Hfe-/- (i-ii) and in ind/Hfe-/- mice (ie, mice harboring both tetO-Hepc1 and rTALAP-1 transgenes and with Hfe-/genotype; iii,iv). All microscopy was performed using a Nikon Eclipse E800 microscope equipped with 10 ×/0.45, 20 ×/0.75, and 40 ×/0.95 oil-immersion objective lenses. Digital images were captured with a Nikon DXM1200 camera, and were acquired and processed with Nikon AC7-1 2.63 software (all from Nikon France, Champigny-sur-Marne, France).
Perls staining and tissue ferroportin protein levels of liver, spleen, and duodenum sections from mice carrying the inducible hepcidin transgenes crossed with Hfe knockout (Hfe-/-) mice and treated for 3 weeks with doxycycline. (A-B) Typical Perls staining (A) and ferroportin immunodetection (B) using anti-mouse ferroportin antibody of liver (i,iii; original magnification × 40 in panel A and × 20 in panel B) and spleen (ii,iv; original magnification × 10) sections from Hfe-/- (i-ii) and ind/Hfe-/- mice (ie, mice harboring both tetO-Hepc1 and rTALAP-1 transgenes and with Hfe-/- genotype; iii-iv). Nonheme iron stains blue. The liver iron content for each animal is shown. (C) Duodenum (original magnification × 20) was stained for iron (ii,iv, Perls DAB staining, nonheme iron stains brown) and ferroportin (i,iii) immunodetection in Hfe-/- (i-ii) and in ind/Hfe-/- mice (ie, mice harboring both tetO-Hepc1 and rTALAP-1 transgenes and with Hfe-/genotype; iii,iv). All microscopy was performed using a Nikon Eclipse E800 microscope equipped with 10 ×/0.45, 20 ×/0.75, and 40 ×/0.95 oil-immersion objective lenses. Digital images were captured with a Nikon DXM1200 camera, and were acquired and processed with Nikon AC7-1 2.63 software (all from Nikon France, Champigny-sur-Marne, France).
Discussion
We report the characterization of a new mouse model that allows inducible expression of the iron-regulatory hormone hepcidin. The effect of short-term induction of hepcidin was evaluated by giving a single dose of the inducer doxycycline to mice carrying transgenes mediating hepcidin expression. We found that induction was associated with decreased serum iron within 6 hours, consistent with a direct role of murine hepcidin in vivo in the development of hypoferremia. This result is in accord with the recent work of Rivera et al who demonstrated that injection of synthetic human hepcidin triggered hypoferremia in mice 1 hour later.29 The delay in decreased serum iron observed in our model is likely explained by the time needed for transactivation, transcription, and processing of hepcidin prior to its secretion in the bloodstream. The rapid effect of hepcidin in these models is most likely due to blockade of export of recycled iron from macrophages, a process that supplies most of the iron needed for erythropoiesis. Hepcidin-induced hypoferremia lasted at least 18 hours after a single dose of inducing drug in our transgenic model, a time-course effect that is compatible with that reported by Rivera et al using human radiolabeled hepcidin.29
The mechanism of regulation of hepcidin gene expression in response to iron status is not yet known. It was recently proposed that circulating diferric transferrin may directly regulate hepcidin gene expression and that transferrin receptor 2 (TFR2) is responsible for homeostatic iron sensing by the liver (for a review, see Frazer and Anderson30 ). Our observation that endogenous hepcidin gene expression was decreased 3 hours after serum iron decreased is consistent with the hypothesis that decreased iron bound to transferrin is involved in rapid down-regulation of hepcidin synthesis.
To study the chronic effects of hepcidin, chronic induction was achieved by giving doxycycline in the drinking water. Under these conditions, mice carrying the inducible transgene showed iron retention in the macrophages (data not shown) and developed iron-limited erythropoiesis 2 weeks after treatment. These characteristics are reminiscent of anemia of inflammation (also referred to as anemia of chronic disease) as already discussed by Rivera et al.31 These authors reported that mice given implants with tumor xenografts engineered to overexpressed human hepcidin developed severe anemia.31 However, although those authors reported that hepcidin was shunting iron away from erythropoiesis by sequestering it predominantly in hepatocytes, we were unable to detect a significant amount of iron in the hepatocytes of our inducible mice (data not shown). Instead, iron was sequestered in macrophages as demonstrated for the inducible/Hfe-/- mice. This discrepancy may be explained by the different mouse strains and the complexity of the model used by Rivera and colleagues, who introduced hepatoma tumor cells into immunodeficient NOD-SCID mice maintained on a high-iron diet.31
We previously reported that hepcidin could prevent iron overload in HFE-associated hemochromatosis by crossing transgenic mice constitutively expressing hepcidin TTR-Hepc1 mice with Hfe-/- mice.20 However, to consider possible therapy for affected patients, it was important to determine whether hepcidin treatment would be beneficial after iron overload was established. Indeed, hemochromatosis remains substantially underdiagnosed and, if untreated, can cause serious illness with complications including liver cirrhosis, fibrosis, and hepatocellular carcinoma. It could be advantageous to manipulate iron homeostasis in patients with more advanced disease.
Therefore, we used an inducible transgene to effect chronic hepcidin replacement in adult animals that already had increased liver iron deposition. We demonstrated that supplementary expression of hepcidin in our murine hemochromatosis model leads to an altered pattern of cellular iron accumulation. Iron sequestration in the macrophage is associated with, and likely explained by, hepcidin-induced ferroportin degradation. The link between ferroportin and hepcidin was first established by Nemeth et al, who demonstrated ex vivo that hepcidin was able to bind to ferroportin, thereby inducing internalization and degradation of ferroportin, resulting in decreased export of cellular iron.9 This finding has been replicated in several transfected cell lines32,33 and, more recently, in bone marrow-derived macrophages expressing endogenous ferroportin.34 In this study, we observed iron retention in splenic macrophages and enterocytes of Hfe-/- mice induced to express hepcidin, providing further evidence that hepcidin-induced ferroportin degradation explains the pleiotropic effects of hepcidin in the regulation of iron homeostasis in vivo.
The altered pattern of iron distribution in Hfe-/- mice expressing the hepcidin transgene is the phenotypic opposite of the liver iron overload that occurs in genetic hemochromatosis associated with insufficient hepcidin. Interestingly, hepatocyte ferroportin expression is not appreciably different from normal in either case.16,17 It is tempting to speculate that hepatocyte iron egress is governed by a hepcidin-ferroportin independent pathway. Alternatively, there may be distinct mechanisms for regulating ferroportin expression or activity (or both) in hepatocytes. However, ferroportin protein is difficult to detect in hepatocytes under normal conditions, and it remains possible that subtle changes in ferroportin expression would be impossible to discern under our experimental conditions.
Iron accumulation in splenic macrophages and consequent decreased serum iron in Hfe-/- mice expressing the inducible hepcidin transgene is reminiscent of the clinical features of patients with autosomal dominant iron overload due to pathogenic mutations in the ferroportin gene (for a review, see Pietrangelo35 ). In most cases, ferroportin mutations result in loss of protein function. Patients typically present with increased iron deposition in Kupffer cells (rather than hepatocytes) and low serum transferrin saturation. Some do not tolerate phlebotomy and develop a mild anemia.36-38 Furthermore, in contrast to the other forms of hemochromatosis associated with hepcidin deficiency, hepcidin levels are elevated in these patients.39 These patients rarely develop liver fibrosis or cirrhosis and they have few, if any, clinical manifestations of iron-storage disease. As recently suggested by Zoller et al, it appears that iron deposition in Kupffer cells is well tolerated.40 Based on these observations, we speculate that the altered pattern of cellular iron distribution following hepcidin treatment could ameliorate hemochromatosis by reducing iron-induced damage of parenchymal cells. Furthermore, the hepcidin-induced down-regulation of ferroportin and iron retention that we observed in enterocytes suggest that further iron absorption will be abrogated, thus preventing additional body iron accumulation. This result contrasts with studies of patients with 3 different types of ferroportin mutations, in which no enterocyte iron retention was observed.41
In conclusion, our results provide further support for the attractive hypothesis that hepcidin acts to decrease intestinal iron absorption and iron egress from the macrophages by down-regulating ferroportin. It provides a rationale for using hepcidin or a hepcidin agonist in the treatment of iron overload disorders.
Prepublished online as Blood First Edition Paper, December 8, 2005; DOI 10.1182/blood-2005-10-4071.
Supported by Inserm and the French Ministry of Education and Research (“Action Concertée Incitative”). D.-Q.L. and J.-C.L.-B. received grants from Agence National de la Valorisation de la Recherche (ANVAR).
L.V. designed and performed research, analyzed data, and wrote the paper; G.N. designed and performed research; D.-Q.L. analyzed data; M.B. performed research; J.-C.L.-B. performed research; N.C.A. provided a mouse model and wrote the paper; K.S. provided a mouse model; H.B. provided a mouse model; F.C.-H. contributed key reagents; A.K. analyzed data; and S.V. designed research, analyzed data, and wrote the paper.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal