Abstract
Cardiovascular disease frequently occurs after lymphoma therapy, but it is common in the general population too. Therefore, risk estimation requires comparison to population-based rates. We calculated risk by standardized incidence ratios (SIRs) and absolute excess risks (AERs) per 10 000 person-years based on general population rates (Continuous Morbidity Registry Nijmegen) in 476 (Dutch and Belgian) patients with aggressive non-Hodgkin lymphoma (NHL) treated with at least 6 cycles of doxorubicin-based chemotherapy in 4 European Organization for Research on Treatment of Cancer (EORTC) trials (1980-1999). Cumulative incidence of cardiovascular disease, estimated in a competing risk model, was 12% at 5 years and 22% at 10 years (median follow-up, 8.4 years). Risk of chronic heart failure appeared markedly increased (SIR, 5.4; 95% CI, 4.1-6.9) with an AER of 208 excess cases per 10 000 person-years, whereas risk of coronary artery disease matched the general population (SIR, 1.2; 95% CI, 0.8-1.8; AER, 8 per 10 000 person-years). Risk of stroke was raised (SIR, 1.8; 95% CI, 1.1-2.4; AER, 15 per 10 000 person-years), especially after additional radiotherapy (> 40 Gy). Preexisting hypertension, NHL at young age, and salvage treatment increased risk of all cardiovascular events; the effect of radiotherapy was dose dependent. In conclusion, patients are at long-term high risk of chronic heart failure after NHL treatment and need therefore life-long monitoring. In contrast, risk of coronary artery disease appeared more age dependent than treatment related.
Introduction
Nowadays, many patients with malignant lymphoma become long-term survivors. During follow-up, therapy-related late sequelae have become an important issue. Whereas secondary cancers were the first to be noticed,1-3 nonmalignant late sequelae, especially related to cardiovascular disease, are considered important as well.3-8 Most reports on cardiovascular disease in long-term follow-up of patients with malignant lymphoma concern patients with Hodgkin lymphoma mainly treated with radiotherapy.7-12 Patients with aggressive non-Hodgkin lymphoma (NHL) are generally older than patients with Hodgkin lymphoma (HL) and are, because of age alone, already at risk for cardiovascular disease. The large majority of these patients will receive doxorubicin-based chemotherapy with cumulative doxorubicin doses up to 300 to 400 mg/m2.13 Moreover, as about half of the patients will not obtain a complete response, and another large percentage will relapse afterward, salvage treatment is often needed. Involved field radiotherapy and autologous stem cell transplantation (ASCT) have been introduced to improve survival but can also become contributing factors to eventual late sequelae.14-18
The availability of a large EORTC (European Organization of Research and Treatment of Cancer) database of patients with aggressive NHL, consistently treated with doxorubicin-based chemotherapy, offered the possibility to explore risk of cardiovascular disease in long-term follow-up. Detailed information on additional treatment was collected to evaluate excess risk as a result of multimodality treatment.
Cardiovascular toxicity, which has mainly been studied in patients with breast cancer and HL, is described to be dose, sex, and age dependent.19,20 In the general population, the incidence of cardiovascular disease is similarly related to age and sex.21,22 Other factors influencing risk of cardiovascular disease, that is, hypertension, socioeconomic status, lifestyle (diet and smoking), familiar predisposition, but also medical care (prevention and intervention) may vary geographically and over time.23-26 Therefore, risk estimation requires comparison to population-based rates. We compared the incidence of cardiovascular disease in our EORTC cohort with general population rates by using a large Dutch population-based morbidity registry.
Patients, materials, and methods
Data collection procedures
A retrospective cohort study was performed in 974 patients with advanced aggressive NHL enrolled in 4 successive EORTC trials between 1980 and 1999. To relate risk to the general (Dutch) population, we selected patients treated in the Netherlands or Belgium (incidence rates in Belgium were supposed to be comparable to those registered in the Netherlands).25-27
Patient records were reviewed by the local investigators (see Appendix A), and the following data were collected: sex, age, International Prognostic Index (IPI),28 smoking habits, preexisting hypertension, date of start of chemotherapy, specification of regimen (first-line and salvage treatment), additional ASCT or radiotherapy (including field, dose, and timing), date of death, cause of death, date of last follow-up, date of diagnosis of secondary event, and cause and specification of cardiovascular disease. No routine electrocardiograph (ECG) or other tests had been performed to detect subclinical cardiotoxicity during follow-up, only clinical diagnoses and the most plausible cause of cardiovascular disease were registered. Unfortunately, no sufficient data on other risk factors such as familiar predisposition, obesity, hypercholesterolemia, hypertriglyceridemia, or diabetes mellitus were available. For details related to the specifically designed case record forms, see Moser et al.6 Approval of the study was obtained from the EORTC Protocol Review Committee and from all local institutions. Informed consent was provided according to the Declaration of Helsinki.
We restricted the analyses to those patients treated with at least 6 cycles of doxorubicin-based chemotherapy and with a minimal follow-up time of 0.5 year. Patients with a history of cardiovascular disease were excluded, except patients with preexisting hypertension.
General population rates
In this study, we used data derived from the Continuous Morbidity Registration of the Department of General Practice (GP) at the University of Nijmegen (CMRN).29,30 In this database every episode of morbidity presented to the GPs in 4 affiliated practices has been registered since 1971. Comparison of recent incidence rates of cardiovascular disease from the CMRN with those of several new registries in the Netherlands, comprising short-term incidence rates, showed that incidence rates of CMRN were similar to the mean of all registries combined, indicating that the CMRN is representative of the Netherlands.31
The relevance and limitations of this database are directly influenced by the Dutch health care system: nearly all residents have a GP and not only those seeking medical care. GPs receive all medical correspondence from attending physicians regarding their patients. Every GP has a stable practice population, being the official person to access specialized medical care. Hence, the data set consists of the entire primary health care provided to the population as well as referrals for health care. The outcome of interest of the CMRN is estimating incidence of the most common diseases in the general population, according to age and sex over time. The diagnoses are registered in a primarily disease-oriented classification based on the International Classification of Health Problems in Primary Care (ICHPPC-2). We selected 5 diagnoses: first myocardial infarction, first attack of angina pectoris, chronic heart failure (also named congestive heart failure), coronary artery disease, and cerebrovascular accidents.
Definitions
According to the definitions used in the CMRN database, we used diagnoses based on the ICHPPC-2. Incidence rates in our cohort and those registered by the CMRN both allowed multiple separate diagnoses per person, but only the first of the given diagnosis was recorded.
Chronic heart failure was defined as clinical congestive heart failure class II or more by the New York Heart Association (NYHA) criteria, meaning that, although the patient could be comfortable at rest, ordinary physical activity caused symptoms. Coronary artery disease, including angina pectoris or myocardial infarctions, was also registered if at least class II NYHA. For angina pectoris, one of the following criteria has to be met: precordial chest pain characteristic for angina pectoris, ischemia on the ECG, or confirmed sclerosis on the angiography. For myocardial infarction, the criteria are precordial pain typical for myocardial infarction longer than 15 minutes and one of the following: abnormal ST-T segments or Q waves on the ECG, raised serum levels of enzymes. Vascular disease was registered as large vessel occlusion causing pain or loss of physical function, needing (urgent) intervention and hospital admission. No distinction was made in outcome: both lethal and nonlethal cardiovascular events were included. Smoking was scored positively when the patient had ever smoked, also when the patient quit during follow-up. Hypertension was scored as present if medically treated and diagnosed before the cardiovascular event.
Treatment
All patients had received at least 6 cycles of chemotherapy containing doxorubicin, up to 400 mg/m2 in the CHVmP/BV regimen given in all 4 trials (consisting of cyclophosphamide, doxorubicin, teniposide, prednisone, bleomycin, and vincristine) and CHVmP (CHVmP/BV without bleomycin and vincristine) in the first trial, and up to 200 mg/m2 with the ProMACE-MOPP regimen (consisting of doxorubicin, cyclophosphamide, etoposide, mechlorethamine, vincristine, procarbazine, methotrexate, and prednisone) in the second trial.32-35 No cumulative total dose doxorubicin-complication relation could be estimated, because dose information of salvage treatment was often lacking.
To relate cardiovascular events to involved field radiotherapy, all fields were retrospectively checked, and the cumulative dose per field (specified as mediastinum, neck, abdomen, or groin) was estimated. The advised dose in all EORTC protocols was 30 Gy for patients with initially bulky disease (> 5 cm) in complete response after first-line chemotherapy, and 40 Gy for partial response (in fractions of 1.5-2 Gy). If large fields were needed, a reduction of the field, focusing on the remaining lesion was suggested for the last 4 to 10 Gy. For extranodal locations dose was limited to 20 to 30 Gy with a boost of 4 to 10 Gy after field reduction. In the salvage setting, often the same dose levels were used.
Statistical analysis
A comparison was made between the incidence of cardiovascular disease in the study population and the incidence in the Dutch population (CMRN database). In this person-years type of analysis, the ratio of observed and expected number of cardiovascular events was determined. The observed-to-expected ratio will further be denoted as the standard incidence ratio (SIR). Expected numbers were computed with the use of age-, sex-, and calendar period-specific incidence rates derived from the CMRN database. Absolute excess risk (AER; per 10 000 person-years) was calculated as the observed number of cardiovascular events in our cohort minus the number expected, divided by number of person-years at risk, multiplied by 10 000. AER expresses the excess cases per 10 000 person-years diagnosed in the study group compared with the general population and is seen as the best way to asses the burden of treatment sequelae in a patient population.
The incidences per person-year were categorized by age in 5-year intervals (running from 15 to 85 years), by sex, and by calendar period in 2- to 3-year intervals (running from 1980 to 2001) in both the study and the CMRN cohort. CMRN incidence rates for coronary artery disease were available from 1971 to 2000 (3-year moving averages) but for stroke and chronic heart failure only from 1985 to the end of 2000. Therefore, the person-time analysis for chronic heart failure and stroke was started at January 1, 1985, and for coronary artery disease at January 5, 1980 (first patient enrolled). In all, patient accumulation of person-time at risk for cardiovascular disease began at 0.5 year after start of NHL treatment and ended at date of diagnosis of the event, date of death, or most recent information on cardiovascular disease occurrence, whichever came first. When analyzing one specific cardiovascular diagnosis, observed numbers were based on all first events of this given diagnosis occurring at least 0.5 year after start of NHL treatment in the study cohort, because the expected numbers of events were recorded correspondingly in the CMRN cohort. The case record forms offered coronary artery disease, myocardial infarction, and angina pectoris as separate items to fill in, which explains why all 3 are overlapping (Table 1). In the analyses of coronary artery disease, time at risk ended at the day of diagnosis of 1 of the 3 events. Confidence limits were calculated using exact Poisson probabilities of observed numbers.36
Standardized incidence ratios and absolute excess risks of cardiovascular disease after NHL treatment
Event . | No. Observed* . | No. Expected . | SIR (95% CI) . | AER per 10 000 person-years . | Median age at event, y (range) . | Median interval of treatment until event, y (range) . |
|---|---|---|---|---|---|---|
| Chronic heart failure | 64 | 12.0 | 5.4 (4.1-6.9) | 208 | 53.6 (38-82) | 3.4 (2.3-4.6) |
| Coronary artery disease | 20 | 30.4 | 0.7 (0.4-0.9) | –38 | 53.1 (34-89) | 2.5 (1.8-3.4) |
| Myocardial infarction | 17 | 14.8 | 1.2 (0.8-2.4) | 8 | 49.3 (38-73) | 2.6 (2.0-3.4) |
| Angina pectoris | 16 | 19.6 | 0.8 (0.4-1.3) | –14 | 54.1 (34-89) | 2.4 (1.8-3.2) |
| Stroke | 11 | 7.3 | 1.5 (1.1-2.7) | 15 | 62.1 (39-79) | 3.7 (1.6-5.1) |
Event . | No. Observed* . | No. Expected . | SIR (95% CI) . | AER per 10 000 person-years . | Median age at event, y (range) . | Median interval of treatment until event, y (range) . |
|---|---|---|---|---|---|---|
| Chronic heart failure | 64 | 12.0 | 5.4 (4.1-6.9) | 208 | 53.6 (38-82) | 3.4 (2.3-4.6) |
| Coronary artery disease | 20 | 30.4 | 0.7 (0.4-0.9) | –38 | 53.1 (34-89) | 2.5 (1.8-3.4) |
| Myocardial infarction | 17 | 14.8 | 1.2 (0.8-2.4) | 8 | 49.3 (38-73) | 2.6 (2.0-3.4) |
| Angina pectoris | 16 | 19.6 | 0.8 (0.4-1.3) | –14 | 54.1 (34-89) | 2.4 (1.8-3.2) |
| Stroke | 11 | 7.3 | 1.5 (1.1-2.7) | 15 | 62.1 (39-79) | 3.7 (1.6-5.1) |
Observed in 476 patients for myocardial infarction (1980-2000) and in 441 patients for chronic heart failure and stroke (1985-2000)
To assess treatment effects on risk of cardiovascular disease among other possible factors, we distinguished different subgroups: patients younger versus older than 55 years at start of treatment, those with versus without preexisting hypertension, who had smoked versus who had never smoked, who received versus did not need (any) salvage treatment, who received additional ASCT in the first line or as salvage treatment versus never received ASCT, and who received additional radiation on the neck or heart region (depending on the event) in the first-line or as salvage treatment versus never received radiotherapy in this region. We repeated the overall and subgroup analyses in those patients with at least 3 (n = 305) and 5 years (n = 240) of follow-up and in those patients treated (and often cured by) only in the first line (n = 253).
Median follow-up, time to occurrence, and survival were estimated as a function of time since start of NHL treatment and analyzed according to the product-limit method first described by Kaplan and Meier,37 censoring for death, lost to follow-up, and event (whatever came first). Cumulative incidences of cardiovascular disease were estimated in the competing risk model by Gray38 with death by any cause as competing event. The Cox proportional-hazards model39 was used to quantify the effects of different treatments on risk within the patient group, adjusting for confounders, as opposed to the person-year analysis in which risk is compared with the general population. Forward stepwise confounder selection, in which the effect of adding one confounder at the time was evaluated, was based on a more than 10% change in the risk estimate of the exposure variable of interest, irrespective of significant values. Variables evaluated in this model were age at start of treatment, trial (because the 4 trials were successively performed, this reflects also the calendar period of treatment), IPI, smoking, pre-existing hypertension, additional salvage (any type), ASCT, and radiotherapy (in region of event). Age was analyzed as a continuous variable; other factors were categorized (yes versus no). Cox models were fitted using SPSS statistical software (SPSS, Chicago, IL).
Results
Within the 4 EORTC trials for advanced aggressive NHL, 592 patients (61%) had been treated in the Netherlands or Belgium between 1980 and 1999. Seventy-one had a follow-up of less than 0.5 year because of early progression or death, and 9 patients had a history of cardiovascular disease. In 476 of the remaining 512 cases follow-up information on survival, treatment, and cardiovascular risk was complete until death or January 1, 2001. The quality of the forms was excellent with only 7% missing data, spread over the various items. The characteristics of the 476 patients according to the selected subgroups are described in Table 2. There were more men than women. The mean age was 49 years, ranging from 16 to 82 years. Most patients had stage III or IV disease (68%) and 45% had a low-intermediate, 34% an intermediate-high, and 21% a high IPI risk profile.
Patient characteristics at start of first-line NHL treatment
Characteristic . | Men, no. . | Women, no. . | Total, no. (%) . |
|---|---|---|---|
| Age | |||
| Younger than 55 y | 178 | 116 | 294 (62) |
| 55 y or older | 114 | 68 | 182 (38) |
| Ann Arbor stage | |||
| I bulky | 11 | 7 | 18 (3) |
| II | 85 | 53 | 138 (29) |
| III | 79 | 43 | 122 (26) |
| IV | 117 | 81 | 198 (42) |
| International Prognostic Index | |||
| Low-intermediate | 136 | 76 | 212 (45) |
| Intermediate | 98 | 63 | 161 (34) |
| Intermediate-high | 37 | 30 | 67 (14) |
| High | 11 | 11 | 22 (7) |
| History of smoking | |||
| No | 119 | 88 | 207 (43) |
| Yes | 120 | 53 | 173 (36) |
| Unknown | 53 | 43 | 96 (21) |
| Hypertension | |||
| No | 266 | 173 | 439 (92) |
| Yes | 18 | 8 | 26 (5) |
| Unknown | 8 | 3 | 11 (3) |
| Stem cell transplantation | |||
| No | 256 | 164 | 420 (88) |
| Yes | 36 | 20 | 56 (12) |
| Radiotherapy on neck | |||
| No | 175 | 112 | 287 (60) |
| Yes | 104 | 84 | 189 (40) |
| Radiotherapy on mediastinum | |||
| No | 215 | 122 | 337 (71) |
| Yes | 77 | 62 | 139 (29) |
| Salvage (any type) | |||
| No | 137 | 98 | 235 (49) |
| Yes | 155 | 86 | 241 (51) |
Characteristic . | Men, no. . | Women, no. . | Total, no. (%) . |
|---|---|---|---|
| Age | |||
| Younger than 55 y | 178 | 116 | 294 (62) |
| 55 y or older | 114 | 68 | 182 (38) |
| Ann Arbor stage | |||
| I bulky | 11 | 7 | 18 (3) |
| II | 85 | 53 | 138 (29) |
| III | 79 | 43 | 122 (26) |
| IV | 117 | 81 | 198 (42) |
| International Prognostic Index | |||
| Low-intermediate | 136 | 76 | 212 (45) |
| Intermediate | 98 | 63 | 161 (34) |
| Intermediate-high | 37 | 30 | 67 (14) |
| High | 11 | 11 | 22 (7) |
| History of smoking | |||
| No | 119 | 88 | 207 (43) |
| Yes | 120 | 53 | 173 (36) |
| Unknown | 53 | 43 | 96 (21) |
| Hypertension | |||
| No | 266 | 173 | 439 (92) |
| Yes | 18 | 8 | 26 (5) |
| Unknown | 8 | 3 | 11 (3) |
| Stem cell transplantation | |||
| No | 256 | 164 | 420 (88) |
| Yes | 36 | 20 | 56 (12) |
| Radiotherapy on neck | |||
| No | 175 | 112 | 287 (60) |
| Yes | 104 | 84 | 189 (40) |
| Radiotherapy on mediastinum | |||
| No | 215 | 122 | 337 (71) |
| Yes | 77 | 62 | 139 (29) |
| Salvage (any type) | |||
| No | 137 | 98 | 235 (49) |
| Yes | 155 | 86 | 241 (51) |
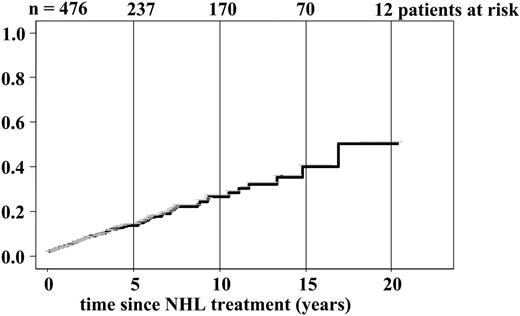
Cumulative incidence of cardiovascular disease since end of first-line NHL treatment.
Cumulative incidence of cardiovascular disease since end of first-line NHL treatment.
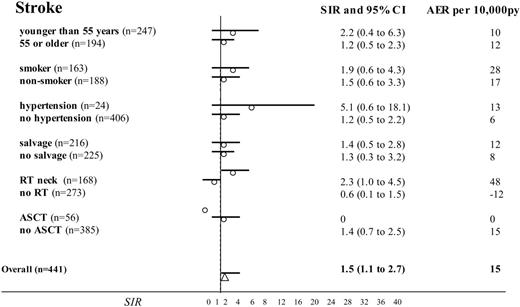
Standardized incidence ratios and absolute excess risks with 95% confidence intervals for stroke after NHL treatment: risk factor sub-analyses. Comparison of 441 NHL patients treated (from 1985 to 2000) with doxorubicin-based chemotherapy with the Dutch population (as population-based incidences of chronic heart failure were available after January 1, 1985). Subgroups were selected to estimate the impact of age during NHL treatment, smoking, preexisting hypertension and additional salvage treatment (any type), radiotherapy (RT) of the neck, and ASCT. (Missing data for smoking in 90 cases and for hypertension in 11 cases.)
Standardized incidence ratios and absolute excess risks with 95% confidence intervals for stroke after NHL treatment: risk factor sub-analyses. Comparison of 441 NHL patients treated (from 1985 to 2000) with doxorubicin-based chemotherapy with the Dutch population (as population-based incidences of chronic heart failure were available after January 1, 1985). Subgroups were selected to estimate the impact of age during NHL treatment, smoking, preexisting hypertension and additional salvage treatment (any type), radiotherapy (RT) of the neck, and ASCT. (Missing data for smoking in 90 cases and for hypertension in 11 cases.)
Half of the patients (51%) received a salvage regimen. Of the 235 patients cured in the first line, 170 received only doxorubicin-based chemotherapy, 25 received chemotherapy followed by ASCT, and the remaining received chemotherapy with radiotherapy on the neck (n = 66) or on the mediastinum (n = 52). If all patients were taken into account, 139 patients received radiotherapy on the mediastinum and 189 on the neck. Fifty-six patients received an ASCT of whom 31 as salvage. The median radiotherapy dose on the neck was 36 Gy (range, 28-60 Gy), the mediastinum 32 Gy (range, 28-56 Gy), abdominal fields 36 Gy (range, 20-42 Gy), and the groins 38 Gy (range, 28-50 Gy). Sixty-one patients were irradiated more than once in the same area: 38 on the same side of the neck, 18 on the mediastinum, and 10 with a field containing the same groin and 5 in the same abdominal region. ASCT was preceded by high-dose chemotherapy; no total body irradiation had been given.
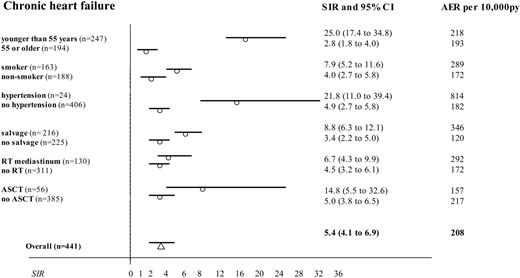
Next, the SIR for stroke was significantly higher in the patients with additional irradiation on the neck compared with those not irradiated in this region (Figure 2; SIR, 2.3 versus 0.6). Notably, radiotherapy on the mediastinum did not significantly influence risk of cardiac disease when all irradiated patients were taken into account (Figures 3 and 4). However, when we performed a subgroup analysis according to radiation dose, strongly increased risks of chronic heart failure, myocardial infarction, and stroke were observed after 40 Gy or more (Table 3). The presence of preexisting hypertension increased the risk of myocardial infarction (Figure 3; SIR, 6.9 versus 0.8) and chronic heart failure (SIR, 22 versus 4.9). NHL treatment at a young age (SIR, 24.8 versus 2.8 for younger than 55 and 55 or older, respectively), and salvage treatment (SIR, 8.8 versus 3.4) raised the risk of chronic heart failure as well (Figure 4). Smoking was associated with a higher risk of chronic heart failure, stroke, and especially myocardial infarction (Figures 2, 3, 4).
Standardized incidence ratios and absolute excess risks of cardiovascular disease after NHL treatment including radiotherapy: a cumulative dose-risk relation
Event, radiation dose . | No. of patients* . | No. observed . | No. expected . | SIR (95% CI) . | AER per 10 000 person-years . |
|---|---|---|---|---|---|
| Chronic heart failure | |||||
| No radiotherapy on mediastinum | 311 | 39 | 8.9 | 4.4 (3.1-6.0) | 167 |
| 30 Gy or less | 30 | 5 | 1.1 | 4.7 (1.0-8.2) | 171 |
| 30-40 Gy | 85 | 11 | 1.6 | 6.8 (3.3-13.3) | 232 |
| More than 40 Gy | 15 | 9 | 0.3 | 32.0 (13.7-57) | 932 |
| Myocardial infarction | |||||
| No radiotherapy on mediastinum | 337 | 10 | 11.6 | 0.9 (0.4-1.6) | –8 |
| 30 Gy or less | 32 | 1 | 1.3 | 0.8 (0.3-2.3) | 1 |
| 30-40 Gy | 89 | 3 | 2.1 | 1.4 (0.4-4.3) | 5 |
| More than 40 Gy | 18 | 3 | 0.4 | 7.5 (1.4-19.9) | 274 |
| Stroke | |||||
| No radiotherapy | 273 | 3 | 4.8 | 0.6 (0.1-1.2) | –12 |
| 30 Gy or less | 48 | 1 | 1.4 | 0.7 (0.1-2.7) | –9 |
| 30-40 Gy | 103 | 2 | 0.9 | 2.2 (0.1-3.1) | 5 |
| More than 40 Gy | 38 | 5 | 0.7 | 8.6 (3.1-18.7) | 260 |
Event, radiation dose . | No. of patients* . | No. observed . | No. expected . | SIR (95% CI) . | AER per 10 000 person-years . |
|---|---|---|---|---|---|
| Chronic heart failure | |||||
| No radiotherapy on mediastinum | 311 | 39 | 8.9 | 4.4 (3.1-6.0) | 167 |
| 30 Gy or less | 30 | 5 | 1.1 | 4.7 (1.0-8.2) | 171 |
| 30-40 Gy | 85 | 11 | 1.6 | 6.8 (3.3-13.3) | 232 |
| More than 40 Gy | 15 | 9 | 0.3 | 32.0 (13.7-57) | 932 |
| Myocardial infarction | |||||
| No radiotherapy on mediastinum | 337 | 10 | 11.6 | 0.9 (0.4-1.6) | –8 |
| 30 Gy or less | 32 | 1 | 1.3 | 0.8 (0.3-2.3) | 1 |
| 30-40 Gy | 89 | 3 | 2.1 | 1.4 (0.4-4.3) | 5 |
| More than 40 Gy | 18 | 3 | 0.4 | 7.5 (1.4-19.9) | 274 |
| Stroke | |||||
| No radiotherapy | 273 | 3 | 4.8 | 0.6 (0.1-1.2) | –12 |
| 30 Gy or less | 48 | 1 | 1.4 | 0.7 (0.1-2.7) | –9 |
| 30-40 Gy | 103 | 2 | 0.9 | 2.2 (0.1-3.1) | 5 |
| More than 40 Gy | 38 | 5 | 0.7 | 8.6 (3.1-18.7) | 260 |
Comparison in 476 patients for myocardial infarction (1980-2000) and in 441 patients for chronic heart failure and stroke (1985-2000)
In patients with more than 3, respectively, 5-year follow-ups, higher risks of chronic heart failure were observed. This trend of greater SIRs with longer follow-up was also seen for myocardial infarction and stroke (Table 4). By reviewing cumulative incidence (Figure 1) this could have been expected, because there was no plateau observed during longer follow-up.
Standardized incidence ratios according to follow-up time: a longer follow-up shows further increase of risk of cardiovascular disease
Event . | SIR, all patients, with median follow-up of 8.4 y . | SIR, patients with at least 3 y of follow-up . | SIR, patients with at least 5 y of follow-up . |
|---|---|---|---|
| Chronic heart failure | 5.4 (4.1-6.9) | 6.2 (4.9-7.9) | 7.3 (5.9-12.1) |
| Myocardial infarction | 1.2 (0.8-1.8) | 1.5 (0.8-2.6) | 1.6 (1.2-6.9) |
| Stroke | 1.8 (1.1-2.4) | 2.1 (1.4-6.1) | 2.5 (1.6-7.9) |
Event . | SIR, all patients, with median follow-up of 8.4 y . | SIR, patients with at least 3 y of follow-up . | SIR, patients with at least 5 y of follow-up . |
|---|---|---|---|
| Chronic heart failure | 5.4 (4.1-6.9) | 6.2 (4.9-7.9) | 7.3 (5.9-12.1) |
| Myocardial infarction | 1.2 (0.8-1.8) | 1.5 (0.8-2.6) | 1.6 (1.2-6.9) |
| Stroke | 1.8 (1.1-2.4) | 2.1 (1.4-6.1) | 2.5 (1.6-7.9) |
For all patients with median follow-up of 8.4 y, n = 476 for myocardial infarction (1980-2000) and 441 chronic heart failure and stroke (1985-2000). Data are standardized incidence ratios (95% CI). For patients with at least 3 y of follow-up, n = 305. For patients with at least 5 y of follow-up, n = 240.
Multivariate Cox regression analysis showed excess risk associated with the same factors that emerged from the stratified analyses comparing SIRs. Age at start of NHL treatment was the most important factor determining risk of any cardiovascular event (HR, 4.1; 95% CI, 2.1-8.9). Preexisting hypertension (HR, 2.1; 95% CI, 1.2-6.7) and salvage treatment (any type; HR, 2.4; 95% CI, 1.9-9.9) did also significantly increase risk of all types of cardiovascular disease. Other significant factors were smoking for coronary artery disease (HR, 1.7; 95% CI, 1.4-5.6) and radiotherapy of the neck for stroke (HR, 1.8; 95% CI, 1.3-9.1). Cardiovascular disease was more often seen in patients with NHL treated in the earlier trials (HR, 0.3; 95% CI, 0.1-0.7) running from 1980 to 1990.
To study the independent role of doxorubicin on risk of cardiovascular disease, we needed a cohort treated only with chemotherapy. However, these were on average the patients with the best prognosis. Therefore, comparison of risk because of additional modalities to this group would be biased by the difference in follow-up. We therefore selected those 253 patients who were treated only in the first line. Here, we compared risk after chemotherapy alone with risk after chemotherapy plus other modalities (Table 5). It appeared that chemotherapy alone (which in all cases consisted of doxorubicin-based combinations) was clearly related to an increased risk of chronic heart failure (SIR, 3.1; 95% CI, 1.9-4.8), with an AER of 127 excess cases per 10 000 person-years.
Standardized incidence ratios and absolute excess risks according to NHL treatment: additional treatment increases risk of cardiovascular disease
Event, treatment . | No. of patients* . | No. observed . | No. expected . | SIR (95% CI) . | AER per 10 000 person-years . |
|---|---|---|---|---|---|
| Chronic heart failure | |||||
| After salvage (any type) | 225 | 38 | 4.3 | 8.8 (6.3-12.1) | 346 |
| After first-line treatment only | 216 | 26 | 7.7 | 3.4 (2.2-4.9) | 120 |
| Only chemotherapy | 162 | 20 | 6.4 | 3.1 (1.9-4.8) | 127 |
| Chemotherapy and RT | 38 | 4 | 1.1 | 3.6 (1.0-9.8) | 120 |
| Chemotherapy and transplantation | 13 | 1 | 0.1 | 7.1 (0.2-39.8) | 81 |
| Chemotherapy, RT, and transplantation | 12 | 1 | 0.1 | 12.5 (0.3-69.7) | 83 |
| Myocardial infarction | |||||
| After salvage (any type) | 241 | 10 | 6.8 | 1.5 (0.7-2.7) | 29 |
| After first-line treatment only | 235 | 7 | 9.0 | 0.8 (0.3-1.6) | 13 |
| Only chemotherapy | 170 | 6 | 6.5 | 0.9 (0.3-2.0) | 5 |
| Chemotherapy and RT | 40 | 1 | 1.8 | 0.6 (0.1-3.1) | 33 |
| Chemotherapy and transplantation | 13 | 0 | 0.5 | 0 | 0 |
| Chemotherapy, RT, and transplantation | 12 | 0 | 0.2 | 0 | 0 |
| Stroke | |||||
| After salvage (any type) | 225 | 4 | 3.2 | 1.3 (0.3-3.3) | 12 |
| After first-line treatment only | 216 | 7 | 5.1 | 1.4 (0.6-2.8) | 8 |
| Only chemotherapy | 135 | 2 | 3.4 | 0.6 (0.1-2.1) | –13 |
| Chemotherapy and RT | 56 | 5 | 1.5 | 3.3 (1.1-7.8) | 145 |
| Chemotherapy and transplantation | 15 | 0 | 0.1 | 0 | 0 |
| Chemotherapy, RT, and transplantation | 10 | 0 | 0.1 | 0 | 0 |
Event, treatment . | No. of patients* . | No. observed . | No. expected . | SIR (95% CI) . | AER per 10 000 person-years . |
|---|---|---|---|---|---|
| Chronic heart failure | |||||
| After salvage (any type) | 225 | 38 | 4.3 | 8.8 (6.3-12.1) | 346 |
| After first-line treatment only | 216 | 26 | 7.7 | 3.4 (2.2-4.9) | 120 |
| Only chemotherapy | 162 | 20 | 6.4 | 3.1 (1.9-4.8) | 127 |
| Chemotherapy and RT | 38 | 4 | 1.1 | 3.6 (1.0-9.8) | 120 |
| Chemotherapy and transplantation | 13 | 1 | 0.1 | 7.1 (0.2-39.8) | 81 |
| Chemotherapy, RT, and transplantation | 12 | 1 | 0.1 | 12.5 (0.3-69.7) | 83 |
| Myocardial infarction | |||||
| After salvage (any type) | 241 | 10 | 6.8 | 1.5 (0.7-2.7) | 29 |
| After first-line treatment only | 235 | 7 | 9.0 | 0.8 (0.3-1.6) | 13 |
| Only chemotherapy | 170 | 6 | 6.5 | 0.9 (0.3-2.0) | 5 |
| Chemotherapy and RT | 40 | 1 | 1.8 | 0.6 (0.1-3.1) | 33 |
| Chemotherapy and transplantation | 13 | 0 | 0.5 | 0 | 0 |
| Chemotherapy, RT, and transplantation | 12 | 0 | 0.2 | 0 | 0 |
| Stroke | |||||
| After salvage (any type) | 225 | 4 | 3.2 | 1.3 (0.3-3.3) | 12 |
| After first-line treatment only | 216 | 7 | 5.1 | 1.4 (0.6-2.8) | 8 |
| Only chemotherapy | 135 | 2 | 3.4 | 0.6 (0.1-2.1) | –13 |
| Chemotherapy and RT | 56 | 5 | 1.5 | 3.3 (1.1-7.8) | 145 |
| Chemotherapy and transplantation | 15 | 0 | 0.1 | 0 | 0 |
| Chemotherapy, RT, and transplantation | 10 | 0 | 0.1 | 0 | 0 |
Comparison in 476 patients for myocardial infarction (1980-2000) and in 441 patients for chronic heart failure and stroke (1985-2000)
Standardized incidence ratios and absolute excess risks with 95% confidence intervals for chronic heart failure after NHL treatment: risk factor sub-analyses. Comparison of 441 NHL patients treated (from 1985 to 2000) with doxorubicin-based chemotherapy with the Dutch population (as population-based incidences of chronic heart failure were available after January 1, 1985). Subgroups were selected to estimate the impact of age during NHL treatment, smoking, preexisting hypertension and additional salvage treatment (any type), radiotherapy (RT) of the mediastinum, and autologous ASCT. (Missing data for smoking in 90 cases and for hypertension in 11 cases.)
Standardized incidence ratios and absolute excess risks with 95% confidence intervals for chronic heart failure after NHL treatment: risk factor sub-analyses. Comparison of 441 NHL patients treated (from 1985 to 2000) with doxorubicin-based chemotherapy with the Dutch population (as population-based incidences of chronic heart failure were available after January 1, 1985). Subgroups were selected to estimate the impact of age during NHL treatment, smoking, preexisting hypertension and additional salvage treatment (any type), radiotherapy (RT) of the mediastinum, and autologous ASCT. (Missing data for smoking in 90 cases and for hypertension in 11 cases.)
Standardized incidence ratios and absolute excess risks with 95% confidence intervals for myocardial infarction after NHL treatment: risk factor sub-analyses. Comparison of 476 NHL patients treated (from 1980 to 2000) with doxorubicin-based chemotherapy with the Dutch population. Subgroups were selected to estimate the impact of age during NHL treatment, smoking, preexisting hypertension and additional salvage treatment (any type), radiotherapy (RT) of the mediastinum, and ASCT. (Missing data for smoking in 96 cases and for hypertension in 11 cases.)
Standardized incidence ratios and absolute excess risks with 95% confidence intervals for myocardial infarction after NHL treatment: risk factor sub-analyses. Comparison of 476 NHL patients treated (from 1980 to 2000) with doxorubicin-based chemotherapy with the Dutch population. Subgroups were selected to estimate the impact of age during NHL treatment, smoking, preexisting hypertension and additional salvage treatment (any type), radiotherapy (RT) of the mediastinum, and ASCT. (Missing data for smoking in 96 cases and for hypertension in 11 cases.)
Discussion
In comparison with the Dutch population, the risk of chronic heart failure appeared markedly increased in patients treated for aggressive NHL. In contrast, the risk of coronary artery disease matched the general population. Coronary artery disease is considered to be the main cause of chronic heart failure in the general population, whereas valvular disease and cardiomyopathy are far less common causes.40-42 In our cohort of patients with NHL causes other than coronary artery disease were frequently reported: cardiomyopathy (21%) and also rhythm disturbances were far more frequently observed than expected.31-33,42 Chronic heart failure (related to left ventricular dysfunction), ECG alterations (rhythm disturbances), and cardiomyopathy have all been described after doxorubicin-containing chemotherapy, not only as early onset complications but also as late events, observed up to 20 years after completion of anthracycline therapy.42-45 Unfortunately, detailed information on type of cardiomyopathy (dilated or obstructive) or on kind of rhythm disturbances was not available in our cohort. Nevertheless, we observed a clearly increased risk (Figure 4; SIR, 3.1; AER, 127 per 10 000 person-years) of chronic heart failure in patients treated only with chemotherapy, even though in most patients the maximum doxorubicin dose of 400 mg/m2 had not been exceeded. Coronary artery disease and other vascular occlusive events according to pathologic findings of accelerated atherosclerosis have been reported as late complications in patients with Hodgkin lymphoma, who have been mainly treated with radiotherapy.9-12 Also, fibrosis of the pericardia, myocardia, and valves are described, leading to serious and often life-threatening cardiac events.46,47 Because more than half of our patients had received additional radiotherapy involving the mediastinum, we had expected more coronary and valvular disease. Remarkably, risk of coronary artery disease in our cohort appeared to be comparable to the Dutch population when both age and sex were taken into account. Only in the small subset of patients who had received 40 Gy or more was an increased risk observed. Cardiotoxicity resulting from radiotherapy has been described as appearing late (time intervals of approximately 7 years before development of clinical signs are reported).47,48 To evaluate whether follow-up time did influence the role of radiotherapy, we reanalyzed our data restricted to those patients who had a follow-up of more than 3 years or even more than 5 years. In these long-term survivors a relative higher risk of chronic heart failure and myocardial infarction was found, but still no significant influence of mediastinal radiotherapy was observed. We cannot exclude, however, that such an effect of increase in risk may emerge after more prolonged follow-up. Another explanation for the lack of radiotherapy-related cardiotoxicity in NHL may be related to the dose and field size of radiotherapy on the mediastinum. In earlier reports, mainly in patients with Hodgkin lymphoma, often higher doses of radiotherapy had been given (without chemotherapy) and outdated techniques were used.9-12,47-50 In our study protocols, the prescribed dose of additional radiotherapy was 30 Gy in the case of complete remission and 40 Gy in the case of partial response. Dose reduction was advised in the case of large-field and extranodal localizations. After the retrospective collection of all fields and cumulative dose, most of our patients appeared to be treated with 30 to 40 Gy. Furthermore, the cumulative radiation dose to the heart region in our cohort was restricted by improved planning techniques, including (partial) shielding, which became standard after 1985.50,51 In contrast, no blocks or dose limitations were used in the neck and groins, resulting in higher cumulative doses and indeed higher risk of occlusive vascular disease. Moderate doses (30-50 Gy) increased the risk of stroke after NHL in our cohort, but truly high risks, like that described by Dorresteijn et al52 in patients with head and neck cancer (who received up to 60 Gy), were not observed.
In a rather elderly patient group, risk of cardiovascular disease can only be correctly described if compared with the general population. However, the way in which population-based rates are selected for comparison needs close attention. First, incidence rates differ over time: for both chronic heart failure and coronary artery disease the incidence dropped around 20% from 1985 to 1998, probably because of a lower prevalence of risk factors and early intervention strategies in Northern Europe.21-26 Furthermore, incidences also vary geographically because of differences in access to medical care but also because of lifestyle, socioeconomic status, and genetic predisposition.22-24 We therefore obtained population-based data covering the same region and used calendar period-specific rates to account for changes over time in the background rate for our cohort. Thus, the lower incidence rates in the Dutch population during the late 1990s did not cause an overestimation of risk among those patients diagnosed in the 1980s. The Dutch (CMRN) incidence rates used were prospectively collected in general practices. Similarly, as defined in our cohort, the CMRN registers all different events (and dates) even when present in the same person, but in the case of more of the same events only the first event in time is recorded. In both the CMRN and our study occurrence of cardiovascular disease was registered during follow-up. The patients with NHL were followed in the routine follow-up of the 4 EORTC-trials; the CMRN cohort was followed by their GP. Because in the Netherlands nearly all residents are followed by their GP and not only those needing medical care, the surveillance bias is limited. The only difference is that the study cohort has been retrospectively reviewed, whereas the CMRN is a prospective registration project since 1971. The ideal way to compare incidence, without surveillance bias, would have been a comparison between 2 cohorts that are both prospectively followed. However, (prospective) studies on late sequelae need long-term follow-up in patients uniformly treated, and these data are rare. The source of information in the study group was the treating physician, consulting the GP of the patient if the patient was no longer under his medical surveillance at time of the survey in 2002 (mainly because of death). Because of the retrospective nature of the survey, we cannot guarantee that the treating physicians actively inquired about cardiovascular disease in all of the patients. However, only hard diagnoses, quoted as serious and disabling, were asked to be registered. This implies a selection of patients with serious cardiotoxicity only (and therefore underestimation of cardiovascular disease incidence) but corresponds with the CMRN registration.
Hequet et al53 analyzed a group of adult patients with lymphoma treated with doxorubicin-based chemotherapy and found only a single case with overt clinical chronic heart failure of the 141 patients but found subclinical cardiomyopathy in 39 cases. Adams et al54 did the same in long-term survivors of HL (median follow-up, 14.3 years) and also concluded that, although most patients are doing fine, subclinical cardiovascular changes are often present and can progress to clinical disease if not treated. If this ratio of chronic heart failure versus subclinical cardiomyopathy would be identical in the long-term follow-up of patients with NHL, close monitoring of these patients would be mandatory. Because most cardiovascular events in our study occurred already in the first years after NHL treatment (median interval, around 3 years), we propose that follow-up of patients with NHL in this period needs to be focused not only on recurrence of disease but also on signs of cardiovascular damage. As the cumulative incidence increased only further over time, we would advise to continue follow-up after 5 years and even 10 years, if possible. Hopefully lifestyle advice, especially quitting smoking, and treatment of hypercholesterolemia and hypertension will decrease the effect of treatment-related risk of cardiovascular morbidity and mortality. According to our study, an elevated risk of chronic heart failure can also be expected after full-dose doxorubicin-based chemotherapy for other cancer types, such as breast cancer. More research in this field, based on long-term follow-up data and comparison to general population incidence rates, is needed.
In conclusion, patients with NHL carry a high risk of chronic heart failure after being cured and need lifelong monitoring in this regard. In contrast, risk of coronary artery disease appeared more age dependent than treatment related.
Appendix
The names of the participants (with institutional affiliations) who entered more than 5 patients are as follows: J. Baars (Dutch Cancer Institute, Amsterdam, The Netherlands), J. Thomas (University Hospital Gasthuisberg, Leuven, Belgium), D. Bron (Jules Bordet Institute, Brussels, Belgium), J. C. Kluin-Nelemans (until 2001 Leiden University Medical Center, Leiden, The Netherlands), W. Schroyens (University Hospital Antwerp, Antwerp, Belgium), K. J. Roozendaal (Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands), J. M. M. Raemaekers (St Radboud University Hospital, Nijmegen, The Netherlands), G. J. Creemers (Catherina Hospital, Eindhoven, The Netherlands), M. B. van 't Veer (Rotterdam Cancer Institute, Rotterdam, The Netherlands), W. Gerrits (Comprehensive Cancer Center West, The Netherlands), G. J. Goverde (St Ignatius Hospital, Breda, The Netherlands), A. van Hoof (University Hospital St Jan, Brugge, Belgium), R. Debock (General Hospital Middelheim, Antwerp, Belgium), M. Fickers (Atrium Medical Center Heerlen, The Netherlands), H. P. Muller (Gooi-Noord Regional Hospital, Blaricum, The Netherlands), H. C. Schouten (University Hospital Maastricht, Maastricht, The Netherlands), H. N. L. M. Bron (Maasland Hospital, Sittard, The Netherlands), J. J. Keuning (St Josef Hospital, Veldhoven, The Netherlands), J. Michel (Medical Center Tivoli, Louviere, Belgium), and A. C. Tagnon (Medical Center Tournai, Tournai, Belgium).
Prepublished online as Blood First Edition Paper, December 8, 2005; DOI 10.1182/blood-2005-08-3392.
Supported by the Dutch Cancer Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The help of all participating centers and local investigators for their attribution is greatly acknowledged (see appendix A). We thank A. W. van den Belt-Dusebout (Department of Epidemiology, the Netherlands Cancer Institute, Amsterdam, the Netherlands) for her help with the person-year analyses and P. J. Perik (Department of Cardiology, University Medical Center, Groningen, The Netherlands), R. van Loon (Department of Cardiology, VU University Medical Center, Amsterdam, The Netherlands), and G. W. van Imhoff (Department of Hematology, University Medical Center, Groningen) for revising our manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal