Abstract
The anemia of chronic disease (ACD) results from 3 major processes: slightly shortened red cell survival, impaired reticuloendothelial system iron mobilization, and impaired erythropoiesis. Hepcidin is an acute-phase protein with specific iron regulatory properties, which, along with the anemia seen with increased hepcidin expression, have led many to consider it the major mediator of ACD. However, if hepcidin is the major factor responsible for ACD, then it should also contribute to the impaired erythropoiesis observed in this syndrome. Erythroid colony formation in vitro was inhibited by hepcidin at erythropoietin (Epo) concentrations less than or equal to 0.5 U/mL but not at Epo 1.0 U/mL. At Epo concentrations of 0.3 U/mL, HCD57 erythroleukemia cells exposed to hepcidin exhibit decreased expression of the antiapoptotic protein pBad compared with controls. These studies suggest that hepcidin may contribute to anemia in ACD not only through effects on iron metabolism, but also through inhibition of erythroid progenitor proliferation and survival.
Introduction
The anemia of chronic disease (ACD) is one of the most common clinical syndromes encountered in the practice of medicine.1 This disorder typically manifests itself as a hypoproliferative anemia accompanied by a low serum iron concentration despite adequate reticuloendothelial iron stores. ACD has generally been considered to result from a combination of pathologic processes which can be linked to the cytokine mediators of inflammation. However, the diagnostic importance of altered iron metabolism in ACD has led many to consider that these features represent the dominant pathophysiologic contributor to this syndrome. Hepcidin is an antibacterial protein that is produced in the liver, circulated in the blood, and excreted in the urine. It is a type II acute-phase protein, the expression of which is induced by interleukin 6 (IL-6), and down-regulated by tumor necrosis factor.2 The specific iron regulatory functions of hepcidin, as well as the anemia syndromes observed in patients and transgenic animals with abnormalities of hepcidin gene expression,3,4 have led many to consider that it is the key to unlocking the “mysteries” of ACD.5,6
However, the pathogenesis of ACD is not purely a matter of iron, but rather involves 3 major processes. A slight shortening of red cell survival creates a demand for a small increase in red cell production by the bone marrow.7 The marrow cannot respond adequately to this demand due to impaired erythropoiesis and impaired mobilization of reticuloendothelial system iron stores.7 The impairment of erythropoiesis, in turn, consists of both a blunted erythropoietin (Epo) response to anemia8,9 and a decreased response of erythroid progenitors to Epo.10-12 A potential role for hepcidin in the iron anomalies of ACD is well supported by data currently available. However, if hepcidin is the major factor responsible for ACD, then it should also contribute to the impaired erythropoiesis observed in this syndrome. In this study, the effects of hepcidin on erythroid colony formation in vitro are examined.
Study design
Human subject participation
Bone marrow aspirates were collected from paid healthy volunteers under protocols approved by the institutional review boards of the investigators' institutions. Informed consent was provided in accordance with the Declaration of Helsinki.
Culture of erythroid colony-forming units (CFU-Es)
Marrow light density mononuclear (LDMN) cells were cultured for CFU-Es in 0.2 mL plasma clots as previously described,11,12 with recombinant human (rh) Epo and 25 amino acid (aa) human hepcidin. After incubation for 7 days in a humidified 95% air/5% CO2 incubator, clots were collected, fixed with 5% glutaraldehyde, and stained with hematoxylin-benzidine.13 CFU-Es were identified.14 In order to compare the results of different experiments, colony formation was expressed as a percentage of control (CFU-E colony formation at rhEpo 1 U/mL without added hepcidin).
Hepcidin was purchased from Alpha Diagnostics (San Antonio, TX). The preparation was reported by the manufacturer to be more than 95% pure. Matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF/MS) showed a major peak at 2790, consistent with the 25 aa hepcidin species, a shoulder at 2807 consistent with methionine oxidation of 25 aa hepcidin, and a lower molecular weight peak at approximately 2691, suggestive of loss of a single amino acid from 25 aa hepcidin. Biologic activity was confirmed by demonstrating that exposure to 100 ng/mL hepcidin for 24 hours decreased expression of ferroportin in marrow LDMN cells.
The possibility of nonspecific peptide effects on CFU-E colony formation or on phosphorylated Bad (pBad) expression by HCD57 cells was evaluated using aa 1-24 of human adrenocorticotropic hormone (ACTH(1-24)).
HCD57 cell line studies
Cell proliferation was evaluated by cell counting; viability was determined by trypan blue exclusion. Cells were lysed in RIPA buffer and the protein extracted for Western blot analysis. Each lane on the gel had 6.54 μg total protein applied. Gels were probed with antibodies against Bad (CalBiochem, La Jolla, CA), phosphorylated Bad (pBad), and Bcl-xL (Upstate Cell Signalling Solutions, Charlottesville, VA). Antiferroportin was purchased from Alpha Diagnostics, and was generated against a 19 aa ferroportin peptide.
Results and discussion
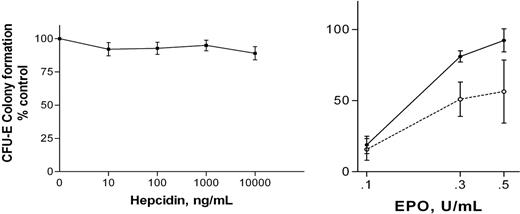
At the standard in vitro rhEpo concentration used for CFU-E colony assays in vitro (1 U/mL), hepcidin had no significant effects on colony formation (Figure 1A). However, ACD is a syndrome associated with relative reductions in Epo concentration.8,9 The effects of 100 ng/mL hepcidin on CFU-E colony formation were evaluated at lower rhEpo concentrations (0.1 U/mL-0.5 U/mL). Colony formation was significantly decreased in the presence of hepcidin (Figure 1B). This effect was not reversed by the addition of 500 μg/mL iron-saturated transferrin to the culture medium.
The regulatory pathways controlling apoptosis and proliferation of HCD57 erythroleukemia cells replicate those observed in primary human erythroid progenitors.15-17 This cell line was studied in order to more fully define the effects of hepcidin on erythropoiesis in a uniform erythroid-cell population. HCD57 cells were incubated in Iscove-modified Dulbecco medium (IMDM) with 10% fetal calf serum (FCS) with 0.3 U/mL rhEpo at 37°C for 24 hours, with or without 100 ng/mL hepcidin. No difference in cell proliferation was seen.
Effects of hepcidin on CFU-E on colony formulation. (A) Effects of hepcidin on CFU-E colony formation at rhEpo 1.0 U/mL. Results are expressed as mean ± SEM; findings from 4 experiments are combined. No values at any hepcidin concentration differ significantly from control (no added hepcidin) or from each other (ANOVA). Mean CFU-E concentration in control clots was 610 per 105 LDMN cells. (B) Effects of 100 ng/mL hepcidin on CFU-E colony formation at reduced rhEpo concentrations are shown. Results are expressed as mean ± SEM; findings from 3 experiments are combined. Colony formation by hepcidin-exposed cells (□, broken line) is significantly less than that of unexposed cells (▪, solid line; P = .031, ANOVA). Mean CFU-E concentration in control clots was 455 per 105 LDMN cells. ACTH(1-24) (100 ng/mL) had no effect on CFU-E colony formation at rhEpo concentrations between 0.1 U/mL and 1.0 U/mL rhEpo.
Effects of hepcidin on CFU-E on colony formulation. (A) Effects of hepcidin on CFU-E colony formation at rhEpo 1.0 U/mL. Results are expressed as mean ± SEM; findings from 4 experiments are combined. No values at any hepcidin concentration differ significantly from control (no added hepcidin) or from each other (ANOVA). Mean CFU-E concentration in control clots was 610 per 105 LDMN cells. (B) Effects of 100 ng/mL hepcidin on CFU-E colony formation at reduced rhEpo concentrations are shown. Results are expressed as mean ± SEM; findings from 3 experiments are combined. Colony formation by hepcidin-exposed cells (□, broken line) is significantly less than that of unexposed cells (▪, solid line; P = .031, ANOVA). Mean CFU-E concentration in control clots was 455 per 105 LDMN cells. ACTH(1-24) (100 ng/mL) had no effect on CFU-E colony formation at rhEpo concentrations between 0.1 U/mL and 1.0 U/mL rhEpo.
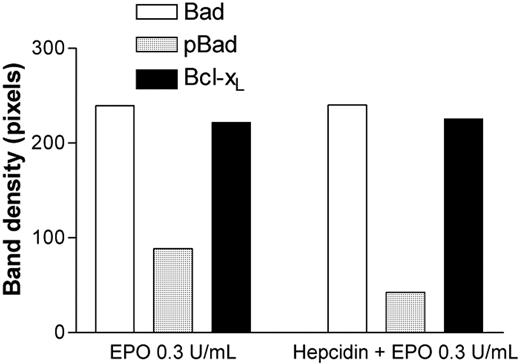
Effects of exposure to 100 ng/mL hepcidin on Bad, pBad, and Bcl-xL expression in HCD57 erythroleukemia cells at rhEpo 0.3 U/mL. pBad expression is significantly reduced relative to total Bad or Bcl-xL expression after exposure to hepcidin (P = .02, ANOVA). ACTH(1-24) (100 ng/mL) had no effect on pBAD expression by HCD57 cells.
Effects of exposure to 100 ng/mL hepcidin on Bad, pBad, and Bcl-xL expression in HCD57 erythroleukemia cells at rhEpo 0.3 U/mL. pBad expression is significantly reduced relative to total Bad or Bcl-xL expression after exposure to hepcidin (P = .02, ANOVA). ACTH(1-24) (100 ng/mL) had no effect on pBAD expression by HCD57 cells.
Apoptosis in HCD57 cells is regulated by the Bad/Bcl-xL pathway.17 Bad, pBad, and Bcl-xL are also expressed by primary human CFU-Es.18 Total Bad and Bcl-xL protein expression were unchanged by exposure to hepcidin; however, the proportion of the antiapoptotic protein pBad was decreased approximately 50% (Figure 2). CFU-E colony formation (as in Figure 1) reflects a combination of both differentiation and proliferation over a 7-day period. The demonstration of a proapoptotic effect of hepcidin does not rule out the possibility of a later antiproliferative hepcidin effect also contributing to the observed results.
There is considerable evidence to support the contention that hepcidin is a major, and possibly the major, contributor to the hypoferremia and increased reticuloendothelial iron stores associated with inflammation, particularly (though not exclusively) that associated with IL-6.2,19 A recent report has demonstrated that hepcidin reduces macrophage iron efflux by binding to the iron transporter ferroportin, causing its internalization and subsequent degradation.20 It is unlikely that the inhibition of CFU-E colony formation reported here results from this effect. Although marrow macrophages are present in the LDMN-cell population, and iron is required for erythropoiesis in vitro,21 the amount of iron-saturated transferrin contributed to the culture medium by FCS is comparable to the amount required for maximal CFU-E colony formation in serum-free medium in the absence of macrophages (300 μg/mL).21 In addition, supplementation of the culture medium by 500 μg/mL iron-saturated transferrin failed to reverse inhibition by hepcidin. The studies reported here do not disprove the possibility that hepcidin-induced impairment of iron flow to erythroid cells contributes to ACD; rather, they suggest an additional mechanism by which hepcidin can inhibit erythropoiesis.
It is important to recognize that not all of the pathogenetic processes for ACD are equally implicated in all clinical scenarios. In a study of anemic children with cancer, the Epo concentrations were appropriate; the major process in these patients was considered to be an impaired erythropoietic response to Epo.22 A similar study in adult patients with lung cancer found that the primary contributor to anemia was impaired erythropoiesis, but also observed a blunted Epo response.23 In contrast, it has been suggested that inavailability of iron is the dominant factor in the anemia of juvenile rheumatoid arthritis.24 A complete picture of the contribution of hepcidin or any other mediator to the pathogenesis of ACD requires identification of its contributions to these different processes.
Prepublished online as Blood First Edition Paper, December 6, 2005; DOI 10.1182/blood-2005-07-2854.
Supported by funds from the Medical Research Service, United States Department of Veterans Affairs, and by grant HL69 418 from the National Institutes of Health.
G.D. developed techniques, performed the experiment, collected study material from donors, and wrote/reviewed the manuscript; E.L. developed techniques, performed the experiment, and wrote/reviewed the manuscript; R.T.M. Jr designed the experiment, developed techniques, analyzed data, collected study material from donors, and wrote/reviewed the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Jack Goodman, University of Kentucky Advanced Science & Technology Commercialization Center, for performing MALDI-TOF/MS on hepcidin. The HCD57 murine erythroleukemia cell line was a generous gift from Dr Stephen Sawyer, Department of Pharmacology and Toxicology, Medical College of Virginia/Virginia Commonwealth University, Richmond.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal