Abstract
Hypoxia is a major pathophysiological condition for the induction of angiogenesis, which is a crucial aspect of growth in solid tumors. In mammalian cells, the transcriptional response to oxygen deprivation is largely mediated by hypoxia-inducible factor 1 (HIF-1), a heterodimer composed of HIF-1α and HIF-1β subunits. However, the response of endothelial cells to hypoxia and the specific involvement of HIF-α subunits in this process are still poorly understood. We show that human umbilical vein endothelial cells (HUVECs) cultured in the absence of growth factors survive and form tubelike structures when cultured under hypoxic, but not normoxic, conditions. HUVECs expressed both HIF-1α and HIF-2α when cultured under hypoxic conditions. Transfection of HIF-1α, but not HIF-2α, siRNA to HUVECs completely abrogated hypoxic induction of cords. Neutralizing antibodies to bFGF, but not IGF-1, VEGF, or PDGF-BB, blocked survival and sprouting of HUVECs under hypoxic conditions, suggesting the existence of an autocrine loop induced by low oxygen levels. Notably, bFGF-dependent induction of cord formation under normoxic conditions required HIF-1α activity, which was also essential for hypoxic induction of bFGF mRNA and protein expression. These results uncover the existence of an HIF-1α–bFGF amplification pathway that mediates survival and sprouting of endothelial cells under hypoxic conditions.
Introduction
Angiogenesis is the result of the combined activity of different cellular components of the tumor microenvironment and of signaling molecules that either activate or inhibit neovascularization.1 Autocrine and paracrine (eg, derived from tumor- and/or stromal-infiltrating cells) production of growth factors promoting angiogenesis ultimately acts on endothelial cells (ECs), which by a process involving invasion of the extracellular matrix, migration, and proliferation gives rise to vessel sprouting and formation of a new vascular bed. In tumor angiogenesis, imbalance between factors promoting and inhibiting vessel formation leads to irregular and disorganized formation of a vascular network, which is nevertheless essential for tumor growth and metastasis.2,3 Hypoxia is the major pathophysiological condition regulating angiogenesis. Increased angiogenesis in response to hypoxia is part of an adaptive response aimed at achieving increased delivery of oxygen and nutrients to tissues.4,5 The exposure of ECs to hypoxia has been shown to occur in vivo as a result of structurally abnormal tumor vasculature.2,6
The transcriptional response of mammalian cells to hypoxia is largely mediated by hypoxia-inducible factor-1 (HIF-1). HIF-1 is a basic helix-loop-helix transcription factor composed of an HIF-1β subunit, which is constitutively expressed, and an HIF-α subunit, which is strongly up-regulated under hypoxic conditions.7 At least 3 isoforms of the HIF-α subunit have been identified, although HIF-1α and HIF-2α or EPAS-1 are the ones that appear to play a predominant role in the transcriptional response to hypoxia. In normoxic conditions, HIF-1α and HIF-2α are degraded by a mechanism involving hydroxylation of 2 prolyl residues, ubiquitylation, and proteasomal degradation through a VHL-dependent pathway.8,9 Levels of HIF-1α are also influenced by genetic alterations, including but not limited to mutations of the VHL gene, growth factors, which increase HIF-1α protein synthesis by a pathway involving PI3K/AKT/mTOR and MAPK, and cytokines produced by both tumor and stromal cells.10-12 While HIF-1α is ubiquitously expressed, HIF-2α was originally identified in ECs and some highly vascularized tissues and hence named endothelial PAS domain protein-1 (EPAS-1).13,14 However, later studies have shown that HIF-2α is also expressed in a variety of other cell types and tissues.15-18 The 2 HIF-α subunits induce transcriptional activation via interaction with hypoxia response elements (HREs), but their role in regulating specific transcriptional responses in different cell types is still poorly understood.19
In this study, we analyzed the response of isolated culture ECs to hypoxia and specifically the relative involvement of HIF-1α and HIF-2α in this process. We found that human umbilical vein endothelial cells (HUVECs), cultured on growth factor–reduced Matrigel in the absence of exogenous growth factors, form tubelike structures when incubated under hypoxic but not normoxic conditions. Interestingly, hypoxic induction of HUVEC cord formation was dependent on HIF-1α activity and induction of bFGF by ECs. Importantly, we found that late but not early hypoxic induction of HIF-1α in HUVECs requires bFGF, whose expression was further amplified by an HIF-1α–dependent pathway. These results uncover the existence of an HIF-1α–bFGF amplification pathway that mediates survival of ECs under hypoxic conditions. In addition, they emphasize the role that HIF-1α plays in the response of ECs to hypoxia and underscore its potential role as therapeutic target for development of antiangiogenic therapies.
Materials and methods
Cell culture and reagents
HUVECs (GlycoTech, Rockville, MD) were cultured on uncoated polystyrene dishes in endothelial basal medium supplemented with EGM-2 Bullet Kit (Cambrex BioScience, Walkersville, MD) containing 2% FBS, heparin, human IGF-1, ascorbic acid, human EGF, human VEGF, human bFGF, hydrocortisonee, and gemtamicin/amphotericin B, as supplied by the manufacturer (referred to as GF+) (Cambrex Bio Science, Baltimore, MD). Cells were maintained at 37°C in a humidified 5% CO2 incubator. Cells were generally used between the third and sixth passage. For experiments under minimal medium conditions (referred to as GF-), HUVECs were maintained in Clonetics EBM-2 medium with 0.1% of serum (Cambrex Bio Science) without addition of exogenous growth factors. Experiments under hypoxic conditions (1% O2) were performed in the hypoxic workstation Invivo2 400 (Biotrace International, Cincinnati, OH). Topotecan and TNP-470 were provided by Developmental Therapeutics Program (DTP), National Cancer Institute (Rockville, MD). Aliquots of stock solutions (10 mM in DMSO, stored at –70°C) were used once and then discarded. Human IGF-1, VEGF, bFGF, and PDGF-BB were purchased from R&D Systems (Minneapolis, MN).
Matrigel angiogenesis assay
All the experiments were performed using growth factor–reduced Matrigel at a concentration of 1 mg/mL (BD Biosciences, Franklin Lakes, NJ). Sixty microliters of Matrigel was added to each well of a 96-well plate on ice, allowed to gel for 15 minutes at room temperature, and then placed in a humidified incubator at 37°C. HUVECs (2 × 104 cells per well) were added to the Matrigel-coated plates in a final volume of 200 μL. Growth factors were added as indicated, and cells were then incubated under hypoxic or normoxic conditions. Sixteen hours later, pictures of each well were taken using a Leica DM IRB inverted microscope equipped with a 40×/0.55 N-PLAN objective lens (Leica, Rockville, MD); pictures were taken with an Olympus DP70 camera (Olympus, Melville, NY) and processed with Bioquant Image Analysis System BQ NOVA PRIME, version 6.75.10 (R&M Biometrics, Nashville, TN). The effect of each treatment was also assessed by measuring the length of cords and the number of junctions formed using the Bioquant Image Analysis System (R&M Biometrics).
Immunoblotting
After appropriate stimulation, HUVECs were collected, washed twice with ice-cold Dulbecco phosphate-buffered saline 1 × (PBS), and pelleted by centrifugation at 240g (1000 rpm) for 5 minutes at 4°C. The cell pellet was subsequently lysed in a buffer containing 1.5 mM NaF, 10 mM KCl, 2 mM DTT, 2 mM sodium vanadate, 4 μg/mL pepstatin, 4 μg/mL leupeptin, 4 μg/mL aprotinin, and diisopropyl fluorophosphate (DFP) and incubated for 10 minutes on ice. Typically, 100 μg protein was separated in a 4% to 20% Tris-glycine gel (Invitrogen, Carlsbad, CA) and electroblotted on a PVDF membrane (Invitrogen). Membranes were blocked for 1 hour at room temperature with blocking buffer A (1 × PBS, 0.1% Tween 20, and 4% nonfat dry milk). To detect HIF-1α and HIF-2α proteins, specific antibodies anti–HIF-1α (BD Biosciences) and anti–HIF-2α (Novus Biologicals, Littleton, CO) were used at 1:300 and 1:1000 dilution, respectively, in blocking buffer B (PBS, 0.1% Tween 20, and 0.4% BSA). After washing 3 times in washing buffer (PBS and 0.1% Tween 20), membranes were incubated for 1 hour at room temperature with a peroxidase-conjugated antimouse antibody (diluted 1:10 000) for HIF-1α and peroxidase-conjugated antirabbit for HIF-2α (Amersham Biosciences, Piscataway, NJ). Membranes were then washed 3 times in washing buffer, and chemiluminescence detection was performed using an enhanced chemiluminescence kit according to the manufacturer's protocol (Amersham Biosciences).
Transient transfection
siRNA oligonucleotides targeting HIF-1α (target sequence: 5′-AAAGGACAAGUCACCACAGGA-3′) or HIF-2α (target sequence: 5′-UCAAGUUGCUGGUCAUCAG-3′) were obtained from Qiagen (Valencia, CA).
HUVECs were transfected with the corresponding siRNA using Oligofectamine (Invitrogen) 48 hours before treatment with the indicated conditions. Cells were plated onto 10 cm2 cell culture dishes and grown to 30% to 50% confluence before transfection. The siRNA was diluted to a final concentration of 20 nM in Opti-Mem I (Invitrogen). Sixty microliters of Oligofectamine transfection reagent (Invitrogen) was added and the mixture incubated at room temperature for 30 minutes. Cells were rinsed in Opti-Mem to remove any residual of serum before the addition of siRNA complexes and then incubated in serumfree condition for 4 hours at 37°C. Media were then changed into EGM-2, and cells were incubated for an additional 48 hours before treatment.
Real-time PCR
Total RNA from HUVECs was obtained using RNA Mini Kit (Qiagen). One μg of total RNA was used to perform reverse transcriptase–polymerase chain reaction (RT-PCR) using RT-PCR kit (PE Biosystems, Foster City, CA) according to the manufacturer's protocol. To measure human HIF-1α and HIF-2α expression, real-time PCR was performed using an ABI-Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA). Typically 5 ng of reverse-transcribed cDNA per sample was used to perform real-time PCR in triplicate samples. Specific primers were obtained from Applied Biosystems. The following primers were used: HIF-1α, forward 5′-CCAGTTAGGTTCCTTCGATCAGT-3′ and reverse 5′-TTTGAGGACTTGCGC TTTCA-3′; HIF 2-α, forward 5′-AGCAGATGGACAACTTGTACCTGA-3′ and reverse 5′-TGTCGCCATCTTGGGTCAC-3′; bFGF, forward 5′-GGCGTGTACATGTGGTCTCAGA-3′ and reverse 5′-TTATGGCTCAC TGCAACCTTGA-3′.
Detection of 18S rRNA, used as internal control, was performed using premixed reagents from Applied Biosystems. Detection of HIF-1α and HIF-2α and 18S rRNA was performed using Syber Green PCR Master Mix (Applied Biosystems).
Values are expressed as fold increases relative to the reference sample (untreated control).
ELISA
Supernatants were collected from HUVECs after 16 hours of incubation under the indicated conditions. Total levels of bFGF protein were measured at the Lymphokine Testing Laboratory, Science Applications International Corporation-Frederick, NCI (Frederick, MD) using human bFGF Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems).
Results
Hypoxia induces human EC cord formation in the absence of exogenous growth factors
To determine the involvement of hypoxia in capillary morphogenesis, we analyzed the ability of HUVECs to form tubelike structures in the presence or absence of exogenously added growth factors under either normoxic (21% O2) or hypoxic (1% O2) conditions. HUVECs were plated in growth factor–reduced Matrigel either in complete medium containing growth factors and 2% FBS (GF+) or growth factor–depleted medium with 0.1% FBS (GF-).
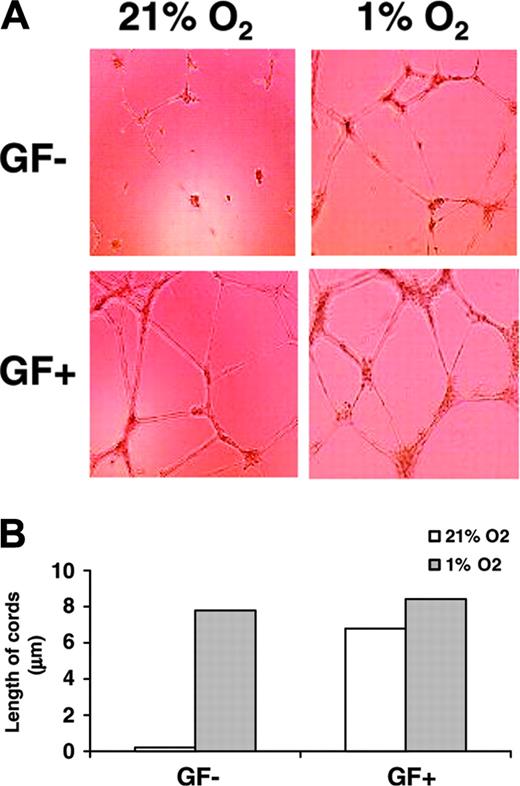
As shown in Figure 1A, HUVECs cultured in GF+ medium form tubelike structures under either normoxic or hypoxic conditions to a comparable extent. In contrast, ECs cultured in GF– medium only formed cords when they were incubated under hypoxic but not normoxic conditions for 16 hours. Hypoxia also significantly increased the number of junctions (data not shown) and the length of cords in HUVECs cultured in GF– medium by approximately 40-fold compared with cells cultured under normoxic conditions (Figure 1B). No significant differences between number of junctions (not shown) and length of cords were observed in HUVECs cultured in GF+ medium between normoxic and hypoxic conditions (Figure 1B).
Hypoxia induces cord formation in HUVECs. (A) HUVECs were seeded on growth factor–reduced Matrigel in GF+ or in GF– medium. Cells were then cultured under 21% O2 (normoxia) or 1% O2 (hypoxia) for 16 hours. Cord formation was examined by phase-contrast microscopy at × 10 magnification. Results are representative of 3 independent experiments. (B) HUVECs were cultured as described in panel A. Every well was divided in 3 different parts, and the length of cords was counted in each part using the Bioquant Image Analysis System.
Hypoxia induces cord formation in HUVECs. (A) HUVECs were seeded on growth factor–reduced Matrigel in GF+ or in GF– medium. Cells were then cultured under 21% O2 (normoxia) or 1% O2 (hypoxia) for 16 hours. Cord formation was examined by phase-contrast microscopy at × 10 magnification. Results are representative of 3 independent experiments. (B) HUVECs were cultured as described in panel A. Every well was divided in 3 different parts, and the length of cords was counted in each part using the Bioquant Image Analysis System.
These results demonstrate that hypoxia induces morphologic changes in HUVECs similar to those observed in the presence of growth factors, suggesting the existence of a hypoxia-dependent autocrine pathway that mediates EC survival and induction of angiogenesis.
HIF-1α, but not HIF-2α, mediates hypoxic induction of cord formation in HUVECs. HUVECs were seeded in a 10 mm dish and transfected with a negative control siRNA (NC) or siRNA specific for HIF-1α or HIF-2α. Cells were then treated in the absence or presence of DFO (100 μM) for 6 hours. HIF-1α and HIF-2α mRNA expression (A) was analyzed by real-time PCR, and HIF-1α and HIF-2α protein accumulation (B) was measured by immunoblotting.(C) HUVECs transfected with the indicated siRNA were subjected to hypoxic conditions for 16 hours in GF– medium, and cord formation was examined. Results are representative of 4 independent experiments. (D) Length of cords from the experiment shown in panel C was quantified using the Bioquant Image Analysis System. Results are presented as mean of 3 different areas examined per well.
HIF-1α, but not HIF-2α, mediates hypoxic induction of cord formation in HUVECs. HUVECs were seeded in a 10 mm dish and transfected with a negative control siRNA (NC) or siRNA specific for HIF-1α or HIF-2α. Cells were then treated in the absence or presence of DFO (100 μM) for 6 hours. HIF-1α and HIF-2α mRNA expression (A) was analyzed by real-time PCR, and HIF-1α and HIF-2α protein accumulation (B) was measured by immunoblotting.(C) HUVECs transfected with the indicated siRNA were subjected to hypoxic conditions for 16 hours in GF– medium, and cord formation was examined. Results are representative of 4 independent experiments. (D) Length of cords from the experiment shown in panel C was quantified using the Bioquant Image Analysis System. Results are presented as mean of 3 different areas examined per well.
HIF-1α, but not HIF-2α, mediates cord formation induced by hypoxia in ECs
To determine whether HIF-1 was involved in the hypoxia-dependent induction of capillary morphogenesis, we used siRNA that specifically targeted HIF-1α or HIF-2α. Transfection of siRNA oligonucleotides targeting HIF-1α or HIF-2α in HUVECs led to specific inhibition of the corresponding mRNA (Figure 2A) and protein (Figure 2B) expression. In particular, HIF-1α mRNA and protein expression were inhibited by 80% and 90%, respectively. HIF-2α expression was inhibited by 70% and 80% at the mRNA and protein level, respectively. In contrast, a scrambled siRNA used as negative control (NC) did not affect mRNA (Figure 2A) or protein (Figure 2B) expression of either HIF-1α or HIF-2α.
To investigate the involvement of HIF-1α and HIF-2α in the hypoxic induction of cord formation, HUVECs were transfected with an NC siRNA oligonucleotide or siRNAs targeting HIF-1α or HIF-2α. Forty-eight hours following transfection, cells were seeded on growth factor–reduced Matrigel and incubated under normoxic or hypoxic conditions for 16 hours. HUVECs formed cords under hypoxic (Figure 2C, left panel) but not normoxic conditions (not shown). Notably, transfection of siRNA targeting HIF-1α, but not HIF-2α or NCs, completely abrogated cord formation induced by hypoxia, suggesting a direct involvement of HIF-1α in this process. Consistently, HIF-1α, but not HIF-2α or NC siRNA, inhibited the number of junctions (data not shown) and the length of cords induced by hypoxia (Figure 2D).
In conclusion, these results demonstrate that HIF-1 is essential for the angiogenic response of HUVECs to hypoxia and that HIF-1α, but not HIF-2α, mediates a hypoxia-dependent autocrine pathway leading to cord formation in human ECs.
Topotecan inhibits HIF-1α, but not HIF-2α, protein expression and cord formation in human ECs
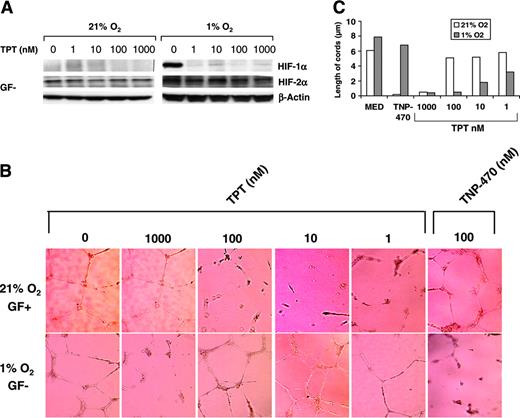
We have previously shown that topotecan, a topoisomerase I poison, inhibits HIF-1α protein accumulation in human cancer cell lines by a mechanism independent of DNA replication-mediated DNA damage.20 To further investigate the role of HIF-1 in the response of ECs to hypoxia, HUVECs were cultured in GF– medium under normoxic or hypoxic conditions in the absence or presence of increasing concentrations of topotecan (1 to 1000 nM), and the levels of HIF-1α and HIF-2α were measured by Western blot. As shown in Figure 3A, HIF-1α was undetectable under normoxic conditions but was significantly induced by incubation under 1% oxygen. HIF-2α was constitutively expressed in HUVECs, and its expression was further increased by incubation under hypoxia. Interestingly, we found that topotecan at concentrations as low as 1 nM completely inhibited hypoxic induction of HIF-1α in HUVECs (Figure 3A). In contrast, topotecan at concentrations as high as 1 μM had minimal or no effect on both the constitutive and the hypoxia-inducible levels of HIF-2α (Figure 3A), demonstrating that topotecan selectively inhibits HIF-1α but not HIF2α in human ECs.
To further investigate the effects of topotecan on hypoxic induction of angiogenic activity in ECs, HUVECs were seeded on growth factor–reduced Matrigel and cultured in GF+ or GF– medium under normoxic or hypoxic conditions, respectively, in the absence or presence of increasing concentrations of topotecan. Topotecan potently inhibited hypoxic induction of cord formation at concentrations as low as 1 to 10 nM (Figure 3B), consistent with the inhibition of HIF-1α protein accumulation shown in Figure 3A. In contrast, HUVEC cord formation induced by GF+ medium under normoxic conditions was only inhibited by topotecan at concentrations in the range of 1 μM. Topotecan also inhibited the number of junctions (data not shown) and the length of cords (Figure 3C) in HUVECs cultured under hypoxic conditions in a dose-dependent fashion. These results suggest that topotecan has a preferential inhibitory effect on hypoxia-dependent induction of EC function. Interestingly, TNP-470, a known antiangiogenic agent, inhibited cord formation under normoxic but not hypoxic conditions (Figure 3B-C), further indicating the existence of distinct pathways mediating EC function in the presence or absence of oxygen.
In conclusion, our results demonstrate that topotecan inhibits HIF-1α, but not HIF-2α, and HIF-1α–dependent functions in human ECs, and they suggest that HIF-1α and HIF-2α are regulated by distinct mechanisms in ECs.
bFGF is required for cord formation induced by hypoxia
Hypoxia is a critical condition for the induction of a number of proangiogenic growth factors in ECs.21-23 It is plausible then to suppose that hypoxia might induce the autocrine production of growth factors responsible for the formation of tubelike structures observed under our experimental conditions.
Topotecan inhibits HIF-1α but not HIF-2α in endothelial cells. (A) HUVECs cultured in GF– medium were incubated under normoxic or hypoxic conditions in the presence or absence of topotecan at the indicated concentrations for 16 hours. HIF-1α and HIF-2α protein accumulation was performed by immunoblotting; β-actin is shown as loading control. (B) HUVECs were seeded on Matrigel in GF+ medium under normoxic condition or in GF– medium under hypoxic conditions for 16 hours in the absence or presence of topotecan (1000 nM to 1 nM) or TNP-470 (100 nM), and cord formation was examined. Results are representative of 3 independent experiments.(C) Length of cords from the experiment shown in panel B was quantified using the Bioquant Image Analysis System.
Topotecan inhibits HIF-1α but not HIF-2α in endothelial cells. (A) HUVECs cultured in GF– medium were incubated under normoxic or hypoxic conditions in the presence or absence of topotecan at the indicated concentrations for 16 hours. HIF-1α and HIF-2α protein accumulation was performed by immunoblotting; β-actin is shown as loading control. (B) HUVECs were seeded on Matrigel in GF+ medium under normoxic condition or in GF– medium under hypoxic conditions for 16 hours in the absence or presence of topotecan (1000 nM to 1 nM) or TNP-470 (100 nM), and cord formation was examined. Results are representative of 3 independent experiments.(C) Length of cords from the experiment shown in panel B was quantified using the Bioquant Image Analysis System.
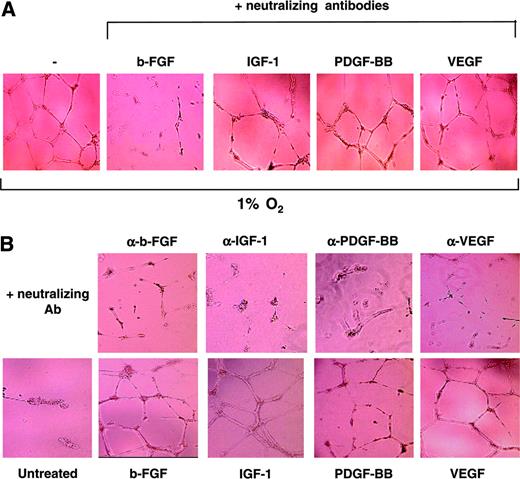
To address this question, HUVECs were seeded on growth factor–reduced Matrigel in the absence or presence of neutralizing antibodies against VEGF, bFGF, IGF-1, and PDGF-BB and cultured under hypoxic conditions for 16 hours. As shown in Figure 4A, addition of neutralizing antibodies for bFGF almost completely abrogated cord formation induced by hypoxia. Accordingly, anti-FGF antibodies also significantly inhibited the number of endothelial cell junctions and the length of cords (data not shown). In contrast, neutralizing antibodies against VEGF, IGF-1, and PDGF-BB had no effect on hypoxic induction of cord formation, indicating that autocrine production of bFGF by HUVECs mediates this function in ECs. To assess whether bFGF was the only growth factor able to induce cord formation in ECs cultured under our experimental conditions, HUVECs were plated on growth factor–reduced Matrigel and treated with the individual growth factors in the absence or presence of the corresponding neutralizing antibody under normoxic conditions. As shown in Figure 4B, bFGF, IGF-1, VEGF, and PDGF-BB were individually able to induce cord formation in ECs cultured in GF– medium. In addition, the induction of cords by each individual growth factor was completely abrogated by addition of the corresponding neutralizing antibody, further validating the specificity of their activity.
In conclusion, these results demonstrate that although bFGF, VEGF, IGF-1, and PDGF-BB all have the ability to induce cords in ECs cultured in GF– medium under normoxic conditions, bFGF is the only growth factor required and sufficient to mediate hypoxic induction of cord formation.
bFGF is required for late but not early induction of HIF-1α in ECs
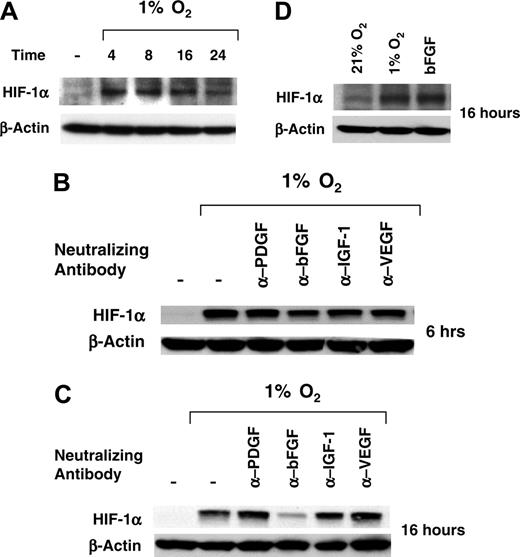
Results shown in Figure 2C indicate that HIF-1α is required for hypoxic induction of cords in HUVECs, raising the possibility of a connection between bFGF and HIF-1α. To address this question, we first determined the time course of hypoxic induction of HIF-1α in HUVECs. As shown in Figure 5A, HUVECs expressed little or no detectable HIF-1α protein under normoxic conditions. HIF-1α was rapidly induced when cells were cultured under hypoxic conditions, with maximal induction at 4 to 8 hours. Sustained levels of HIF-1α were still detectable at 16 hours, with a slight decrease observed at 24 hours. Next, HUVECs were cultured under normoxic or hypoxic conditions in the absence or presence of neutralizing antibodies for bFGF, VEGF, IGF-1, and PDGF-BB for 6 or 16 hours, and the levels of HIF1-α were measured by Western blot. HIF-1α was not detectable under normoxic conditions both at 6 or 16 hours (Figure 5 B-C, lane 1). Hypoxia induced HIF-1α protein accumulation at 6 hours (Figure 5B, lane 2), which was not affected by addition of any of the neutralizing antibodies tested (Figure 5B, lanes 3-6). In contrast, addition of anti-FGF antibodies, but not antibodies against VEGF, IGF-1, or PDGF-BB, almost completely abrogated hypoxic induction of HIF-1α at 16 hours (Figure 5C), indicating that bFGF is involved in late induction of HIF-1α by hypoxia. To directly test the ability of bFGF to induce HIF-1α, HUVECs were cultured under normoxic conditions in the absence or presence of bFGF or under hypoxic conditions for 16 hours. As shown in Figure 5D, bFGF induced HIF-1α protein accumulation in cells cultured under normoxic conditions (lane 3) to a comparable extent to what was observed in cells cultured under hypoxic conditions (lane 2) but did not further increase HIF-1α levels induced by hypoxia (data not shown), demonstrating that bFGF can induce HIF-1α in an oxygen-independent fashion in HUVECs.
bFGF is involved in hypoxic induction of cords in HUVECs. (A) HUVECs seeded on Matrigel in GF– medium were incubated under hypoxic conditions for 16 hours in the presence or absence of specific neutralizing antibodies for IGF-1, VEGF, bFGF, and PDGF-BB (100 ng/mL). (B) HUVECs were treated with 20 ng/mL IGF-1, VEGF, bFGF, or PDGF-BB, in the presence or absence of the specific neutralizing antibodies (100 ng/mL) for 16 hours, and cord formation was examined. Results are representative of 4 independent experiments.
bFGF is involved in hypoxic induction of cords in HUVECs. (A) HUVECs seeded on Matrigel in GF– medium were incubated under hypoxic conditions for 16 hours in the presence or absence of specific neutralizing antibodies for IGF-1, VEGF, bFGF, and PDGF-BB (100 ng/mL). (B) HUVECs were treated with 20 ng/mL IGF-1, VEGF, bFGF, or PDGF-BB, in the presence or absence of the specific neutralizing antibodies (100 ng/mL) for 16 hours, and cord formation was examined. Results are representative of 4 independent experiments.
HIF-1α is required for bFGF-dependent induction of cord formation in ECs
Results shown so far indicate that both bFGF and HIF-1α are required for hypoxia-induced capillary morphogenesis in human ECs. Furthermore, our data suggest that bFGF and HIF-1α might be involved in an autocrine loop that is required for sustained induction of angiogenic activity. To further address the existence of a connection between bFGF and HIF-1α, we tested whether induction of cord formation by bFGF in HUVECs required HIF-1α. HUVECs were transfected with siRNAs targeting HIF-1α, HIF-2α, or NCs and plated on growth factor–reduced Matrigel in the absence or presence of bFGF under normoxic conditions for 16 hours.
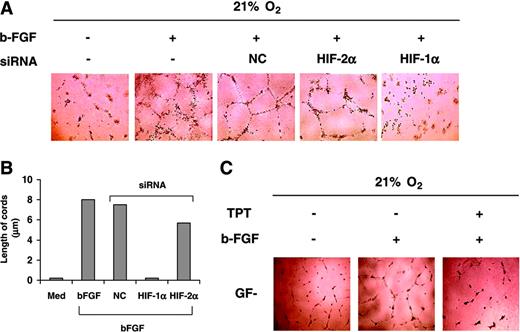
As shown in Figure 6A, bFGF induced tubelike structures in HUVECs, which were completely abrogated by transfection of siRNA targeting HIF-1α, but not HIF-2α or NCs, suggesting a direct connection between bFGF and HIF-1α. siRNA targeting HIF-1α also significantly decreased length of cords in HUVECs (Figure 6B), whereas siRNA targeting HIF-2α had a marginal but detectable effect. Consistent with these results, topotecan, which inhibits HIF-1α but not HIF-2α in HUVECs, also inhibited by 90% cord formation induced by bFGF under normoxic conditions (Figure 6C).
In conclusion, these results demonstrate that HIF-1α is involved in bFGF signaling in ECs, further supporting the existence of an autocrine pathway that amplifies and sustains bFGF signaling in ECs.
HIF-1α is required for hypoxic induction of bFGF
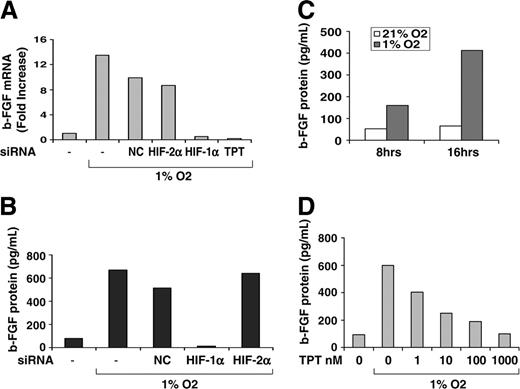
Finally, we asked whether HIF-1α was required for hypoxic induction of bFGF in ECs. HUVECs were cultured on growth factor–reduced Matrigel under normoxic or hypoxic conditions following transfection with siRNAs targeting HIF-1α, HIF-2α, or NCs. As shown in Figure 7A, HUVECs expressed significantly higher levels of bFGF mRNA when cultured under hypoxic relative to normoxic conditions. Transfection of siRNA targeting HIF-1α, but not HIF-2α or NCs, or treatment with topotecan dramatically decreased bFGF induction by hypoxia. Interestingly, when HUVECs were cultured on plastic rather than Matrigel, induction of bFGF mRNA by hypoxia was much less consistent (data not shown), indicating that interaction with extracellular matrix may be essential for a proper activation of ECs in response to hypoxia. Inhibition of bFGF mRNA expression was paralleled by a decrease of hypoxic induction of bFGF protein in the culture supernatant of HUVECs following transfection with HIF-1α, but not HIF-2α or NC, siRNA (Figure 7B). To further define the time course of bFGF induction under hypoxic conditions, HUVECs were cultured under normoxic or hypoxic conditions for 8 and 16 hours, and bFGF protein levels were tested in the culture supernatants. As shown in Figure 7C, hypoxia induced a time-dependent increase of bFGF production, which was already detectable at 8 hours (3-fold above control) and was more pronounced at 16 hours (up to 8-fold above control). Consistent with results shown in Figure 7A, topotecan inhibited hypoxic induction of bFGF protein in a dose-dependent fashion (Figure 7D), with inhibition already observed in the low nanomolar range (1 to 10 nM).
bFGF neutralizing antibody inhibits HIF-1α protein accumulation at a late time. (A) HUVECs were seeded in GF– medium and then cultured under normoxic or hypoxic conditions for 4, 8, 16 and 24 hours. HIF-1α protein accumulation was assessed by immunoblotting (B-C) HUVECs were seeded in GF– medium and then cultured under normoxic or hypoxic conditions for 6 or 16 hours in the presence or absence of specific neutralizing antibodies for IGF-1, VEGF, bFGF, and PDGF-BB (100 ng/mL). HIF-1α protein accumulation was assessed by immunoblotting. (D) HUVECs were seeded in GF– medium and then cultured for 16 hours under normoxic conditions in the presence or absence of bFGF (20 ng/mL) or under hypoxic conditions. HIF-1α protein accumulation was assessed by immunoblotting; β-actin is shown as loading control.
bFGF neutralizing antibody inhibits HIF-1α protein accumulation at a late time. (A) HUVECs were seeded in GF– medium and then cultured under normoxic or hypoxic conditions for 4, 8, 16 and 24 hours. HIF-1α protein accumulation was assessed by immunoblotting (B-C) HUVECs were seeded in GF– medium and then cultured under normoxic or hypoxic conditions for 6 or 16 hours in the presence or absence of specific neutralizing antibodies for IGF-1, VEGF, bFGF, and PDGF-BB (100 ng/mL). HIF-1α protein accumulation was assessed by immunoblotting. (D) HUVECs were seeded in GF– medium and then cultured for 16 hours under normoxic conditions in the presence or absence of bFGF (20 ng/mL) or under hypoxic conditions. HIF-1α protein accumulation was assessed by immunoblotting; β-actin is shown as loading control.
In conclusion, these results demonstrate that HIF-1α mediates hypoxic induction of bFGF mRNA and protein accumulation in HUVECs.
Discussion
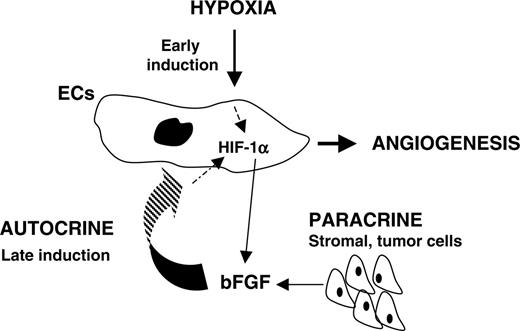
We have investigated the role that hypoxia and HIF-1 play in endothelial cell function. Survival of ECs cultured under normoxic conditions is dependent upon addition of exogenous EC-specific growth factors. We found that hypoxia can overcome this requirement and supplement ECs of necessary growth factors essential for survival and sprouting. More importantly, we discovered the existence of a crosstalk between HIF-1α and bFGF that mediates a hypoxic-dependent autocrine pathway leading to HUVEC survival and angiogenesis (Figure 8).
HIF-1α siRNA and TPT inhibit cord formation induced by bFGF. (A) HUVECs transfected with the indicated siRNA were seeded on Matrigel in GF– medium and treated in the presence or absence of bFGF (20 ng/mL) under normoxic conditions for 16 hours, and cord formation was examined. (B) Length of cords from the experiment shown in panel A was quantified using the Bioquant Image Analysis System as described above. (C) HUVECs were cultured as indicated in panel A under normoxic conditions for 16 hours in the presence or absence of bFGF (20 ng/mL) or TPT (0.1 μM), and cord formation was examined. Results are representative of 4 independent experiments.
HIF-1α siRNA and TPT inhibit cord formation induced by bFGF. (A) HUVECs transfected with the indicated siRNA were seeded on Matrigel in GF– medium and treated in the presence or absence of bFGF (20 ng/mL) under normoxic conditions for 16 hours, and cord formation was examined. (B) Length of cords from the experiment shown in panel A was quantified using the Bioquant Image Analysis System as described above. (C) HUVECs were cultured as indicated in panel A under normoxic conditions for 16 hours in the presence or absence of bFGF (20 ng/mL) or TPT (0.1 μM), and cord formation was examined. Results are representative of 4 independent experiments.
The relative contribution of HIF-1α and HIF-2α to the transcriptional response of ECs to hypoxia is still poorly understood. In most normal and transformed cell lines, HIF-1α has been identified as the master regulator of the transcriptional response to oxygen deprivation. In contrast, in VHL-deficient renal cancer cell lines HIF-2α appears to be essential for a transcriptional program mediating survival and angiogenesis,24 although these cells may also overexpress HIF-1α and the relative contribution of HIF-α subunits to renal carcinogenesis is still poorly understood. Cell-type specificity has been advocated to explain the relative functional activity of HIF-1α and HIF-2α. HIF-2α was originally identified as EC-specific factor (and called EPAS), and its tissue-restricted expression was thought to determine, at least in part, its involvement in the transcriptional response to hypoxia.19 Surprisingly, we found that HIF-1α, but not HIF-2α, was involved in a hypoxic-dependent pathway mediating EC survival and sprouting. This conclusion is supported by experiments performed using siRNA targeting HIF-1α, which completely blocked hypoxic induction of cord formation in HUVECs. In addition, siRNA targeting HIF-1α also inhibited hypoxic induction of several HIF-1-inducible genes, such as CXCR4, c-met, and glut-1, in HUVECs (data not shown). Consistent with these results, we found that topotecan, a small molecule previously shown by our group to inhibit HIF-1α in human caner cell lines,20 also inhibited HIF-1α, but not HIF-2α, and HIF-1-dependent responses in HUVECs cultured under hypoxic conditions. The specific inhibition of HIF-1α, but not HIF-2α, by topotecan suggests not only that these 2 subunits have different mechanisms of regulation but also that they have distinct functions in ECs. The functional role that HIF-1α plays in human ECs has been recently addressed by Manalo et al,25 who have shown that HIF-1α mediates a cell-autonomous transcriptional response in human pulmonary artery ECs under both normoxic and hypoxic conditions. In addition, Tang et al26 have shown that conditional knockout of HIF-1α in mouse ECs profoundly disrupts EC behavior during angiogenesis and their response to VEGF signaling, further emphasizing the essential role that HIF-1α plays in EC biology and angiogenesis. On the contrary, HIF-2α has been implicated in vascular remodeling during vasculogenesis and in the response to oxidative stress.27 Indeed, EPAS-1–/– embryos display severe vascular defects due to improper interaction among blood vessels once they are formed.18,28 It is also notable that, although coexpressed in the same cell type, HIF-1α and HIF-2α do not compensate for each other in modulating the hypoxic response.29 These data suggest that a complex network of environmental conditions (eg, hypoxia and growth factors) and a developmentally determined genetic program must be taken into account to better understand the cellular response of ECs to hypoxia and the relative contribution of HIF-α subunits.
HIF-1α siRNA and TPT inhibit bFGF mRNA and protein accumulation. (A-B) HUVECs transfected with the indicated siRNA were seeded on Matrigel in GF– medium and cultured under normoxic or hypoxic conditions for 16 hours in the presence or absence of TPT (0.5 μM). Expression of bFGF mRNA (A) was determined by real-time PCR (expressed as fold increase relative to untreated cells), while bFGF protein production (B) was measured in supernatants as described in “Material and methods.” (C-D) HUVECs seeded as in panel A were cultured either under normoxic or hypoxic conditions for 8 and 16 hours (C) or in the presence or absence of increasing concentrations of TPT (1 to 1000 nM) for 16 hours (D). Production of bFGF protein was measured in the supernatants as described.
HIF-1α siRNA and TPT inhibit bFGF mRNA and protein accumulation. (A-B) HUVECs transfected with the indicated siRNA were seeded on Matrigel in GF– medium and cultured under normoxic or hypoxic conditions for 16 hours in the presence or absence of TPT (0.5 μM). Expression of bFGF mRNA (A) was determined by real-time PCR (expressed as fold increase relative to untreated cells), while bFGF protein production (B) was measured in supernatants as described in “Material and methods.” (C-D) HUVECs seeded as in panel A were cultured either under normoxic or hypoxic conditions for 8 and 16 hours (C) or in the presence or absence of increasing concentrations of TPT (1 to 1000 nM) for 16 hours (D). Production of bFGF protein was measured in the supernatants as described.
Tumor angiogenesis is essential for growth of cancer cells beyond a few millimeters, and identification of novel antiangiogenic agents is actively pursued for their potential therapeutic activity as anticancer agents.30 Our results indicating that HIF-1α is an essential mediator of the response of ECs to hypoxia further supports its potential role as a therapeutic target both in cancer cells as well as in stromal-infiltrating cells, including but not limited to ECs. We have previously reported that topotecan inhibits HIF-1α protein accumulation in the U251 human glioma cell line by a mechanism independent of DNA replication–mediated DNA damage.20,31 In addition, we have shown that topotecan administered on a chronic schedule (1 mg/kg/d for 10 days) had a profound antitumor effect in U251 human xenografts.32 Concomitant with the antitumor activity, topotecan inhibited microvessel density, as measured by CD31 staining, and HIF-1α expression in tumor tissue. However, it was not clear from our studies whether the antiangiogenic activity of topotecan was due to a direct effect on ECs or to an indirect mechanism mediated by inhibition of HIF-1 in tumor cells. A potential antiangiogenic activity of camptothecin analogs has been previously suggested.33 However, most of the effects described were observed at concentrations well above the pharmacologic levels that can be achieved in patients, and a putative34 mechanism of action had not been elucidated. We provide evidence here that topotecan inhibits HIF-1α expression in ECs and also inhibits hypoxic induction of HIF-1–dependent responses. Strikingly, topotecan had a dramatic effect on ECs cultured under hypoxic conditions, 100- to 1000-fold higher than that observed in cells cultured under normoxic conditions in the presence of growth factors. These results suggest that topotecan might have a selective effect on hypoxia-dependent angiogenic activity that could be therapeutically exploited. A protective effect of growth factors in cells cultured in GF+ medium under normoxic conditions might account at least in part for the lack of inhibition of angiogenesis by topotecan. However, the finding that TNP-470, a known antiangiogenic agent,35 inhibited angiogenic activity in ECs under normoxic but not hypoxic conditions argues against this possibility. The differential effect of topotecan and TNP-470 on ECs cultured under normoxic or hypoxic conditions in the presence or absence of growth factors further indicates the existence of distinct pathways acting in different culture conditions, and they emphasize the potential pitfalls of in vitro screening and testing of antiangiogenic agents.
The complex network of cell-cell interaction in the extracellular matrix of the tumor microenvironment leads to production of signaling molecules that ultimately regulate EC function. It is conventionally thought that survival factors for ECs are produced by stromal-infiltrating cells and/or by tumor cells.36 Under our experimental conditions, we found that ECs cultured in GF-depleted medium are able to survive when incubated under hypoxia but not normoxia. This finding led us to investigate the existence of an autocrine mechanism responsible for survival of ECs under hypoxia. Our results demonstrate that hypoxic induction of bFGF in an HIF-1–dependent fashion was required and sufficient to mediate EC survival. Surprisingly, we also found that HIF-1α was required for bFGF-dependent EC cord formation, suggesting the existence of an amplification pathway involving HIF-1α and bFGF, which mediates a sustained activation of survival and angiogenesis in ECs. Our model emphasizes the role that bFGF may play in the response of ECs to hypoxia but does not rule out the involvement of other growth factors as well, which may vary depending on culture conditions. However, recent evidence also implicates HIF-1α and bFGF in the delayed response of ECs to radiation therapy and in protecting ECs from radiation-induced cell death.37,38 Interestingly, we found that bFGF was required for delayed induction of HIF-1α under hypoxic conditions, as indicated by inhibition of HIF-1α protein accumulation in cells treated with neutralizing antibodies for bFGF. In addition, increased expression of bFGF has been recently described in stromal cells cultured under hypoxic conditions.39 Finally, HIF-1α–dependent alterations of heparan sulfate proteoglycan (HS) composition on the EC surface, which enhance the cell responsiveness to bFGF, has also been demonstrated,40 further emphasizing the connection between hypoxia, HIF-1α, and bFGF.41,42 It remains to be established whether HIF-1–dependent induction of bFGF observed under our experimental conditions is mediated by a direct transcriptional activation or by indirect mechanisms. A formal HRE has not been identified so far in regulatory regions of bFGF, raising the possibility that indirect pathways may play a role.
Schematic representation of an HIF-1α–bFGF autocrine/paracrine loop in ECs.
The crosstalk between HIF-1α and EC-specific growth factors needs to be further elucidated for a better understanding of the response of ECs to hypoxia. Our results emphasize the role that HIF-1α may have as a therapeutic target of the tumor microenvironment not only in tumor cells but also in stromal-infiltrating cells, including ECs.
Prepublished online as Blood First Edition Paper, November 22, 2005; DOI 10.1182/blood-2005-09-3541.
Supported in part by the National Cancer Institute, National Institutes of Health, under contract no. N01-CO-12400, and in part by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge Gurmeet Kaur and Jagadambal Thillainathan (National Cancer Institute at Frederick, MD) for helpful technical advice.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal