Abstract
The SNO-Hb hypothesis holds that heme-bound nitric oxide (NO) present in the β subunits of T-state hemoglobin (Hb) will be transferred to the β-93 cysteine upon conversion to R-state Hb, thereby forming SNO-Hb. A deficiency in the ability of Hb to facilitate this intramolecular transfer has recently been purported to play a role in pulmonary hypertension and sickle cell disease. We prepared deoxygenated Hb samples with small amounts of heme-bound NO and then oxygenated the samples. Electron paramagnetic resonance (EPR) spectroscopy was used to (1) determine the concentration of iron nitrosyl Hb (Fe-NO Hb), (2) show that the NO is evenly distributed among α and β subunits, and (3) show that the Hb undergoes a change in its quaternary state (T to R) upon oxygenation. We did not observe a decrease in the concentration of Fe-NO Hb on oxygenation, which is inconsistent with the prediction of the SNO-Hb hypothesis.
Introduction
In 1996, Stamler's group1 published a revolutionary work stating that, rather than simply destroying NO activity through the deoxygenation reaction, hemoglobin (Hb) is capable of preserving, transporting, and exporting NO activity. It has been proposed that these novel activities of Hb are mediated by SNO-Hb, which is stabilized in the (high-oxygen affinity) R-state and destabilized in the (low-oxygen affinity) T-state.1-3 NO binds rapidly to the heme of deoxygenated Hb to form Fe-NO Hb. According to the SNO-Hb hypothesis, when Fe-NO Hb is oxygenated (and undergoes a T to R transition) some of the NO on the heme is transferred to the β-93 cysteine to form SNO-Hb.2-4 Upon re-deoxygenation, some of the NO (actually NO+) on the cysteine is transferred back to heme and some is exported by the red blood cell to effect vasodilation.2-5
The amount of heme-bound NO that undergoes allosterically controlled intramolecular transfer is claimed to be dependent on the amount of NO bound per hemoglobin tetramer.2,3 Previously, a decrease in yield of SNO-Hb upon oxygenation of an initially deoxygenated Fe-NO Hb sample was attributed to loss of allosteric control when the NO/Hb ratio was too high.2 More recently, the SNO-Hb hypothesis has been refined wherein it is stated that only NO bound to hemes of the β subunits (to form β-nitrosyl Hb) can efficiently undergo allosterically controlled intramolecular transfer.3,6 Due to the relative dissociation rates, α-nitrosyl Hb is more stable than β-nitrosyl Hb.7-9 In the T-state, a percentage of the α-nitrosyl proximal histidine bonds break forming pentacoordinate α-nitrosyl Hb (as opposed to the hexacoordinate form) that has a characteristic hyperfine splitting signature in electron paramagnetic resonance (EPR).8,10 The efficiency of transfer from heme to thiol was predicted to be β-nitrosyl > hexacoordinate α-nitrosyl > pentacoordinate α-nitrosyl.6 It has recently been suggested that inability of Hb to facilitate allosterically controlled intramolecular transfer contributes to the pathology of certain diseases. The mutation in sickle cell Hb was suggested to impede intramolecular transfer.11 In addition, sustained hypoxia purported to be associated with pulmonary hypertension was suggested to result in the build up of α-nitrosyl species with concomitant loss of β-nitrosyl Hb and SNO-Hb.6
Working together with other laboratories on oxygen cycling-induced changes in heme nitrosylation/S-nitrosation, we did not find evidence for allosterically controlled intramolecular transfer.12 Our observations refuting the idea of allosterically controlled intramolecular transfer were criticized based on there being (at least in some cases) unequal distribution of NO among Hb tetramers and prolonged incubations, which would tend to result in a buildup of α-nitrosyl species.3 The current study was designed to test whether allosterically controlled intramolecular transfer occurs when samples are prepared to maximize the amount of β-nitrosyl Hb and to equally distribute small amounts of NO among Hb tetramers.
Study design
Normal adult Hb from red blood cells was obtained as described previously.13,14 Hb samples were deoxygenated by gentle flow of an inert gas and stirring. All experiments were performed at room temperature. Determination of percentages of oxygenated, deoxygenated, and methemoglobin was performed using near infrared spectroscopy.13 SNO-Hb standards for validation studies were prepared as described previously.15 The CO/CuCl/cysteine (3C) assay was also used for validation studies as described.16
The NO donor ProliNO (Cayman Chemical, Ann Arbor, MI) was prepared in 0.01 M NaOH and a small aliquot was rapidly mixed with the deoxygenated Hb for a final concentration of NO of about 5 μM. The half-life of ProliNO is 2 seconds at 37°C, which allowed NO to be evenly distributed among Hb tetramers in our experiments. After 1 minute, aliquots of the sample were frozen for EPR analysis and prepared for measurements of SNO-Hb using SNO stabilization solution and the tri-iodide chemiluminescence method as described previously.12,17 EPR using a Bruker EMX 10/12 (Bruker, Billerica, MA) was performed as described previously.18 Samples were oxygenated by exposure to air and gentle stirring for 5 minutes after which they were assayed for oxygen saturation, Fe-NO Hb, and SNO-Hb. The amount of Fe-NO Hb and its distribution among hexa/pentacoordinate species and subunits was analyzed by fitting to basis spectra and performing a double integral as described previously.19
Results and discussion
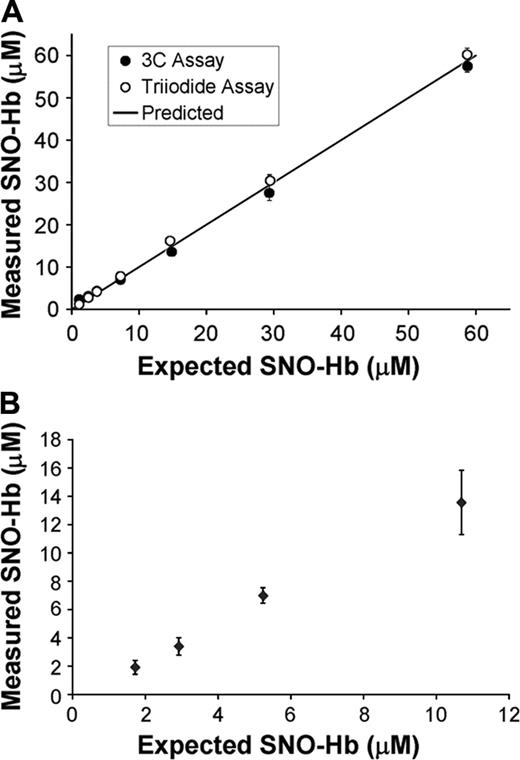
Although EPR is widely accepted as a sensitive and specific method to measure Fe-NO Hb, there has been some disagreement over the accuracy of the tri-iodide method in determining the concentration of SNO-Hb.20 Figure 1A shows that the tri-iodide method compares well with another recently developed assay.16 In Figure 1B, we show that the tri-iodide method is capable of accurately measuring SNO-Hb in the range of concentrations and NO/Hb ratios expected in our oxygenation studies as previously demonstrated.15
Validation of the tri-iodide method. (A) A SNO-Hb standard sample that was 125 μM in heme and 59 μM in nitrosated thiol was serially diluted 1:1 reaching a final concentration of 0.9 μM (SNO). For each concentration, the sample was split in 2 and assayed by the tri-iodide method (○) or the 3C method (•). The measured concentration of SNO is plotted against the expected amount based on the amount that the initial sample was diluted. The solid line shows the predicted amount. The initial concentration of SNO-Hb was taken as the average of that using the 3C and tri-iodide method (57 μM and 60 μM). (B) A SNO-Hb standard sample (57 μM in SNO and 1 mM in heme) was spiked into high concentrations of NO-free Hb with a heme concentration 5 mM to give expected final concentrations of SNO between 1 and 12 μM (SNO/heme between 0.00025 and 0.003). The amount of SNO-Hb in the spiked samples was measured using the tri-iodide method and plotted against the expected values.
Validation of the tri-iodide method. (A) A SNO-Hb standard sample that was 125 μM in heme and 59 μM in nitrosated thiol was serially diluted 1:1 reaching a final concentration of 0.9 μM (SNO). For each concentration, the sample was split in 2 and assayed by the tri-iodide method (○) or the 3C method (•). The measured concentration of SNO is plotted against the expected amount based on the amount that the initial sample was diluted. The solid line shows the predicted amount. The initial concentration of SNO-Hb was taken as the average of that using the 3C and tri-iodide method (57 μM and 60 μM). (B) A SNO-Hb standard sample (57 μM in SNO and 1 mM in heme) was spiked into high concentrations of NO-free Hb with a heme concentration 5 mM to give expected final concentrations of SNO between 1 and 12 μM (SNO/heme between 0.00025 and 0.003). The amount of SNO-Hb in the spiked samples was measured using the tri-iodide method and plotted against the expected values.
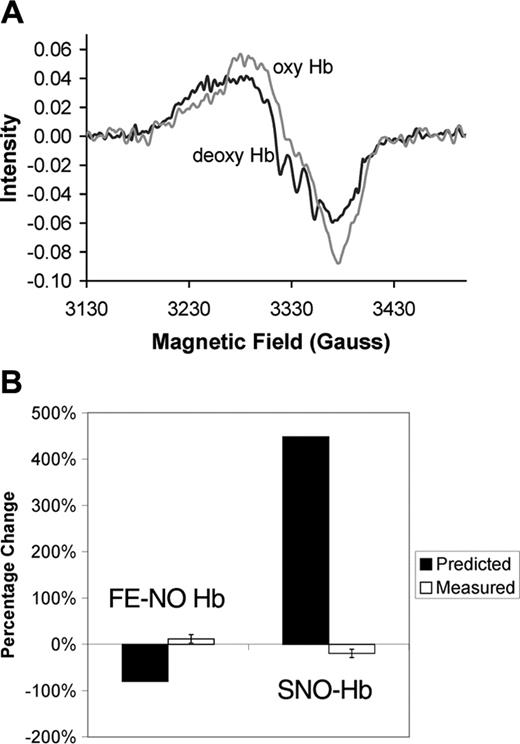
Lack of allosterically controlled intramolecular transfer. (A) Typical EPR spectra of Hb before and after oxygenation. The triplet hyperfine structure characteristic of pentacoordinate α-nitrosyl Hb present in the deoxygenated sample (deoxy Hb) disappears upon oxygenation (oxyHb). For this sample, the total Hb concentration was 4.5 mM and was 99.6% deoxygenated when ProliNO was added. Before oxygenation the total Fe-NO Hb was 3.8 μM, of which 53% was on the β subunits and 24% was of the pentacoordinate α-nitrosyl form. After oxygenation, the total Fe-NO Hb was measured to be 4.1 μM, of which 47% was on the β subunits and 0% was of the pentacoordinate α-nitrosyl form. (B) Measured changes in Fe-NO Hb and SNO-Hb versus those predicted by the SNO-Hb hypothesis. The predictions are shown assuming 80% transfer from heme to thiol. Thus, the prediction is that the amount of Fe-NO Hb would go from 3.7 μM to 0.74 μM upon oxygenation, giving a percentage change of 2.96/3.7 = 80%. The SNO-Hb is predicted to go from 0.66 μM to 3.62 μM (2.96 μM is transferred from the heme iron), giving a percentage change of 2.96/0.66 = 448%. These predictions are based on data presented by Gow and Stamler that measured the amount of SNO-Hb yield as a function of the NO/Hb ratio.2 Below a NO/Hb ratio of 1, as the NO/Hb ratio increased, the SNO-Hb yield decreased.2 At the lowest NO/Hb ratio tested of 0.01, the reported yield was 80%.2 Our samples had an NO/Hb ratio below 0.001. Error bars shown in measured values correspond to 1 standard deviation.
Lack of allosterically controlled intramolecular transfer. (A) Typical EPR spectra of Hb before and after oxygenation. The triplet hyperfine structure characteristic of pentacoordinate α-nitrosyl Hb present in the deoxygenated sample (deoxy Hb) disappears upon oxygenation (oxyHb). For this sample, the total Hb concentration was 4.5 mM and was 99.6% deoxygenated when ProliNO was added. Before oxygenation the total Fe-NO Hb was 3.8 μM, of which 53% was on the β subunits and 24% was of the pentacoordinate α-nitrosyl form. After oxygenation, the total Fe-NO Hb was measured to be 4.1 μM, of which 47% was on the β subunits and 0% was of the pentacoordinate α-nitrosyl form. (B) Measured changes in Fe-NO Hb and SNO-Hb versus those predicted by the SNO-Hb hypothesis. The predictions are shown assuming 80% transfer from heme to thiol. Thus, the prediction is that the amount of Fe-NO Hb would go from 3.7 μM to 0.74 μM upon oxygenation, giving a percentage change of 2.96/3.7 = 80%. The SNO-Hb is predicted to go from 0.66 μM to 3.62 μM (2.96 μM is transferred from the heme iron), giving a percentage change of 2.96/0.66 = 448%. These predictions are based on data presented by Gow and Stamler that measured the amount of SNO-Hb yield as a function of the NO/Hb ratio.2 Below a NO/Hb ratio of 1, as the NO/Hb ratio increased, the SNO-Hb yield decreased.2 At the lowest NO/Hb ratio tested of 0.01, the reported yield was 80%.2 Our samples had an NO/Hb ratio below 0.001. Error bars shown in measured values correspond to 1 standard deviation.
Figure 2A shows typical EPR spectra from a partially nitrosylated sample before and after oxygenation. The deoxygenated sample (deoxy Hb) presents a triplet hyperfine structure characteristic of pentacoordinate α-nitrosyl Hb. This hyperfine structure disappears upon oxygenation as expected due to a change in the quaternary state of the Hb (T to R). The SNO-Hb hypothesis predicts that, upon the oxygen-induced quaternary change, the total amount of nitrosyl Hb should be substantially reduced. This prediction is not observed.
Table 1 summarizes the characteristics of the partially nitrosylated Hb samples prepared under deoxygenated conditions followed by oxygenation. Because the total amount of NO released is so small (NO/Hb = 0.00085) and the NO donor is mixed before it releases NO, there should be virtually no Hb tetramers with more than one NO bound. As expected by the equal association rate of NO with hemes α and β subunits,21 the initial sample contains 50% nitrosylation on the β subunits—the most one can expect for a deoxygenated sample. A small amount of SNO-Hb was detected by chemiluminescence in the initial samples, perhaps due to a small amount of nitrite contamination.22 Upon oxygenation, contrary to the prediction of the SNO-Hb hypothesis, the concentration of Fe-NO Hb did not decrease. The slight increase observed may have been due to the reaction of deoxygenated Hb with the small amount of nitrite present (the concentration of nitrite was 2.0 ± 1.1 μM before oxygenation and 1.3 ± 0.6 μM afterward).22 Effective conversion from the T to R quaternary states is expected for the percentages of oxygenation and deoxygenation and low amounts of methemoglobin (MetHb) shown in Table 1. This allosteric transition is confirmed by the disappearance of the pentacoordinate α-nitrosyl species upon oxygenation, which is destabilized in the R-state. Overall, the data shown in Figure 2 and Table 1 show that we did not observe the loss of Fe-NO Hb and gain in SNO-Hb predicted by the SNO-Hb hypothesis.
Hemoglobin properties before and after oxygenation
Property . | Deoxygenated . | Oxygenated . |
|---|---|---|
| Fe-NO Hb, μM | 3.7 ± 0.2 | 4.3 ± 0.5 |
| SNO-Hb, μM | 0.66 ± 0.08 | 0.56 ± 0.10 |
| HbO2/total Hb, % | 0.1 ± 0.2 | 97.2 ± 1.6 |
| DeoxyHb/total Hb, % | 99.4 ± 0.2 | 2.8 ± 1.6 |
| MetHb/total Hb, % | 0.6 ± 0.2 | 0.0 ± 0.0 |
| β-Nitrosyl Hb*, % | 50 ± 3 | 45 ± 6 |
| Pentacoordinate α-nitrosyl Hb*, % | 24 ± 3 | 0.3 ± 0.4 |
Property . | Deoxygenated . | Oxygenated . |
|---|---|---|
| Fe-NO Hb, μM | 3.7 ± 0.2 | 4.3 ± 0.5 |
| SNO-Hb, μM | 0.66 ± 0.08 | 0.56 ± 0.10 |
| HbO2/total Hb, % | 0.1 ± 0.2 | 97.2 ± 1.6 |
| DeoxyHb/total Hb, % | 99.4 ± 0.2 | 2.8 ± 1.6 |
| MetHb/total Hb, % | 0.6 ± 0.2 | 0.0 ± 0.0 |
| β-Nitrosyl Hb*, % | 50 ± 3 | 45 ± 6 |
| Pentacoordinate α-nitrosyl Hb*, % | 24 ± 3 | 0.3 ± 0.4 |
Total Hb = 5.2 ± 0.1 mM. Total NO/Hb = 0.00085 ± 0.00005. Data shown as average of 7 trials from 2 samples deoxygenated on different days ± SD. Percentage of hexacoordinate α-nitrosyl Hb is equal to that of 100% minus the sum of the β-nitrosyl Hb and pentacoordinate α-nitrosyl Hb.
Compared to total nitrosyl Hb.
Figure 2B presents a summary of the measured percentage changes in Fe-NO Hb and SNO-Hb upon oxygenation. In addition, the predicted percentage changes based on an 80% transfer efficiency2 are also shown. It has been almost 10 years since the SNO-Hb hypothesis was originally proposed,1 yet no conclusive evidence using the widely accepted technique of EPR has been put forth to support the notion of an allosterically controlled intramolecular transfer mechanism. Results have been presented using EPR to support the intramolecular transfer but most of these have included nitrite in the Hb preparations3,4 and we have previously suggested that the added nitrite was potentially responsible for some of the phenomena attributed to intramolecular transfer.12 One set of EPR data has been presented in the absence of nitrite, yet the growth in Fe-NO Hb presented was said to be due to slow loss of SNO-Hb on aging without indication to its relation to allosteric control.3 Other evidence for intramolecular transfer has relied on detection using photolysis chemiluminescence, but this technique reports levels of Fe-NO Hb in human blood that are at least 10 times that measured by EPR.4,6,23 We conclude that allosterically controlled intramolecular transfer from heme to thiol does not occur under normal conditions and therefore a defect in this processing of NO by Hb does not contribute to pathologic states.
Prepublished online as Blood First Edition Paper, December 8, 2005; DOI 10.1182/blood-2005-10-4104.
Supported by National Institutes of Health grant HL58091 (D.B.K.-S.). Further support is acknowledged through Career Award K02 HL078706 (D.B.K.-S.).
I.A. and K.T.H. worked out details of the experiments and performed some of them; S.B. and J.H. performed some of the experiments; and D.B.K.-S. designed the experiments and wrote the paper.
K.T.H. and I.A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Neil Hogg for critical reading of a draft of this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal