Dendritic cells (DCs) are famously known for their role as initiators of adaptive immune responses. However, DCs can also exert tolerogenic functions, the basis of which is much less well understood. In this issue of Blood, Orabona and colleagues reveal that a balance between KARAP/DAP12 and IRF-8/ICSBP signals governs the indoleamine 2,3-dioxygenase–dependent tolerogenic DC potential in humans and in mice.
Dendritic cells (DCs) represent a heterogeneous population of cells comprising CD11chigh conventional DCs (cDCs) and CD11cdim plasmacytoid DCs.1 Besides their widely recognized role as critical initiators of antigen-specific T-cell responses, DCs can also exert some tolerogenic function. Specifically, the CD8α+ subset of cDCs is endowed with tolerogenic ability under steady-state conditions, which can be further increased upon binding of interferon-γ (IFN-γ) or CTLA-4.2 Although they seem naturally prone to immunogenicity, CD8α– cDCs can also be driven to tolerogenic activity upon CTLA-4 and IFN-γ binding.2 Indoleamine 2,3-dioxygenase (IDO), an enzyme that catabolizes tryptophan (the rarest of all amino acids), is involved in DC tolerogenic function both by tryptophan deprivation as well as by increasing the concentration of downstream toxic metabolites, such as kynurenines.2
The first part of the present study was designed to identify critical genes involved in the induction of an IDO-dependent tolerogenic program in mouse cDCs. This was achieved by correlating variations in gene expression with acquisition of IDO competence, upon comparison of the transcriptomes of mouse DCs subjected to various treatments. Induction of tolerogenic activity in CD8α+ or CD8α– cDCs was systematically associated with down-regulation of the KARAP/DAP12 (also known as Tyrobp) molecule. Moreover, artificial inhibition of KARAP/DAP12 expression by means of siRNA transfection led to an up-regulation of IDO-dependent DC tolerogenic properties, through posttranslational activation of the enzyme. These results confirm the importance and prove the physiologic relevance of previous work by the authors showing that the tolerogenic potential of CD8α+ DCs strictly correlates with IDO activity and the level of KARAP/DAP12 expression in loss-of-function or transgenic KARAP/DAP12 mice.3 FIG1
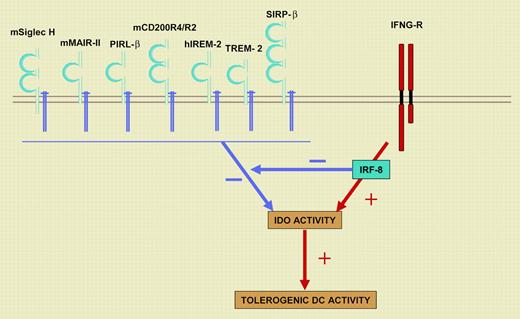
A balance between KARAP/DAP12- and IRF-8/ICSBP–dependent signals governs the tolerogenic DC potential. Cell-surface receptors that noncovalently associate with KARAP/DAP12 on DCs are indicated. h indicates human; m, mouse; Siglec H, Sialic acid–binding Ig-like lectin H; MAIR-II, myeloid-associated immunoglobulin-like receptor II; PIRL-β, paired immunoglobulin-like type 2 receptor β; IREM-2, immune receptor expressed on myeloid cells 2; TREM-2, triggering receptor expressed by myeloid cells 2; and SIRP-β, signal regulatory protein-β. Signals through KARAP/DAP12 antagonize IDO function and DC tolerogenic potential, whereas IFN-γ, through IRF-8/IBCSP, acts in an opposite manner and down-regulated KARAP/DAP12 transcription. IFNG-R indicates interferon-γ receptor.
A balance between KARAP/DAP12- and IRF-8/ICSBP–dependent signals governs the tolerogenic DC potential. Cell-surface receptors that noncovalently associate with KARAP/DAP12 on DCs are indicated. h indicates human; m, mouse; Siglec H, Sialic acid–binding Ig-like lectin H; MAIR-II, myeloid-associated immunoglobulin-like receptor II; PIRL-β, paired immunoglobulin-like type 2 receptor β; IREM-2, immune receptor expressed on myeloid cells 2; TREM-2, triggering receptor expressed by myeloid cells 2; and SIRP-β, signal regulatory protein-β. Signals through KARAP/DAP12 antagonize IDO function and DC tolerogenic potential, whereas IFN-γ, through IRF-8/IBCSP, acts in an opposite manner and down-regulated KARAP/DAP12 transcription. IFNG-R indicates interferon-γ receptor.
The authors further show that IRF-8/ICSBP acts as a repressor of the KARAP/DAP12 gene but promotes transcription of the INDO gene, encoding IDO. IRF-8/ICSBP thus favors DC tolerogenic activity by inducing expression of IDO and by releasing its function from the negative control exerted by KARAP/DAP12. These results thus suggest a model in which a dynamic balance between KARAP/DAP12- and IRF-8/ICSBP–dependent signals antagonize or promote DC tolerogenic function by acting on IDO expression and/or function (Figure 1). Of importance, Orabona et al further argue that the KARAP/DAP12 and IRF-8/ICSBP balance is conserved through evolution, as they obtained similar results using monocyte-derived humans DCs.
KARAP/DAP12 is a transmembrane signaling polypeptide, initially described on human natural killer (NK) cells and since shown to associate with more than 25 surface receptors on myeloid and lymphoid cells.4 KARAP/DAP12 bears an intracytoplasmic immunoreceptor tyrosine–based activation motif (ITAM) that couples the engagement of the associated cell-surface receptor to protein tyrosine kinase–dependent pathways, involving Src and Syk family members. IRF-8/ICSBP is a transcription factor that controls CD8α and plasmacytoid DC development. IRF-8/ICSBP is a member of the IFN regulatory factors (IRFs) that is induced and activated downstream of Jak family protein tyrosine kinases, upon engagement of IFN-γ receptor. The description of this novel balance between 2 distinct protein tyrosine-kinase pathways may open new avenues for the pharmacologic modulation of DC immunogenic versus tolerogenic potential. Yet, a number of issues remain to be addressed, in particular at the level of the control of IDO activity. Whereas IFN-γ–dependent pathways are transcriptional activators of the INDO gene, the role of IDO posttranscriptional modifications, and the role of KARAP/DAP12 in those, has to be clarified. In addition, the identification of the ligand-receptor pairs that are coupled to KARAP/DAP12-dependent signals in DCs and turn down DC tolerogenic activity is an attractive new path for the dissection of mechanisms regulating tolerance versus autoimmunity. ▪
Comment on Orabona et al, page 2846

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal