Abstract
Using the European Group for Blood and Marrow Transplantation (EBMT) registry, we retrospectively studied 19 patients with AL (amyloid light-chain) amyloidosis who underwent allogeneic (allo; n = 15) or syngeneic (syn; n = 4) hematopoietic stem cell transplantation (SCT) between 1991 and 2003. For allo-SCT, full-intensity conditioning was used in 7 patients and reduced-intensity conditioning (RIC) in 8 patients. Engraftment was durable in 12 of those 15 patients. The median follow-up time is 19 months. Kaplan-Meier probabilities of overall and progression-free survival were 60% and 53% at 1 year, respectively. Overall, 40% of patients died of transplant-related mortality (TRM). Best hematologic response after SCT was complete remission (CR) and partial remission (PR) in 8 and 2 patients, respectively, leading to an organ response in 8 of these patients. Seven of the 10 patients in remission are long-term survivors. In 5 of 7 evaluable patients in CR, chronic graft-versus-host disease (GvHD) was observed, indicating the contribution of immune effects to disease control. The main clinical problem was cardiac failure in patients with poor performance status due to amyloidosis or in combination with severe infections. These data suggest that allo-SCT might be a promising and potentially curative treatment modality for selected patients with AL amyloidosis. (Blood. 2005;107:2578-2584)
Introduction
Systemic AL (amyloid light-chain) amyloidosis is a protein conformation disorder caused by a clonal plasma cell dyscrasia. Symptoms result from fibrillar extracellular deposits in kidney, heart, liver, gut, peripheral nervous system, and other tissues. The deposits disrupt organ function and ultimately lead to death. The prognosis of systemic AL amyloidosis is poor; probably less than 5% of all patients survive 10 years or longer.1 Because it is a rare disease and basically related to multiple myeloma (MM), most of the treatment approaches have been adopted from the experiences in MM. Using conventional chemotherapy with melphalan/prednisone, the median survival was 18 months.2 Treatment with high-dose melphalan (HDM) and autologous stem cell transplantation (auto-SCT) can stabilize and even reverse the disease course. In 40% to 50% of patients, complete hematologic remission can be achieved,3,4 which leads to improvement of organ function in two thirds of these patients. A case control study showed the superiority of HDM compared with alkylator-based conventional chemotherapy regimens.5 However, HDM causes a high transplant-related mortality (TRM) up to 43%.6-8 Major progress to decrease mortality of high-dose chemotherapy has been achieved by definition of risk groups to identify patients who will not benefit from HDM.3,9 The treatment of patients with unresponsive disease or relapse after auto-SCT has only been evaluated in a few prospective studies so far. Thalidomide is an effective second-line therapy for MM10 and has been tested as a single agent in patients with AL amyloidosis in a phase 2 trial.11 Complete remission (CR) was not reached in any patient and 50% of patients experienced grade 3/4 toxicity. The combinations of dexamethasone-alpha interferon12 or thalidomide/dexamethasone were also used and found to be effective but rather toxic in newly diagnosed patients.13 The feasibility of a second auto-SCT after relapse as well as tandem auto-SCT has also been tested.14,15 A few anecdotal reports on successful allogeneic SCT (allo-SCT) have raised the possibility that allo-SCT could be a curative option for patients with AL amyloidosis.16-19 Here, we review retrospectively 19 patients with AL amyloidosis reported to the European Group for Blood and Marrow Transplantation (EBMT) registry who had undergone allo-SCT or syngeneic SCT (syn-SCT).
Patients, materials, and methods
This study was conducted on behalf of the Chronic Leukemia Working Party of the EBMT. All EBMT centers report a minimal essential data set (MED-A forms) into a central database. After identification of eligible patients, centers were contacted to get information about patients' history, amyloidosis manifestations, transplant course, detailed parameters of organ response, as well as hematologic remission and follow-up. Missing information and data inconsistency were clarified by individual requests. Data were stored at the Leiden EBMT Data Centre for analysis. Informed consent was obtained locally according to the regulations applicable at the time of transplantation. Approval for this study was obtained from the Institutional Review Board of the University of Heidelberg.
Definitions and diagnostic criteria
All patients have been evaluated for AL amyloidosis or MM by standard investigations. MM stage I was distinguished from AL amyloidosis if the bone marrow infiltration by plasma cells was at least 30% or monoclonal light-chain excretion in urine was greater than 1 g/d.20,21 The diagnosis of AL amyloidosis was done by positive Congo red staining of tissue biopsies. All patients had a monoclonal gammopathy in serum and/or urine. In 16 of 19 patients, immunohistochemistry was additionally performed to confirm the AL type of amyloidosis.22 One further patient showed a typical clinical symptom of AL amyloidosis (macroglossia).23 No patient had polymerase chain reaction (PCR) screening to exclude hereditary amyloidosis.24 Overall, in 2 patients (unique patient numbers [UPNs] 21405051 and 38906001) who received transplants in 1997 and 1998, diagnosis of AL amyloidosis was based on the presence of a plasma cell dyscrasia and positive Congo red staining. Performance status (PS) was assessed according to World Health Organization criteria (good: PS < 2; poor: PS ≥ 2). In our study, the definition of patients with high risk for TRM were age older than 50 years at SCT using matched unrelated donors or older than 55 years using matched related donors, poor PS, more than 2 organs involved, New York Heart Association classification stage more than II, and previous auto-SCT (excluding patients with a planned auto-allo approach). The definition of reduced-intensity conditioning (RIC) was determined by contributing centers and based on the current EBMT guidance. Toxicity was analyzed with National Cancer Institute Common Toxicity Criteria (CTC) version 2.0.
Hematologic response, relapse, and disease progression were defined according to published MM criteria25 in all patients surviving to at least day +100, and organ response was evaluated as early as 3 months after allo-SCT as published.22 Overall survival (OS) was measured in months and defined as the time from the date of transplantation to the date of death or last follow-up. Progression-free survival (PFS) was defined as the time from transplantation until date of progression or death from any cause or last follow-up. TRM was defined as death due to any cause other than disease progression or relapse occurring at any time after transplantation.
Graft-versus-host disease grading and therapy
The local investigators used standard criteria to grade acute and chronic graft-versus-host disease (GvHD).26 Treatment of acute and chronic GvHD was per each institution's standard practice guidelines.
Chimerism analysis
Chimerism analysis was performed per each institution's standard practice guidelines.
Statistical analysis
OS and PFS were estimated by the Kaplan-Meier method, and TRM and disease-related mortality were summarized using cumulative incidence estimates. Data were analyzed as of January 25, 2005. The package CMPRSK (by R. Gray; version 2.1-2, 2002; run on R, version 1.6.2) was used for the computation of cumulative incidence curves.27
Results
Patient characteristics
Between 1987 and 2003, 20 patients with AL amyloidosis who underwent allo- or syn-SCT have been reported to the EBMT. Treatment centers are listed in the “Acknowledgments.” One patient who received a transplant in 1987 had to be excluded from further analysis because of an incomplete data set. Four of the cases had been published as case reports and were updated for this analysis (UPN 23923221,28 UPN 23491761,16 UPN 7179454,17 UPN 609276218 ). Pretransplantation patient characteristics are listed in Table 1. Of 19 patients with AL amyloidosis (median age 47 years; range, 30-63 years), 13 patients had AL amyloidosis with monoclonal gammopathy and 6 patients had MM with symptomatic AL amyloidosis. All 4 MM patients classified as stage I had as single diagnostic criterion a monoclonal light-chain excretion in the urine greater than 1 g/d. Chromosomal analysis has been performed in 8 patients: 5 patients had a normal karyotype, 2 patients had deletion of chromosome 13 (UPN 16092762 and 1600231), and 1 patient showed deletion of chromosome 21 (UPN 23925391). Four patients underwent syn-SCT. Fifteen patients received an allo-SCT and are described in detail in this paper.
Patient characteristics prior to allo- or syn-SCT
UPN allogeneic . | Diagnosis . | Age at dx, y . | Type of paraprotein . | Dominant organ affected* . | Other organs involved* . | No. involved organs* . | Conventional chemo . | Auto-SCT/no. auto-SCTs . | PS* . | Time from dx to SCT, mo . | Graft/donor . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-RIC | |||||||||||
| 26256341 | AL | 33 | Kappa | Kidney | Liver | 3 | VMCP | No | Good | 9 | BM/MRD |
| 23491761 | AL | 55 | IgG lambda | Kidney | None | 1 | No | No | Good | 3 | BM/MRD |
| 24006981 | AL | 30 | IgG lambda | Kidney | Liver, PNP | 2 | No | No | Good | 63 | PB/MRD |
| 16092762 | AL | 42 | Kappa | None† | Gut | 2 | No | Yes/1 | Good | 44 | BM/MUD |
| 7179454 | AL/MM IIA | 44 | IgG kappa | Macroglossia | Skin, gut, kidney, spleen | 2 | C-VAMP | No | Good | 9 | BM/MRD |
| 23924891 | AL/MM IA | 46 | Lambda | Kidney | None | 1 | VAD, IDM | No | Good | 19 | BM/MRD |
| 23925391 | AL/MM IA | 48 | IgG + IgD lambda | Kidney | None | 1 | VAD, IDM | No | Good | 9 | BM/MRD |
| RIC | |||||||||||
| 38906001 | AL | 42 | IgG lambda | Kidney | Heart | 2 | No | No | Poor | 6 | PB/MRD |
| 25596342 | AL | 43 | IgG lambda | Liver | Kidney, heart | 3 | MP, dexa | Yes/1 | Good | 30 | PB/MRD |
| 1600237 | AL | 46 | IgG lambda | Kidney | Heart, gut | 3 | No | Yes/1 | Poor | 13 | PB/MRD |
| 81100832 | AL | 53 | IgG lambda | Kidney | Heart | 2 | No | Yes/1 | Poor | 9 | PB/MRD |
| 23908912 | AL | 54 | Kappa | Muscle | None | 1 | VAD | Yes/1 | Good | 29 | PB/mmRD |
| 1600231 | AL/MMIIIB | 45 | Kappa | Muscle, macroglossia | Gut, kidney | 3 | Dexa/VAD | Yes/1 | Good | 10 | PB/MUD |
| 33998213 | AL/MM IA | 47 | IgG lambda | Kidney | None | 1 | VAD, MP, IFN, Thal | Yes/2 | Good | 122 | PB/MRD |
| 77247721 | AL/MM IA | 56 | Lambda | Heart | Soft tissue | 2 | No | No | Poor | 3 | PB/MRD |
| Syngeneic | |||||||||||
| 23923221 | AL | 32 | Lambda | Kidney | Heart, Liver, spleen, PNP | 5 | MP | No | Good | 11 | BM/syn |
| 61402181 | AL | 52 | Lambda | PNP | Joints, gut | 2 | MP | No | Good | 13 | PB/syn |
| 21405051 | AL | 62 | Lambda | PNP | Gut, heart | 3 | MP | No | Poor | 4 | PB/syn |
| 26203731 | AL | 29 | Lambda | Heart‡ | None | 1 | No | No | Poor | 2 | PB/syn |
UPN allogeneic . | Diagnosis . | Age at dx, y . | Type of paraprotein . | Dominant organ affected* . | Other organs involved* . | No. involved organs* . | Conventional chemo . | Auto-SCT/no. auto-SCTs . | PS* . | Time from dx to SCT, mo . | Graft/donor . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-RIC | |||||||||||
| 26256341 | AL | 33 | Kappa | Kidney | Liver | 3 | VMCP | No | Good | 9 | BM/MRD |
| 23491761 | AL | 55 | IgG lambda | Kidney | None | 1 | No | No | Good | 3 | BM/MRD |
| 24006981 | AL | 30 | IgG lambda | Kidney | Liver, PNP | 2 | No | No | Good | 63 | PB/MRD |
| 16092762 | AL | 42 | Kappa | None† | Gut | 2 | No | Yes/1 | Good | 44 | BM/MUD |
| 7179454 | AL/MM IIA | 44 | IgG kappa | Macroglossia | Skin, gut, kidney, spleen | 2 | C-VAMP | No | Good | 9 | BM/MRD |
| 23924891 | AL/MM IA | 46 | Lambda | Kidney | None | 1 | VAD, IDM | No | Good | 19 | BM/MRD |
| 23925391 | AL/MM IA | 48 | IgG + IgD lambda | Kidney | None | 1 | VAD, IDM | No | Good | 9 | BM/MRD |
| RIC | |||||||||||
| 38906001 | AL | 42 | IgG lambda | Kidney | Heart | 2 | No | No | Poor | 6 | PB/MRD |
| 25596342 | AL | 43 | IgG lambda | Liver | Kidney, heart | 3 | MP, dexa | Yes/1 | Good | 30 | PB/MRD |
| 1600237 | AL | 46 | IgG lambda | Kidney | Heart, gut | 3 | No | Yes/1 | Poor | 13 | PB/MRD |
| 81100832 | AL | 53 | IgG lambda | Kidney | Heart | 2 | No | Yes/1 | Poor | 9 | PB/MRD |
| 23908912 | AL | 54 | Kappa | Muscle | None | 1 | VAD | Yes/1 | Good | 29 | PB/mmRD |
| 1600231 | AL/MMIIIB | 45 | Kappa | Muscle, macroglossia | Gut, kidney | 3 | Dexa/VAD | Yes/1 | Good | 10 | PB/MUD |
| 33998213 | AL/MM IA | 47 | IgG lambda | Kidney | None | 1 | VAD, MP, IFN, Thal | Yes/2 | Good | 122 | PB/MRD |
| 77247721 | AL/MM IA | 56 | Lambda | Heart | Soft tissue | 2 | No | No | Poor | 3 | PB/MRD |
| Syngeneic | |||||||||||
| 23923221 | AL | 32 | Lambda | Kidney | Heart, Liver, spleen, PNP | 5 | MP | No | Good | 11 | BM/syn |
| 61402181 | AL | 52 | Lambda | PNP | Joints, gut | 2 | MP | No | Good | 13 | PB/syn |
| 21405051 | AL | 62 | Lambda | PNP | Gut, heart | 3 | MP | No | Poor | 4 | PB/syn |
| 26203731 | AL | 29 | Lambda | Heart‡ | None | 1 | No | No | Poor | 2 | PB/syn |
dx indicates diagnosis; Non-RIC, non-reduced-intensity conditioning; VMCP, vincristin/melphalan/cyclophosphamide/prednisone; BM, bone marrow; MRD, matched related donor; PNP, polyneuropathy; PB, peripheral blood; MUD, matched unrelated donor; C-VAMP, cyclophosphamide/vincristine/adriamycine/melphalan/prednisone; VAD, vincristine/adriamycin/dexamethasone; IDM, intermediate-dosed melphalan; MP, melphalan/prednisone; dexa, dexamethasone; mmRD, mismatched related donor; IFN, interferon alpha; and Thal, thalidomide.
At allo- or syn-SCT.
After cardiac transplantation.
After liver transplantation.
Indications for allo-SCT defined by the treating physicians were “young age” and relapsed or refractory disease. Dominant organ affection at SCT was mainly kidney. Five patients had amyloid cardiac disease. One patient had prior cardiac transplantation (UPN 16092762) and another patient was on dialysis at the time of SCT (UPN 1600237). The median number of organs involved was 2. The median duration from diagnosis to SCT was 9 months (range, 3-122 months). PS was graded as poor in 4 patients.
Pretreatment and conditioning regimens
Four patients were untreated. Eight patients had been treated with conventional chemotherapy and 3 patients with HDM and auto-SCT as front-line treatment, 2 of them followed directly by the allo-SCT (UPN 1600231; 81100832). Another patient had received HDM and auto-allo-SCT as salvage therapy (UPN 33998213). Altogether, 7 patients had been treated with HDM and auto-SCT. The median time interval between auto- and allo-SCT was 10 months (range, 2-30 months). Overall, only 4 patients were responsive to chemotherapy, but no patient reached CR before allo-SCT. The conditioning regimens are shown in Table 2.
Patient course and outcome after allo- or syn-SCT
UPN allogeneic . | Year of allo-SCT . | Disease status at SCT . | Conditioning . | Ex vivo T-cell depletion . | Engraftment . | aGvHD/grade . | cGvHD . | Best hematologic remission to SCT . | Best organ response to SCT (evaluable organs) . | Observation, d . | Current status (cause of death) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-RIC | |||||||||||
| 26256341 | 1995 | NR | 10 Gy TBI/Cy | No | NE | No | NE | NE | NE | 28 | TRM (GI hemorrhage) |
| 23491761 | 1995 | Untreated | 8 Gy TBI/Mel | No | Yes | Yes/2 | Lim | CR | RESP (kidney) | 1253 | TRM (pneumonia) |
| 24006981 | 1997 | Untreated | 10 Gy TBI/Cy/Mel | Yes | Yes, lost graft at 6 mo | No | NE | PR | RESP (kidney, liver, PNP) | 2371 | PR |
| 16092762 | 2002 | Relapse | Bu/Cy/ATG | No | Yes | No | No | CR * | NE | 1016 | CR * |
| 7179454 | 1994 | PR | 12 Gy TBI/Mel | No | Yes | Yes/1 | Lim | CR | RESP (tongue) | 3633 | CR |
| 23924891 | 1998 | PR | 12 Gy TBI/Cy | Yes | Yes | Yes/3 | NE | CR | RESP (kidney) | 106 | TRM (EBV lymphoma) |
| 23925391 | 1999 | NR | 12 Gy TBI/Cy | Yes | Yes | Yes/4 | NE | NE | NE | 54 | TRM (cerebral aspergillosis) |
| RIC | |||||||||||
| 38906001 | 1998 | Untreated | 2 Gy TBI | No | Yes, lost graft at 2 mo | No | NE | NE | NE | 51 | TRM (cardiac death) |
| 25596342 | 2002 | Relapse | Flu/Mel/campath | No | Yes | No | NE | NE | NE | 83 | Death from AL amyloidosis (liver failure) |
| 1600237 | 2003 | PR | 2 Gy TBI/Flu/ATG | No | Yes | Yes/2 | Lim | CR | SD (heart) | 595 | Relapse |
| 81100832 | 2003 | PR | Flu/Mel/ATG | No | Yes | Yes/2 | Ext | PR | RESP (kidney) | 166 | TRM (cardiac death) |
| 23908912 | 2003 | Relapse | 2 Gy TBI/Flu/ATG | No | Yes | Yes/2 | Ext | CR | RESP (muscle) | 560 | CR |
| 1600231 | 2002 | PR | 2 Gy TBI/Flu | No | Yes | No | No | CR | RESP (muscle, tongue, kidney) | 853 | CR |
| 33998213 | 2002 | Relapse | Cy/Flu/ATG | No | Yes | No | Lim | CR | RESP (kidney) | 931 | CR |
| 77247721 | 2002 | Untreated | Mel | Yes | Yes | No | No | NR | RESP (heart) | 573 | Death from AL amyloidosis (cardiac death) |
| Syngeneic | |||||||||||
| 23923221 | 1991 | NR | 12 Gy TBI/Cy | No | Yes | NA | NA | CR | RESP (kidney, PNP) | 4667 | CR |
| 61402181 | 1997 | NA | Mel | No | Yes | NA | NA | CR | RESP (gut, PNP, joints) | 2902 | CR |
| 21405051 | 1997 | NR | Mel | No | NE | NA | NA | NE | NE | 7 | TRM (cardiac arrest, sepsis) |
| 26203731 | 2000 | PROG | Mel | No | NE | NA | NA | NE | NE | 18 | TRM (cardiac arrest, sepsis) |
UPN allogeneic . | Year of allo-SCT . | Disease status at SCT . | Conditioning . | Ex vivo T-cell depletion . | Engraftment . | aGvHD/grade . | cGvHD . | Best hematologic remission to SCT . | Best organ response to SCT (evaluable organs) . | Observation, d . | Current status (cause of death) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-RIC | |||||||||||
| 26256341 | 1995 | NR | 10 Gy TBI/Cy | No | NE | No | NE | NE | NE | 28 | TRM (GI hemorrhage) |
| 23491761 | 1995 | Untreated | 8 Gy TBI/Mel | No | Yes | Yes/2 | Lim | CR | RESP (kidney) | 1253 | TRM (pneumonia) |
| 24006981 | 1997 | Untreated | 10 Gy TBI/Cy/Mel | Yes | Yes, lost graft at 6 mo | No | NE | PR | RESP (kidney, liver, PNP) | 2371 | PR |
| 16092762 | 2002 | Relapse | Bu/Cy/ATG | No | Yes | No | No | CR * | NE | 1016 | CR * |
| 7179454 | 1994 | PR | 12 Gy TBI/Mel | No | Yes | Yes/1 | Lim | CR | RESP (tongue) | 3633 | CR |
| 23924891 | 1998 | PR | 12 Gy TBI/Cy | Yes | Yes | Yes/3 | NE | CR | RESP (kidney) | 106 | TRM (EBV lymphoma) |
| 23925391 | 1999 | NR | 12 Gy TBI/Cy | Yes | Yes | Yes/4 | NE | NE | NE | 54 | TRM (cerebral aspergillosis) |
| RIC | |||||||||||
| 38906001 | 1998 | Untreated | 2 Gy TBI | No | Yes, lost graft at 2 mo | No | NE | NE | NE | 51 | TRM (cardiac death) |
| 25596342 | 2002 | Relapse | Flu/Mel/campath | No | Yes | No | NE | NE | NE | 83 | Death from AL amyloidosis (liver failure) |
| 1600237 | 2003 | PR | 2 Gy TBI/Flu/ATG | No | Yes | Yes/2 | Lim | CR | SD (heart) | 595 | Relapse |
| 81100832 | 2003 | PR | Flu/Mel/ATG | No | Yes | Yes/2 | Ext | PR | RESP (kidney) | 166 | TRM (cardiac death) |
| 23908912 | 2003 | Relapse | 2 Gy TBI/Flu/ATG | No | Yes | Yes/2 | Ext | CR | RESP (muscle) | 560 | CR |
| 1600231 | 2002 | PR | 2 Gy TBI/Flu | No | Yes | No | No | CR | RESP (muscle, tongue, kidney) | 853 | CR |
| 33998213 | 2002 | Relapse | Cy/Flu/ATG | No | Yes | No | Lim | CR | RESP (kidney) | 931 | CR |
| 77247721 | 2002 | Untreated | Mel | Yes | Yes | No | No | NR | RESP (heart) | 573 | Death from AL amyloidosis (cardiac death) |
| Syngeneic | |||||||||||
| 23923221 | 1991 | NR | 12 Gy TBI/Cy | No | Yes | NA | NA | CR | RESP (kidney, PNP) | 4667 | CR |
| 61402181 | 1997 | NA | Mel | No | Yes | NA | NA | CR | RESP (gut, PNP, joints) | 2902 | CR |
| 21405051 | 1997 | NR | Mel | No | NE | NA | NA | NE | NE | 7 | TRM (cardiac arrest, sepsis) |
| 26203731 | 2000 | PROG | Mel | No | NE | NA | NA | NE | NE | 18 | TRM (cardiac arrest, sepsis) |
Non-RIC indicates non-reduced-intensity conditioning; NR, no response; TBI, total body irradiation; Cy, cyclophosphamide; NE, not evaluable; TRM, transplant-related mortality; Mel, melphalan; Lim, limited; CR, complete response; RESP, response; PR, partial response; NA, not available; Bu, busulphan; ATG, antithymocyte globulin; EBV, Epstein-Barr virus; Flu, fludarabine; AL, amyloid light-chain; SD, stable disease; Ext, extensive; and PROG, progression.
Immunofixation negative with persistent abnormal free light-chain ratio in the serum.
Donors and graft composition
Donors of stem cells were HLA-identical siblings in 12 cases, a mismatched related sibling in 1 case, and matched unrelated voluntary donors in 2 cases. Six patients had received bone marrow and 9 patients peripheral stem cell grafts. In 4 patients, grafts had undergone ex vivo and in 6 patients in vivo T-cell depletion (TCD). The median CD34+ and CD3+ cell counts transplanted were 4.4 × 106 cells/kg (range, 3.3 × 106 to 12.4 × 106 cells/kg; 11 patients evaluable) and 1.9 × 108 cells/kg (range, 0.001 × 108 to 9.5 × 108 cells/kg; 9 patients evaluable), respectively.
Engraftment, chimerism, and acute toxicities
The posttransplantation patient characteristics are given in Table 2. Fourteen of 15 patients engrafted. One patient died before engraftment (day +28 without hematologic recovery). Chimerism data were available for 10 patients. Eight patients had 100% donor cells on day +28 or +100. One patient (UPN 38906001) who was treated with RIC rejected the graft and had autologous recovery. Another patient (UPN 24006981) had mixed chimerism on day +100, lost the graft 6 months after T-cell depleted allo-SCT, and showed autologous recovery as well.
The median number of days with absolute neutrophil counts (ANCs) less than 0.5 × 109/L (500/μL) was 15 days (range, 0-29 days) and with platelet counts less than 20 × 109/L (20 000/μL) was 7 days (range, 0-44 days). The median number of red blood cell transfusions was 6 (range, 0-34) and of platelet transfusions was 3 (range, 0-22). The patient who did not engraft until day +28 after a conventional conditioning and bone marrow SCT died from gastrointestinal hemorrhage. No further bleeding complications have been reported. For the different conditioning forms, the hematologic toxicity parameters were as follows: for conventional conditioning, ANCs less than 0.5 × 109/L (500/μL) for a median of 18 days (range, 17-21 days) and platelet counts less than 20 × 109/L (20 000/μL) for a median of 12 days (range, 0-23 days); for RIC, ANCs less than 0.5 × 109/L (500/μL) for a median of 11 days (range, 0-29 days) and platelet counts less than 20 × 109/L (20 000/μL) for a median of 1 day (range, 0-44 days).
We observed febrile infections in 8 patients and cytomegalovirus reactivation in 4 patients. Nonhematologic toxicity greater than grade 2 by CTC criteria was documented in 6 patients (worsening of renal function in 1 patient, renal failure leading to dialysis and cardiac death in 1 patient, cardiac arrhythmia in 1 patient, orthostatic hypotension in 1 patient, ascites in 1 patient, fatal gastrointestinal hemorrhage in 1 patient).
Graft-versus-host disease
Clinically relevant acute GvHD (grade II-IV) occurred in 6 of 14 patients at a median of 29 days (range, 15-65 days) after allo-SCT and was severe in 2 patients (grade III-IV). The patient with acute GvHD grade IV did not respond to steroids and died at day +54 due to cerebral aspergillosis. Overall, 6 of 10 evaluable patients developed chronic GvHD: 1 patient had de novo chronic GvHD, and 5 patients had preceding acute GvHD. Four patients required treatment. Three of 4 patients responded to treatment. One of these 6 patients died +41 months due to pneumonia acquired outside a hospital.
Hematologic remission and organ response
Four patients were not evaluable for hematologic remission and organ response due to early death. Best hematologic remission included CR in 8 patients, partial remission (PR) in 2 patients, and nonresponse in 1 patient. Free light-chain assay in the serum was additionally used in 6 patients and confirmed CR in 5 patients. One patient had an abnormal kappa-lambda ratio, although, becoming immunofixation-negative and developing organ progression (Table 2; UPN 16092762). However, we rated this patient as still in CR because EBMT criteria were used for this analysis.
The median time from allo-SCT to CR was 3 months (range, 2-15 months). In 5 of 7 evaluable patients in CR, chronic GvHD was observed. Four of 4 evaluable patients with kappa isotype achieved CR (including the 2 patients with chromosome 13 deletion) compared with 4 of 8 patients with lambda isotype. One patient had a relapse 11 months after allo-SCT and is currently being treated with thalidomide/dexamethasone. Donor lymphocyte infusions have not been performed in any patient.
Organ responses were observed in 9 patients and stable disease in 1 patient. One patient had a cardiac transplantation and no further evaluable organ (UPN 16092762). Evaluable organ involvement is shown in Table 2.
Overall survival and treatment- and disease-related mortality
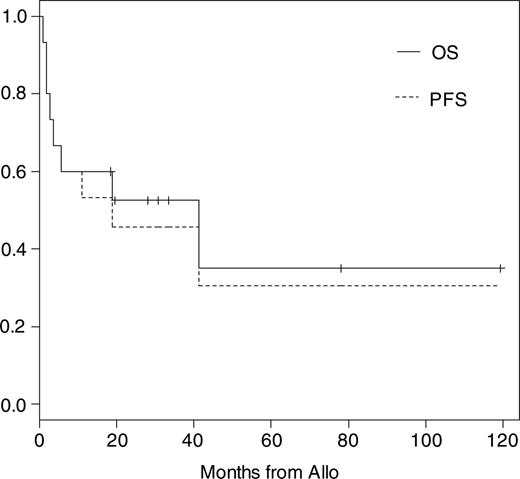
Eleven of 15 patients have to be classified as high-risk patients for TRM as described in “Definitions and diagnostic criteria.” The median follow-up time of all patients is 19 months (range, 1-121 months) and of surviving patients 31 months (range, 19-121 months). Kaplan-Meier probabilities of OS and PFS were 60% (95% confidence interval [CI], 40% to 91%) and 53% (95% CI, 33% to 86%) at 1 year and 52% (95% CI, 32% to 86%) and 46% (95% CI, 26% to 80%) at 2 years, respectively (Figure 1). The median OS and PFS are 42 and 19 months, respectively. The overall day +100 mortality was 27%. Three of 6 patients died before day +100 after conditioning including total body irradiation (TBI) with 8 to 12 Gy. Causes of TRM were acute GvHD grade IV and cerebral aspergillosis in 1 patient, gastrointestinal hemorrhage in 1 patient who did not engraft until day +28, and EBV-related lymphoma in the third patient, who received a transplant of an ex vivo T-cell-depleted graft. One patient died at 41 months of pneumonia associated with chronic GvHD. Another 2 patients died of cardiac failure at days +51 and +166 while suffering from kidney failure or extensive chronic GvHD. Both had cardiac involvement before allo-SCT, which probably contributed to their death. Including these patients the overall TRM was 40%. Overall disease-related mortality was 13% and estimated at 1 year at 6.7% (95% CI, 1% to 48%).
Kaplan-Meier estimates of overall and progression-free survival after allo-SCT.
Kaplan-Meier estimates of overall and progression-free survival after allo-SCT.
Clinical results of T-cell depletion
Four patients received an ex vivo T-cell-depleted transplant and engrafted. Two patients developed severe acute GvHD and died early (days +54 and +106). The latter patient additionally developed an EBV lymphoma. The third patient lost the graft 6 months after transplantation and is alive in PR. The fourth patient had no response and died from organ progression 19 months after SCT. None of the patients developed chronic GvHD.
In a further 6 patients, conditioning included in vivo TCD, which was used as part of RIC in 5 of these patients. Two patients died of organ progression or TRM, 1 patient is alive in relapse, and 3 patients are alive in CR about 3 years after allo-SCT.
Clinical results in 4 patients untreated before allo-SCT
Two of 4 patients untreated before allo-SCT lost their graft either after TCD or minimal conditioning with 2-Gy TBI. Despite losing the graft after 6 months, autologous reconstitution occurred in 1 patient who is in PR and is a long-term survivor. The other patient also had autologous recovery. She had a poor PS at SCT and died of TRM. The third patient received a T-cell-depleted SCT, had no response of his myeloma, and died of organ progression. The fourth patient was a long-term survivor in CR and died of an infection related to chronic GvHD.
Syngeneic transplants
Patient characteristics are given in Table 1. Three of the 4 patients had been unresponsive to melphalan/prednisone chemotherapy, 1 patient was untreated and had a previous liver transplantation. Two of the 4 patients had had a poor PS with advanced heart disease at the time of SCT and died early (days +7 and +18) of TRM (sepsis with cardiac arrest). As a noninfectious complication, nausea CTC grade III was observed in 1 patient. Two patients are long-term survivors (follow-up of +97 and +157 months), both are in CR and have organ response. Time from SCT to CR was 4 and 13 months, respectively.
Discussion
The treatment of AL amyloidosis aims at eradication of the clonal plasma cell disorder to avoid further amyloid formation and deposition. This is the first series describing allo-SCT in this disorder. Our data and a recently published case report19 suggest a potent “graft-versus-plasma-cell-dyscrasia effect” in AL amyloidosis patients.
We retrospectively evaluated the results of 15 well-documented patients who received allografts from either related or unrelated HLA-matched donors. As best hematologic remissions, 8 CR were induced by allo-SCT. After a median observation of 31 months of surviving patients, only 1 relapse occurred using the EBMT criteria. The free light-chain assay is able to detect persistent plasma cell disease with a high sensitivity29 and led to the identification of another patient with amyloidosis activity who had been considered in CR. Previously, a consensus opinion regarding remission criteria in AL amyloidosis had been published,22 including normalization of the free light-chain levels as a further CR criterion.
The importance of achieving CR for OS and organ response has already been shown in the autologous setting.3 Our data confirm that achievement of CR is a main predictor for long-term survival after allo-SCT as well. Currently, 7 patients are alive; 5 are in CR between 19 and 121 months following allo-SCT with organ response. CR was probably associated with the presence of chronic GvHD. As in other hematologic diseases,30,31 our analysis shows that patients not sensitive to chemotherapy are able to achieve CR after allo-SCT. We assume that this is mainly due to immunologic effects. Due to the small patient number and heterogeneous types of conditioning used in this study, it is difficult to draw firm conclusions about the optimal time point of allo-SCT.
The CR rate in our study is encouraging and warrants further investigation. However, TRM was high with 40% and, in our opinion, several factors were contributing. First, 11 of 15 patients in our study have to be classified as high-risk patients for TRM. Therefore, the mortality of 40% is comparable to allo-SCT in other high-risk patients with malignant or nonmalignant diseases.32-35 Performance status is an important predictor for TRM, and a poor PS in AL amyloidosis patients is mainly caused by cardiac involvement. Of 6 patients with poor PS at allo- or syn-SCT, 5 died of cardiac events. As a second factor, TBI with 8 to 12 Gy was associated with high day +100 mortality in our analysis. In MM and auto-SCT, TBI has mostly been deleted from conditioning regimens because of a significantly higher toxicity and lack of improvement of long-term results.36-38 In contrast, TBI-including regimens have not been proven to be inferior to chemotherapy-only regimens in allo-SCT.39 The importance of “full-intensity” or “reduced-intensity” conditioning remains a matter of debate. To reduce toxicity RIC could be a promising attempt in AL amyloidosis patients. Nonrelapse mortality has been reported to be significantly lower in RIC compared with conventional conditioning.40,41 The risk of severe acute GvHD is also reduced but rates of chronic GvHD have been observed to be similar.42,43 In our study, only 2 of 8 patients treated with RIC died of TRM compared with 4 of 7 treated with non-RIC. Of note, these 2 patients were in poor PS at SCT. Third, ex vivo TCD resulted in a worse clinical outcome. Comparable results have been observed in MM.44 In contrast, 7 of 11 patients without ex vivo TCD achieved a CR and 5 are long-term survivors.
In conclusion, we believe that allo-SCT is a promising therapeutic option for patients with AL amyloidosis. In this study we observed a potent graft-versus-plasma cell-dyscrasia effect. Full-intensity conditioning with TBI or ex vivo TCD were associated with an unfavorable outcome. Patients could be eligible for allo-SCT if they have not achieved a CR 6 months after HDM with auto-SCT, are still in a good PS, and have an HLA-matched donor. A prospective phase 2 study for allo-SCT using RIC is planned by the EBMT.
Appendix
Members of the CLWP: D. Niederwieser (chair), P. Corradini (secretary), J. Apperley, W. Arcese, S. Argiris, R. Arnold, J. Aschan, A. Bacigalupo, G. Bandini, N. Basara, A. Bekassy, M. Bernardi, B. Sirohi, B. Bjorkstrand, D. Blaise, C. Boque, J. Bourhis, R. Brand, L. Brinch, P. Browne, M. Brune, D. Bunjes, Buzyn, J. Cahn, C. Cordonnier, E. Carreras, M. Cavo, Y. Chalandon, R. Clark, G. Cook, M. Cook, J. Cornelissen, F. Cremer, T. De Witte, A. Devergie, A. M. Dickinson, P. Dreger, H. Einsele, E. Thiel, A. Fauser, W. Fibbe, T. Fischer, L. Fouillar, F. Frassoni, G. Gahrton, J. Garcia, E. Gluckman, H. Goker, J. Goldman, H. Goldschmidt, E. Goulmy, N. Graeme, P. Guardiola, C. Guglielmi, F. Guilhot, A. Hagman, J.L. Harous-seau, A. Gratwhol, J. Hernandez, B. Hertenstein, E. Holler, J. Hows, S. Iacobellli, A. U. Ispizua, P. Jacobs, J. Gunnar, P. Kalhs, R. Knobler, G. Kobbe, H. K. Kolb, K. Kolbe., E. Koller, N. Kroger, M. Lalancette, G. Lambertenghi, M. Lawton, S. Lenhoff, P. Ljungman, F. Locatelli, H. Lokhorst, S. Mackinnon, G. Martinnelli, S. Mccann, M. Michallet, D. Milligan, W., C. Morris, D. Nachbaur, A. Nagler, D. Niederwieser, E. Olavarria, F. Onida, K. Paloczi, Pautas., R. Peceny, M. Petrini, M. Plawler, K. Porkka, Posthuma, A. Rahemtulla, A. Rambaldi, S. Richards, O. Ringden, E. Roosnek, J. F. Rossi, C. Ruiz, T. Ruutu, J. San Miguel, A. Schattenberg, N. Schimtz, M. Schleu, C. Schmid, S. Slavin, D. Stelljes, J. Vanrood, L. Verdonck, W. Wiktor-Jedrzejczak, W. Nuernberger, and R. Zander.
Prepublished online as Blood First Edition Paper, November 17, 2005; DOI 10.1182/blood-2005-06-2462.
A complete list of the Chronic Leukemia Working Party's membership is provided in the “Appendix.”
S.O.S. designed and performed the study and wrote the manuscript; G.G. codesigned the study and assisted in writing the manuscript; U.H. and H.G. assisted in performing the study and writing the manuscript; D.N. codesigned the study; S.I. performed statistical analysis; H.L. (4 patients included), A.B. (3 patients included), and V.L. (2 patients included) analyzed and provided data of patients; G.B., A.C., E.C., A.F., L.G., P.J., N.K., G.L., N.R., and P.Z. (1 patient each) analyzed and provided data of patients.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The following centers contributed patients to this study. Belgium: Cliniques Universitaires St Luc Brussels, AZ Stuivenberg, Antwerpen. France: Hopital Necker, Paris; Pitie Salpetriere, Paris; Hopital Henri Mondor, Creteil. Germany: Dept of Hematology, University of Leipzig; University Hospital Eppendorf; BMT Centre, Hamburg. Italy: Institute of Hematology, Bologna University; Centro Trapianti di Modollo Osseo, Cagliari. Netherlands: University Medical Centre Utrecht. South Africa: Department of Haematology and BMT Unit, Cape Town. Spain: Institute of Hematology Barcelona. United Kingdom: Oxford Radcliffe Hospital; Nottingham City Hospital.
We thank Anja van Biezen and Miriam van Gestel from the EBMT data registry in Leiden, The Netherlands, for their assistance with data collection.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal