Bennett and colleagues report that approximately one third of children and adolescents with severe or refractory chronic immune thrombocytopenic purpura (ITP) showed a sustained response to treatment with the monoclonal anti-C20 antibody, rituximab. This prospective study is notable for several reasons and it also serves to highlight several unanswered questions as to the drug's mechanism of action and place in ITP management.
The benefit of treating children with ITP remains contentious, and the severity of bleeding in the enrolled subjects just prior to enrollment is unstated. Nevertheless, the pretreatment platelet count (mean of 9-10 × 109/L), duration of disease (4 years on average), number of prior treatments (mean of 4), severity of bleeding after therapy (grades 3-4 in 60% of nonresponders), and inclusion of patients with Evans syndrome (which has a worse prognosis) document the severity of the disease in this cohort and the real benefit of ameliorating thrombocytopenia. These demographics help to explain the lower response rate than reported previously, but they also clearly establish that rituximab induces sustained meaningful responses in a subset of severely affected young patients.FIG1
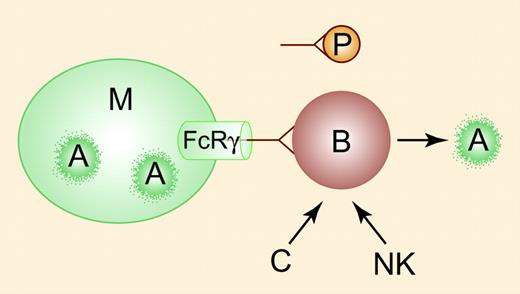
Rituximab (anti-CD20) induces B-cell apoptosis (A, right). The rapid response seen in some patients with ITP may reflect competition between clearance of antibody-coated B cells and antibody-coated platelets (P) by Fcγ receptors (cylinder) expressed on macrophages (M). Phagocytosis of anti-CD20 – coated B cells (A, left) may augment apoptosis mediated by complement (C) and natural killer (NK) cells (right). Illustration by Paulette Dennis.
Rituximab (anti-CD20) induces B-cell apoptosis (A, right). The rapid response seen in some patients with ITP may reflect competition between clearance of antibody-coated B cells and antibody-coated platelets (P) by Fcγ receptors (cylinder) expressed on macrophages (M). Phagocytosis of anti-CD20 – coated B cells (A, left) may augment apoptosis mediated by complement (C) and natural killer (NK) cells (right). Illustration by Paulette Dennis.
That responses occur at a median of one week seems difficult to reconcile with B-cell apoptosis, which is presumed to mediate delayed and durable response.1 The possibility that the initial effect may be through competition between the clearance of antibody-coated peripheral B cells2 (which are considerably reduced in contrast to relatively stable total IgG levels) and platelets, analogous to anti-D, has not been established, nor is it obvious why early responses are generally sustained unless the 2 processes are intertwined (see left side of figure).
Hematologists' use of rituximab differs considerably, ranging from use always before splenectomy to exactly the opposite. This is based on its cost and variable reimbursement, uncertain long-term effects, but largely on the wish of increasing numbers of patients to avoid splenectomy. However, it may be premature to conclude that rituximab might be preferable to splenectomy in younger patients. The high rate of first infusion-related complications such as serum sickness teaches that treatment should be confined to those truly in need of therapy. Even the longest follow-up of rituximab-treated patients is far less than for splenectomy, so the durability of response and incidence of delayed immunologic complications remain to be defined. One is still left wondering if and, if so, how a nonselective inhibitor of B-cell costimulatory help places a “hole” so selectively in the B-cell antibody repertoire and whether a similar effect on other unusually sensitive or low-prevalence B-cell populations may impair the development of a normal immune repertoire, especially in younger patients.
Last, the authors describe the pharmacokinetics of rituximab in children. This not only serves to highlight that children are not simply small adults but also reminds us that we have adopted a lymphoma-style regimen to treat an autoimmune disease because no phase 2 trials have been performed in ITP. Neither the optimal dose, frequency of administration, nor duration of treatment has been thoroughly delineated.3 Studies that better define the effect on rituximab on immunoglobulin gene repertoire4 and expression of platelet antibody-producing B cells may alleviate this glaring gap in our knowledge, alter treatment in ways that increase response rates and lessen concerns about persistent immune paresis,5 and identify patients whose disease is more amenable to this modality.4 ▪
Comment on Bennett et al, page 2639

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal