Abstract
Cutaneous T-cell lymphomas (CTCLs) are malignancies of T cells that have a special affinity for the skin. We have previously reported that much of the T-cell receptor repertoire is altered in CTCL, and both malignant and nonmalignant clones are numerically expanded, presumably in response to T-cell trophic cytokines. We therefore examined levels of the T-cell trophic cytokines IL-2, IL-4, IL-7, IL-12, IL-13, and IL-15 in plasma in 93 CTCL patients and healthy controls. Only IL-7 levels were elevated in CTCL. We next looked at lesional skin from patients with CTCL and found elevated levels of IL-7 mRNA. Explant cultures of normal and lesional CTCL skin biopsies revealed significantly more IL-7 protein production in CTCL skin. Additionally, cultures of CTCL skin released greater numbers of T cells than normal skin; this was blocked by the addition of an IL-7 neutralizing antibody. Finally, these cultures induced proliferation of normal peripheral skin-homing T cells that were added to the cultures. These observations led us to postulate that IL-7 produced by skin cells contributes to the survival and proliferation of T cells within skin lesions and is likely the source of elevated circulating IL-7 in CTCL. (Blood. 2006;107:2440-2445)
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a heterogeneous group of lymphoproliferative disorders of the skin1 and are regarded as a subset of extranodal non-Hodgkin T-cell lymphomas of skin-homing memory T cells.2 Among CTCL patients with peripheral blood involvement, there are greater numbers of T cells expressing the skin-homing cutaneous lymphocyte antigen (CLA) and the chemokine receptor CCR4 than are present in healthy donors.3 Furthermore, the CCR4 ligand CCL17 is highly expressed on the endothelial cells in CTCL skin lesions.3 These findings, together with the increased expression of E selectin and ICAM-1 in CTCL lesions,4,5 suggest that the appropriate microenvironment exists for the entry of skin-homing T cells into CTCL lesions.3 These malignant T cells may be found singly or collectively within the epidermis and admixed with an infiltrate of mononuclear cells within the papillary dermis underlying the involved epidermis.
We recently reported that in all cases of advanced CTCL, and many cases of early disease, there is a significant disruption of the diversity of the T-cell repertoire in peripheral blood.6 T-cell receptor beta-variable (BV) spectratyping revealed diminished complexity in many BV families,6 and this correlated with diminished T-cell receptor excision circle (TREC) levels.7 Both observations are consistent with the idea that some normal T cells are being removed from circulation and other T cells are proliferating to fill the space that this removal creates in the T-cell compartment. The idea that there may be a proliferative stimulus in the peripheral blood of CTCL patients led us to examine peripheral blood plasma for the presence of T-cell trophic cytokines Patients and healthy controls were studied, and plasma levels of interleukin-2 (IL-2), IL-4, IL-7, IL-12, IL-13, and IL-15 were measured. In preliminary studies, only IL-7 was reproducibly increased in patients with CTCL as compared with healthy controls.
It has been appreciated for many years that resident cells of skin, including keratinocytes and fibroblasts, can produce a wide variety of cytokines.8-11 One such cytokine is IL-7. IL-7 is a single-chain 25-kDa molecule that is important for both T- and B-cell growth and development.12-18 It is unique in its ability to both increase the generation of naive T cells by the thymus12-16 and promote the survival of mature T cells19-22 in the blood and lymph nodes, thus maintaining homeostasis in the T-cell compartment. IL-7 increases the survival of T cells in part by increasing the expression of antiapoptotic factor Bcl-2.23 Interestingly, elevated levels of plasma IL-7 have been found in conditions of T-cell depletion, including after chemotherapy and HIV infection,24-26 and IL-7 levels are inversely correlated with CD4 levels.24,25 These studies support the notion that increased production of IL-7 may be a homeostatic mechanism for regulating T-cell proliferation and possibly thymic output.24,25 IL-7 is also involved in the growth and survival of Sézary cells.27,28 Because CTCL cells may remain restricted to the skin during the course of the disease, locally produced IL-7 may be important for the survival of T cells.
In this study, we investigated plasma IL-7 levels in 93 CTCL patients and further measured lesional IL-7 mRNA expression levels in skin lesions from 10 CTCL patients; both were compared with normal plasma and skin, respectively. In addition, we cultured explants of normal and CTCL skin on specialized matrices that we have previously shown to support the survival of resident cells in normal skin.29 These cultures were assayed for IL-7 protein and the ability of the conditioned medium to support T-cell growth. Our results show that IL-7 is significantly increased in the plasma of CTCL patients and that CTCL skin contains mRNA for IL-7 and produces protein identical to that of IL-7. This IL-7 was shown to be functional, because its presence demonstrably enhanced T-cell growth and blocking IL-7 reversed this property. In summary, we have analyzed IL-7 production in CTCL and normal skin and investigated its possible role in production and proliferation of lesional lymphocytes.
Materials and methods
Patients and healthy donors
Patients with CTCL who provided informed consent were recruited from the Cutaneous Oncology Clinic at the Dana-Farber Cancer Institute. Ninety-three patients with CTCL (51 men and 42 women; median age, 61 years; range, 19-94 years) were recruited for analysis of peripheral blood. The subject profiles were as follows: stage I (35 men and 28 women; median age, 59.5 years; range, 19-90 years), stage II (6 men and 2 women; median age, 66.5 years; range, 31-82 years), stage III (8 men and 7 women; median age, 63 years; range, 30-94 years), stage IV (2 men and 5 women; median age, 65 years; range, 50-78 years). Diagnoses were based on clinical criteria as well as on histologic and immunohistologic assessment of skin specimens. CTCL was classified according to the TNM (primary tumor, regional nodes, metastasis) classification. Blood specimens obtained from 20 healthy volunteers (10 men and 10 women; median age, 40 years; range, 24-53 years) were also studied for comparison.
After participants provided informed consent, biopsy samples were taken from skin lesions of 18 CTCL patients (10 men and 8 women; median age, 62 years; range, 29-94 years) under local lidocaine anesthesia. None of the patients had received ultraviolet treatment, systemic drug therapy, or topical corticosteroids for at least 3 weeks before the investigation. Ten normal human skin samples were obtained as discarded tissue from cutaneous surgeries and were cut into 6 × 6 mm pieces, the same size as the biopsy specimens from CTCL patients. All studies using blood and skin biopsy samples were approved by the Dana-Farber Cancer Institute Institutional Review Board.
Plasma preparation
Plasma samples were isolated from heparinized venous blood by density gradient centrifugation over Ficoll (Histopaque; Sigma, St Louis, MO). All plasma samples were stored at -80°C prior to use.
Quantification of cytokines
Cytokine levels in plasma and skin culture supernatants were measured by quantitative sandwich enzyme-linked immunosorbent assay (ELISA). IL-2, IL-4, IL-7, IL-12, IL-13, and IL-15 ELISAs were purchased from R&D Systems (Minneapolis, MN). Samples were thawed at room temperature and assayed in duplicate. The reproducibility of the ELISA was assessed through the incorporation of a control plasma sample in each assay.
Quantification of levels of cytokine mRNA
For quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis, biopsy specimens from 10 CTCL patients and 10 healthy donors were snap-frozen in liquid nitrogen until use. After the specimen was homogenized, total RNA was extracted using the Clontech RNA purification kit (Clontech, Palo Alto, CA), according to the manufacturer's instructions; 2 to 5 μg total RNA (A260/A280 = 1.7 to 2.0) was reverse transcribed with oligo-dT primers and Powerscript Reverse Transcriptase (Clontech) in a final volume of 20 μL; 1 μL of the cDNA was amplified by PCR in a final volume of 50 μL with SYBR Green PCR Core reagents (Biosystems, Warrington, United Kingdom) and 1 M of primers. Samples were screened for the expression of β-actin as a housekeeping gene. The primer pairs specific for IL-7 and for β-actin were as follows (5′-3′): IL-7, TGTTGAACTGCACTGGCCAG and GCAACTGATACCTTACATGG30 ; β-actin, GTGGGGCGCCCCAGGCACCA and CTCCTTAATGTCACGCACGATTTC. A series of standard dilutions of a plasmid were used to quantify cytokines and enzyme. Specific signals for all transcripts were readily detected in human normal fibroblasts. Standard dilutions are amplified with pGEM-T Easy Vector Systems (Promega, Madison, WI) from the PCR amplifiers above. PCR was conducted with 40 cycles, which were within the linear amplification range for PCR reactions. For all of these samples, PCR was conducted twice. The specificity of the PCR products was confirmed by sequence analysis.
Preparation of keratinocyte and fibroblast cell cultures from skin biopsy
Keratinocytes were isolated by incubation of healthy-control skin specimens in 2.4 U/mL dispase II (Roche, Indianapolis, IN) overnight at 4°C; subsequent removal of the epidermis sheet with tweezers was followed by incubation for 5 minutes at 37°C in PBS containing 0.25% trypsin and 0.1% EDTA. Keratinocytes were grown in Keratinocyte-SFM (Gibco, Carlsbad, CA) supplemented with 75 μg/mL bovine pituitary extract (Gibco), 0.2 ng/mL EGF (Gibco), 0.3 mM CaCl2, and 100 IU/mL penicillin and 100 μg/mL streptomycin (PCN/Strep). Fibroblasts were isolated by mincing healthy-donor dermal skin fragments and incubating the fragments in HBSS with 2.5 mg/mL trypsin and 5 mg/mL collagenase for 1 hour. Fibroblasts were cultured in DMEM/F12 (Gibco) supplemented with 15% FCS (Sigma), 10 ng/mL EGF, and PCN/Strep.29 Third-passage samples from normal keratinocytes and fibroblasts were used to quantify cytokine mRNA. After cells were harvested for 2 days, they were washed twice with PBS and collected, and mRNA was purified as described above.
Preparation of 3-dimensional skin explant cultures
To analyze cytokines produced by skin in a culture condition that mimics lymphocyte-tissue interactions in the skin, we developed a 3-dimensional skin explant culture system (detailed by Clark et al29 ). Briefly, 9 × 9 × 1.5 mm Cellfoam matrices (Cytomatrix, Woburn, MA) were autoclaved and incubated in a solution of 100 μg/mL rat tail collagen I (BD Biosciences, Bedford, MA) in PBS for 30 minutes at 37°C. A punch biopsy was taken from lesional CTCL skin or from normal discarded skin, subcutaneous fat was removed, and the tissue was minced into explants approximately 2 × 2 × 2 mm. Three skin explants were placed on the surface of each matrix, and the culture was maintained in Iscove modified medium (Mediatech, Herndon, VA) with 10% heat-inactivated fetal bovine serum (FBS) (Sigma), PCN/Strep, and 3.5 μL/L β-mercaptoethanol. The culture was maintained for 5 weeks, and one half of the skin-culture supernatant was collected and replaced 3 times weekly. The produced T cells were collected once a week for up to 5 weeks. In this system, keratinocytes and dermal fibroblasts grow and spread into Cellfoam matrices, and skin-residing T cells are observed to spill out of the matrices into the culture wells. Skin lesions themselves are simulated for more than 5 weeks. We also cultured these skin explants with anti-human IL-7 neutralizing antibody or recombinant human IL-7, IL-4, and/or IL-13; anti-IL-7 antibody (25 ng/mL) or recombinant IL-7, IL-4, and IL-13 (25 ng/mL) were mixed in the culture medium from day 1. Eight CTCL skin samples and 8 control samples were collected for this experiment.
T-cell proliferation
CFSE (5 (and 6)-carboxyfluorescein diacetate, succinimidyl ester) was purchased from Molecular Probes (Eugene, OR). Peripheral blood mononuclear cells were obtained from discarded donated blood separated by density gradient centrifugation over Ficoll. T cells were purified with magnetic bead selection using the pan-T-cell isolation kit (Miltenyi Biotec, Auburn, CA). CLA-positive subsets were isolated by subsequent incubation of T cells with fluorescein isothyocyanate (FITC)-conjugated anti-human CLA antibody (BD Pharmingen, San Diego, CA) followed by anti-FITC microbeads and magnetic selection. Positively selected cells were collected as the CLA-positive enriched fraction. These cells were then washed with ice-cold PBS and labeled by CFSE (final concentration, 1 μM) in PBS. Cells were incubated for 15 minutes at 37°C, washed once, and incubated again for 30 minutes at 37°C in Iscove modified medium. Cells were resuspended to a concentration of 1 × 106 in 40 μL in Iscove modified medium. Cellfoam matrices colonized with either normal or CTCL skin cells were transferred to empty tissue culture wells, and CFSE (carboxyfluorescein diacetate succinimidyl ester)-labeled CLA-positive T cells were injected into the matrices and incubated for 1 hour at 37°C. Finally, 2 mL Iscove modified medium was added carefully, and the culture was incubated at 37°C for 1 week. One half of the medium was removed and replaced every other day, and produced T cells were collected on day 7. Cells were acquired in a FACScan, and CFSE levels were analyzed using CellQuest software (both Becton Dickinson, Mountain View, CA). We also cultured the skin explant with anti-human IL-7 neutralizing antibody (25 ng/mL) from day 1.
Statistical analyses
Linear regression models were fitted to the plasma IL-7 data from all patients. The models included the log10 of the plasma IL-7 levels as the dependent variable and age, sex, and stage as the independent variables. The Wilcoxon-Mann-Whitney test was used to evaluate differences in mRNA expression levels, IL-7 concentration in the supernatant from skin explants and normal skin cells, and T-cell yields between CTCL and healthy-control skin samples.
Results
Plasma cytokine levels
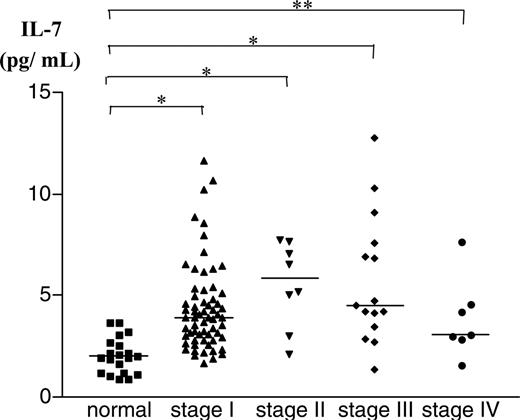
Plasma cytokine levels of 93 patients and 20 controls were measured by ELISA. Levels of IL-2, IL-4, IL-12, and IL-13 were all below the level of detection. In contrast, IL-7 and IL-15 were both detectable in plasma of most patients and controls. There was no significant difference in the circulating levels of IL-15 between healthy controls and CTCL patients (data not shown). However, IL-7 values from plasma were elevated in patients with CTCL, and these results were further analyzed. The log transform, when applied to the plasma IL-7 data, made the data symmetric. Therefore, a linear regression model was fitted to the log10 of plasma IL-7 data, with age, sex, and stage as covariates (Figure 1 and Table 1). All stages of CTCL showed significant differences compared with the healthy cohort, but no significant differences were seen for age and sex. A multiple comparison procedure was used to determine the differences between the stages; however, no significant differences were seen. Therefore, patients with staging data were combined into a single group and compared with the group of healthy volunteers. The linear regression model was fitted again with the following covariates: age, sex, and presence or absence of disease, defined as CTCL versus normal. The results are shown in Figure 1. Only the patients' identity as CTCL versus normal was a significant covariate.
Statistical analysis of plasma IL-7 levels
Variable . | Parameter estimate . | Standard error . | t value . | P . |
|---|---|---|---|---|
| Intercept | 0.340 | 0.89 | 3.84 | <.001 |
| Age | −0.001 | 0.001 | −0.93 | .357 |
| Sex | −0.010 | 0.039 | −0.26 | .792 |
| CTCL versus normal | 0.370 | 0.058 | 6.38 | <.001 |
Variable . | Parameter estimate . | Standard error . | t value . | P . |
|---|---|---|---|---|
| Intercept | 0.340 | 0.89 | 3.84 | <.001 |
| Age | −0.001 | 0.001 | −0.93 | .357 |
| Sex | −0.010 | 0.039 | −0.26 | .792 |
| CTCL versus normal | 0.370 | 0.058 | 6.38 | <.001 |
The DF (degree of freedom) for each category is 1.
Levels of IL-7 in plasma from patients with CTCL. Plasma samples were collected from 93 CTCL patients and 20 healthy controls. IL-7 levels were measured by ELISA. IL-7 levels were significantly higher in plasma samples from patients with CTCL. *P < .001; **P < .01.
Levels of IL-7 in plasma from patients with CTCL. Plasma samples were collected from 93 CTCL patients and 20 healthy controls. IL-7 levels were measured by ELISA. IL-7 levels were significantly higher in plasma samples from patients with CTCL. *P < .001; **P < .01.
Expression of IL-7 mRNA. Expression in normal and CTCL skin (A) and in normal keratinocytes and fibroblasts (B) was analyzed by quantitative PCR. The data are shown as a relative quantification of IL-7 mRNA expression levels divided by levels of β-actin mRNA. Expression of IL-7 mRNA was significantly higher in CTCL skin lesions than in samples of normal skin (A). *P < .001.
Expression of IL-7 mRNA. Expression in normal and CTCL skin (A) and in normal keratinocytes and fibroblasts (B) was analyzed by quantitative PCR. The data are shown as a relative quantification of IL-7 mRNA expression levels divided by levels of β-actin mRNA. Expression of IL-7 mRNA was significantly higher in CTCL skin lesions than in samples of normal skin (A). *P < .001.
Expression of IL-7 mRNA in skin
We next studied the expression of IL-7 mRNA by quantitative PCR analysis. Figure 2A shows the levels of mRNA expression in normal skin (n = 10) and CTCL lesional skin (n = 10). The nonparametric 2-sided Wilcoxon-Mann-Whitney test was used to compare the cytokine levels of CTCL lesions with those of normal skin samples; the 2-sided P values were less than .001. These data demonstrate that the message for IL-7 is constitutively expressed at a low level in human normal skin but at a significantly higher level in CTCL skin lesions. We found no statistically significant differences between the expression of IL-7 by keratinocytes and fibroblasts cultured from normal skin (P = .382) (Figure 2B); thus, the source of IL-7 in CTCL skin is obscure.
IL-7 concentration in supernatants from CTCL and normal skin explant cultures
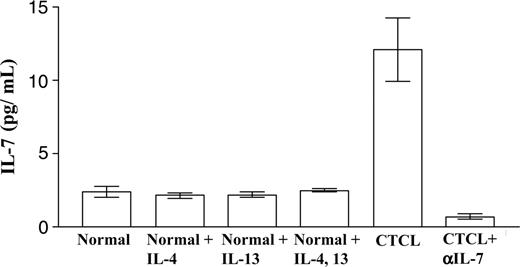
Supernatants from 3-dimensional skin explant culture systems were collected at week 3 and analyzed for IL-7 production by ELISA. The 2-sided Wilcoxon-Mann-Whitney test was used to assess the differences between 8 healthy controls and 8 CTCL patients. The IL-7 concentration in medium conditioned by CTCL explants was significantly higher than in healthy controls (P < .001) (Figure 3). Lesional lymphocytes in CTCL produce T helper (Th) 2 cytokines, and we reasoned that the production of these in situ might increase the levels of IL-7 present. However, levels of IL-7 from normal skin explant cultures, treated with or without Th2 cytokines, were never as high as those observed in CTCL samples. In addition, we found no detectable levels of IL-2 and IL-15 in skin explant cultures from either normal or CTCL skin (data not shown).
Production of T cells by skin matrix explant cultures
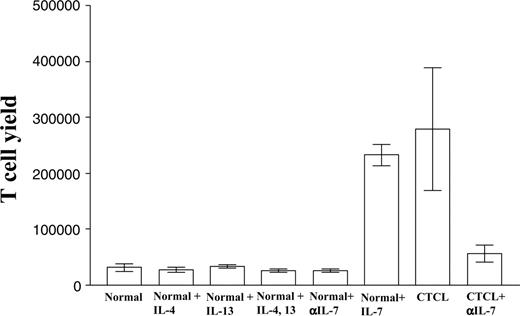
We have reported elsewhere that skin explants cultured on matrices gradually release large numbers of skin-resident T cells into the medium over a period of weeks.29 We harvested and counted the T cells produced by skin matrix cultures from both healthy and CTCL patients once each week. The 2-sided Wilcoxon-Mann-Whitney test was used to assess the differences between 8 healthy controls and 8 CTCL patients. The number of T cells released from CTCL skin matrices was significantly higher than that released from normal skin cultures (P < .001). Normal skin cultures treated with the Th2 cytokines IL-4 and IL-13 did not produce more T cells; however, as we expected, normal skin cultures treated with recombinant human IL-7 released increased numbers of T cells. These cultures released T cells at levels close to those seen in CTCL skin explants. We hypothesized that IL-7 produced by the CTCL explant cultures could be responsible for the increased number of T cells observed. Consistent with our hypothesis, the addition of anti-human IL-7 neutralizing antibody to CTCL skin matrix significantly decreased the T-cell yield from these cultures (Figure 4).
IL-7 concentration in supernatants from skin explant cultures. Normal and CTCL skin explants were cultured under various conditions. The concentration of IL-7 in the supernatant was analyzed by ELISA. IL-7 levels were significantly higher in CTCL samples than in healthy controls. Treatment with the Th2 cytokines IL-4 and/or IL-13 did not significantly increase IL-7 production.
IL-7 concentration in supernatants from skin explant cultures. Normal and CTCL skin explants were cultured under various conditions. The concentration of IL-7 in the supernatant was analyzed by ELISA. IL-7 levels were significantly higher in CTCL samples than in healthy controls. Treatment with the Th2 cytokines IL-4 and/or IL-13 did not significantly increase IL-7 production.
Proliferation of normal T cells induced by CTCL skin cells
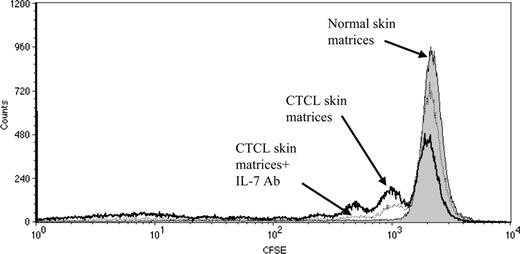
The data obtained thus far suggested that CTCL skin is an environment that supports proliferation of T cells. To test the comparative ability of normal and CTCL skin explant cultures to support proliferation of a relevant population of normal T cells, we labeled normal peripheral blood CLA-positive T cells with CFSE and incubated them for 1 week in matrices colonized with either normal or CTCL skin cells. CD3+ CLA+ CFSE+ lymphocytes incubated in CTCL skin matrices divided up to 3 times during the culture period (Figure 5, open histograms, bold line); this cellular division of T cells could be blocked significantly by addition of antibodies to IL-7 (open histograms, broken line). Normal skin matrices did not support the proliferation of T cells (solid histograms). Similar results were obtained in 3 independent experiments.
Production of T cells by skin matrix explant cultures. CTCL and normal skin explants were cultured under various conditions, and T-cell production was assayed. The number of T cells produced from CTCL skin explants was significantly higher than from normal skin explants. Normal skin explants treated with Th2 cytokines IL-4 and/or IL-13 did not produce more T cells. Normal skin explants treated with recombinant human IL-7 produced more T cells than untreated cultures. The addition of anti-human IL-7 neutralizing antibody to CTCL skin explants decreased the numbers of T cells produced.
Production of T cells by skin matrix explant cultures. CTCL and normal skin explants were cultured under various conditions, and T-cell production was assayed. The number of T cells produced from CTCL skin explants was significantly higher than from normal skin explants. Normal skin explants treated with Th2 cytokines IL-4 and/or IL-13 did not produce more T cells. Normal skin explants treated with recombinant human IL-7 produced more T cells than untreated cultures. The addition of anti-human IL-7 neutralizing antibody to CTCL skin explants decreased the numbers of T cells produced.
Proliferation of normal blood CLA-positive T cells incubated in matrices colonized with skin cells from CTCL lesions or normal skin. CFSE-labeled CLA-positive T cells were isolated from peripheral blood and incubated for 1 week in matrices colonized with skin cells from either CTCL or normal skin. T cells were analyzed by flow cytometry. CD3+ CLA+ CFSE+ lymphocytes divided up to 3 times in CTCL skin matrices (open histograms, bold line); this cellular division of T cells could be blocked significantly by addition of antibodies to IL-7 (open histograms, broken line). Normal skin matrices did not support cell proliferation (filled histograms).
Proliferation of normal blood CLA-positive T cells incubated in matrices colonized with skin cells from CTCL lesions or normal skin. CFSE-labeled CLA-positive T cells were isolated from peripheral blood and incubated for 1 week in matrices colonized with skin cells from either CTCL or normal skin. T cells were analyzed by flow cytometry. CD3+ CLA+ CFSE+ lymphocytes divided up to 3 times in CTCL skin matrices (open histograms, bold line); this cellular division of T cells could be blocked significantly by addition of antibodies to IL-7 (open histograms, broken line). Normal skin matrices did not support cell proliferation (filled histograms).
Discussion
In this study, we measured the levels of T lymphotropic cytokines in the plasma of healthy controls and a large population of CTCL patients. Of 6 cytokines measured, only IL-7 showed statistically significant and reproducible elevations in patients with CTCL. These elevations were found at all stages, and in fact stage was not a significant covariate. We further demonstrated that increased levels of IL-7 mRNA were present in lesional CTCL skin as compared with normal skin and that explants of CTCL skin released more IL-7 into the medium than did normal skin explants. In parallel, CTCL explant cultures resulted in the release of many more T cells from skin than normal explants. Importantly, this yield of T cells from CTCL explants could be reduced significantly if antibodies to IL-7 were added. Finally, we demonstrated that CTCL explant cultures could support the presumably antigen-independent proliferation of normal skin-homing memory T cells, while normal skin explants could not. This too could be blocked by antibodies to IL-7. Taken together, these data point to production of IL-7 by CTCL skin as an important feature of this disease.
IL-7 is thought to be produced predominately by epithelial and stromal cells in the thymus, lymph nodes, and bone marrow31-33 and is a critical factor in both B- and T-cell development.12-18,34,35 Furthermore, it enhances both thymic and extrathymic lymphopoesis in the context of diseases that result in lymphopenia. IL-7 treatment can induce the expansion of naive T cells without antigenic stimulation36,37 or differentiation into memory T cells.38,39
We reported previously that the diversity of the T-cell repertoire in CTCL is significantly contracted6 despite the presence of relatively normal absolute T-cell counts.40,41 We have postulated that this contraction of the T-cell repertoire contributes to the immune suppression and significant infection-related mortality that characterizes advanced disease. We also reported that the levels of TRECs in patients with CTCL were decreased, even in early-stage disease.7 Especially in patients with advanced-stage CTCL, TREC levels in normal nonmalignant T cells were clearly reduced, a finding consistent with the interpretation that many normal T cells had been removed from the T-cell compartment and that the remaining cells had expanded clonally to fill the empty space created by their removal.7 These results led us to consider the presence of IL-7 and other T-cell trophic cytokines in CTCL patients. Plasma levels of IL-2, IL-4, IL-7, IL-12, IL-13, and IL-15 were examined in CTCL patients and healthy controls. Only IL-7 and IL-15 could be reproducibly measured in the plasma of patients and controls. While IL-15 levels did not differ between patients and controls, plasma IL-7 levels were significantly higher in CTCL patients than in healthy controls. This increase appeared to be independent of stage, although there was a trend toward higher levels in patients with stage II and III disease. We next investigated whether CTCL skin could be the source of the increased circulating levels of IL-7 in these patients. Using a sensitive and specific quantitative PCR assay, we found higher levels of IL-7 mRNA in CTCL skin than in normal skin.
Because cytokine mRNA expression and protein production do not always correlate, we felt it was necessary to measure cytokine protein production in CTCL skin. Direct quantitative IL-7 protein measurements from skin were impractical, so we employed a 3-dimensional matrix skin explant culture system to simulate intact normal and CTCL skin.29 Periodically, the medium conditioned by these explants was sampled and tested for cytokines including IL-7. Our results indicated that IL-7 was produced at much higher levels in CTCL explant cultures as compared with normal skin explant cultures.
CTCL is thought to be a Th2 disease, and clonal CTCL cells produce Th2 cytokines.42-45 To assess whether Th2 cytokines produced by clonal CTCL cells can stimulate IL-7 production by resident skin cells, we incubated normal skin explant cultures with the Th2 cytokines IL-4 and/or IL-13. We found no production of IL-7 at levels comparable to those observed in CTCL explant cultures (Figure 3), suggesting that these T-cell cytokines do not stimulate skin cells in the explant cultures to produce IL-7. When fibroblasts and keratinocytes from CTCL lesions were grown in 2-dimensional culture in the absence of lymphocytes, these cultures produced only low levels of IL-7 (data not shown). Subsequent addition of autologous CD3+ T cells to these 2-dimensional CTCL skin cell cultures did not restore IL-7 production (data not shown). These results suggest that close cell-to-cell interactions between lymphocytes and skin-construct fibroblasts and keratinocytes in a 3-dimensional arrangement might be required for efficient production of IL-7. We have reported previously that explants of skin placed on the Cellfoam 3-dimensional matrices produce significant numbers of T cells that maintain a CLA+ CCR4+ skin-homing phenotype.29 CTCL skin explant cultures produced 8-fold more cells than cultures of normal skin (Figure 4). Treatment of normal skin explant cultures with IL-4 and/or IL-13 did not significantly increase T-cell yields. However, treatment of normal skin explants with IL-7 increased T-cell production to levels observed in CTCL skin. Last, addition of anti-human IL-7 neutralizing antibody to CTCL explants dramatically decreased the number of cells produced (Figure 4). Interestingly, when we cultured psoriasis skin on the same matrix, IL-7 levels in the supernatant were as low as those from normal skin explants, and the number of T cells produced from psoriasis skin explants was similar to that from normal skin explants (data not shown).
These data demonstrate that skin-derived IL-7 can promote the proliferation of lymphocytes in CTCL skin lesions. To confirm that CTCL skin-derived IL-7 can induce the proliferation of skin-homing T cells, we added CLA-positive T cells from peripheral blood to matrices colonized with skin cells from either normal or CTCL skin. Skin-homing T cells present in normal skin also express high levels of CLA.3,46 Our data clearly demonstrated that IL-7 produced in CTCL skin can support the proliferation of CLA-positive T cells derived from peripheral blood, a process that can be inhibited by antibody to IL-7 (Figure 5). We performed the same experiment with psoriasis skin; however, we did not detect any proliferation for the CLA-positive T cells cultured on the psoriasis skin explants. Thus, the contribution of IL-7 to lymphocyte proliferation appears to be unique to CTCL skin. In conclusion, in CTCL skin lesions where T cells may remain restricted to the skin throughout the course of the disease, local production of IL-7 can strongly influence their survival and proliferation.
Prepublished online as Blood First Edition Paper, December 1, 2005; DOI 10.1182/blood-2005-03-1139.
Supported by a Specialized Program of Research Excellence (SPORE) in Skin Cancer from the National Cancer Institute/National Institutes of Health (NCI/NIH).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Abrar Qureshi, Marianne Tawa, and Carrie E. Wechsler for sample collection and Nancy K. Voynow for critical reading of this manuscript. Samples of normal human skin were graciously provided by Dr Thomas Cochran of the Boston Center for Plastic Surgery.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal