Abstract
Human early thymic precursors have the potential to differentiate into multiple cell lineages, including T cells and plasmacytoid dendritic cells (pDCs). This decision is guided by the induction or silencing of lineage-specific transcription factors. The ETS family member Spi-B is a key regulator of pDC development, whereas T-cell development is critically dependent on GATA-3. Here we show that triggering of the Notch1 signaling pathway by Delta-like1 controls the T/pDC lineage decision by regulating the balance between these factors. CD34+CD1a- thymic progenitor cells express Notch1, but down-regulate this receptor when differentiating into pDCs. On coculture with stromal cell lines expressing either human Delta-like1 (DL1) or Jagged1 (Jag1) Notch ligands, thymic precursors express GATA-3 and develop into CD4+CD8+TCRαβ+ T cells. On the other hand, DL1, but not Jag1, down-regulates Spi-B expression, resulting in impaired development of pDCs. The Notch1-induced block in pDC development can be relieved through the ectopic expression of Spi-B. These data indicate that DL1-induced activation of the Notch1 pathway controls the lineage commitment of early thymic precursors by altering the levels between Spi-B and GATA-3. (Blood. 2006;107:2446-2452)
Introduction
Dendritic cells (DCs) are specialized in the capture and subsequent presentation of antigenic peptides to T cells. Different subsets of DCs have been characterized, which all have distinct cell surface phenotypes, functions, and anatomic localizations.1 The most recent identified member of the DC lineage is the plasmacytoid DC (pDC),2 also referred to as natural type 1 interferon (IFN)-producing cell3 or type 2 DC precursors.4 On activation with IL-3 and CD40L or virus mature pDCs induce naive T cells to produce either interleukin 4 (IL-4) and IL-54 or IL-10 and IFN-γ,5,6 respectively. Human pDCs lack expression of typical myeloid markers CD13, CD33, CD11c, and the mannose receptor, but do express lymphoid-related genes including SPIB, PTCRA, IGLL1, and show presence of immunoglobulin heavy-chain D-J rearrangements.2,7-9 This indicates that pDCs may have a lymphoid origin. Indeed, it was recently documented that common lymphoid precursors (CLPs) can give rise to pDCs. However, pDCs are not exclusively of lymphoid origin because pDCs can also develop from Flt3+ common myeloid precursors (CML).10 These data suggest that a pDC developmental program can be imposed on both myeloid and lymphoid precursors.11,12 In line with this, Flt3 ligand induces the development of pDCs from progenitor cells, in both humans13 and mice.14
We and others have shown that pDCs are present in the thymus.8,9 Although the function of thymic pDCs in T-cell development is still unclear, they are able to suppress HIV-1 replication in thymocytes.15 In addition, we documented that thymic precursors can give rise to pDCs in vitro and in vivo and that the thymus is able to support pDC development.16,17 This indicates that thymic pDCs develop within the thymus from early thymic progenitors (ETPs). The fact that multiple hematopoietic lineages can develop from thymic precursors as shown for both mouse ETP and human CD34+ cells18-20 suggests that the thymic precursor pool contains multipotent precursors. Indeed, tripotential T/NK/DC precursors are present in the mouse21-23 and bipotential T/NK precursors have been found in the human thymus.24
It is clear that transcriptional programs drive development of hematopoietic cells.25-27 Notch1 and GATA-3 are essential for T-cell development,26,28 and we recently documented that the ETS transcription factor Spi-B is a key regulator of pDC development.7,29 Forced expression of Spi-B stimulates the development of pDCs from hematopoietic CD34+CD38- precursors while strongly inhibiting the development of alternative lineage choices. Moreover, specific degradation of Spi-B mRNA by RNA interference (RNAi) strongly impairs the ability of hematopoietic progenitor cells to differentiate into pDCs. Additional transcription factors involved in pDC development are interferon regulatory factor 8 (IRF-8), also called ICSBP, and one or more members of the basic helix-loop-helix (bHLH) factor family.16,30,31 pDCs are not present in IRF-8-deficient mice30,31 and a role of E-proteins in pDC development can be inferred from our demonstration that the inhibitors of DNA binding 2 (Id-2) and -3, antagonists of E-proteins, strongly block pDC but not myeloid DC development.16 Based on the premise that Spi-B can form a complex with IRF-8 and this complex may also contain the E-protein E47,32,33 it is tempting to speculate that pDC development is driven by Spi-B/IRF-8/E47 complexes.
Notch1 has emerged as a key factor for T-cell development. The evolutionary conserved Notch signaling pathways have been described to control T-cell commitment,34 the αβ versus γδ35 and Th1 versus Th236 lineage decisions in T-cell development. Four Notch receptors (Notch1-4) and 5 ligands (Jagged [Jag] 1, 2 and Delta-like [DL] 1, 3, 4) have been found in mammals.37 Triggering of Notch by its ligands induces translocation of the intracellular part of the receptor to the nucleus and subsequent activation of downstream targets, including HES-1 and pTα.38,39 Notch1 signaling is implicated in the T/B-cell lineages decision because Notch1-deficient mice lack T cells and instead show dramatically increased B-cell development in the thymus.40 This prompted us to investigate whether Notch1 signaling also regulates the T-cell versus pDC switch. In this study we show that differentiation of human thymic precursors toward pDCs is strongly blocked by DL1/Notch1 signaling via down-modulation of Spi-B, whereas the T lineage-specific factor GATA-3 is up-regulated. The data presented here indicate that the DL1/Notch1 signaling pathway directs the T/pDC lineage decision by controlling the levels of the downstream lineage-specific factors GATA-3 and Spi-B.
Materials and methods
Reagents and monoclonal antibodies
Monoclonal antibodies to CD1a, CD3, CD4, CD5, CD7, CD8, CD123, TCRαβ, and TCRγδ conjugated to PE, PerCP, PeCy7, APC, or APCCy7 were purchased from Becton Dickinson (San Jose, CA). Anti-BDCA2-APC was obtained from Miltenyi Biotec (Bergisch Gladbach, Germany). The cytokines IL-7 and stem cell factor (SCF) were obtained from R&D Systems (Abingdon, United Kingdom). Flt3L was a kind gift from Dr G. Wagemaker (Erasmus University, Rotterdam, The Netherlands). The γ-secretase inhibitor IX (DAPT) was purchased from Calbiochem (San Diego, CA) and used at a concentration of 10 μM.
Constructs, cell lines, and retroviral production
The OP9-control, OP9-DL1, and OP9-Jag1 cell lines were generated by transduction of the murine bone marrow stromal cell line OP9 (kindly provided by Dr T. Nakano, Osaka University, Osaka, Japan)41 with, respectively, the empty LZRS IRES neo retroviral vector or with the LZRS IRES neo vector engineered to express human Delta-like1 (DL1) or Jagged1 (Jag1), both provided by Dr L. Parreira (Universidade de Lisboa, Lisbon, Portugal). Transduced cells were selected based on their resistance for neomycin by culturing for 2 to 3 weeks in the presence of 1.5 mg/mL geneticin (G418; Invitrogen, Carlsbad, CA). Cells were maintained in MEMα (Invitrogen) with 20% FCS (Hyclone, Logan, UT).
cDNA sequences encoding the intracellular domain of Notch1 (icNotch1, provided by Dr B. Verhasselt, University of Ghent, Belgium) and GATA-3 (provided by P-H. Romeo, Institut Cochin, Paris, France) were subcloned into LZRS IRES GFP or LZRS IRES YFP. The LZRS Spi-B IRES GFP construct was described previously.7 Retroviral supernatants were produced as described42 using the 293T-based Phoenix packaging cell line.43
Isolation of CD34+ cells from postnatal thymus
The use of postnatal thymus tissue was approved by the Medical Ethical Committee of the Academic Medical Center. Thymocytes were obtained from surgical specimens removed from children up to 3 years of age undergoing open heart surgery, with informed consent from patients in accordance with the Declaration of Helsinki. The tissue was disrupted by mechanical means and pressed through a stainless steel mesh to obtain a single-cell suspension, which was left overnight at 4°C. The following day thymocytes were isolated from a Ficoll-Hypaque density gradient (Lymphoprep; Nycomed Pharma, Oslo, Norway). Subsequently, CD34+ cells were enriched by immunomagnetic cell sorting, using a CD34 cell separation kit (varioMACS, Miltenyi Biotec). The CD34+ thymocytes were stained with antibodies against CD34, CD1a, CD56, and BDCA2. CD34+CD1a-CD56-BDCA2- and CD34+CD1a+CD56-BDCA2- (further referred to as CD34+CD1a- and CD34+CD1a+) populations were sorted to purity. For the isolation of pDCs, BDCA4+ cells were enriched by immunomagnetic cell sorting, using a BDCA4+ cell separation kit (varioMACS, Miltenyi Biotec). The BDCA4+ cell fraction was labeled with anti-CD123 and anti-CD45RA antibodies and CD123hiCD45RA+ cells were sorted to purity. Cells were sorted using a FACSAria (Becton Dickinson); purity of the sorted cells in all experiments was greater than 99%.
Isolation of CD34+ cells from fetal liver
Human fetal tissues were obtained from elective abortions. The use of fetal tissue was approved by the Medical Ethical Committee of the Academic Medical Center and was contingent on obtaining informed consent, in accordance with the Declaration of Helsinki. Gestational age was determined by ultrasonic measurement of the diameter of the skull and ranged from 14 to 20 weeks. Fetal liver CD34+ cells were isolated as described previously.16
Retroviral transduction and differentiation assays
For transduction experiments CD34+CD1a- postnatal thymocytes were cultured overnight in Yssel medium44 with 5% NHS, 20 ng/mL SCF, and 10 ng/mL IL-7. The following day cells were incubated for 6 to 7 hours with virus supernatant in retronectin-coated plates (30 μg/mL; Takara Biomedicals, Otsu, Shiga, Japan). The development of pDCs and T cells was assessed by coculturing 5 × 104 CD34+CD1a- progenitor cells with 5 × 104 OP9 cells in MEMα (Invitrogen) with 20% FCS (Hyclone), 5 ng/mL IL-7, and 5 ng/mL Flt3L. Differentiation assays for pDCs were analyzed after 1 week of coculture. T-cell cultures were refreshed every 2 to 3 days and progenitor cells were transferred to fresh stromal cells every 4 to 5 days of culture. Flow cytometric analyses were performed on an LSRII FACS analyzer (Becton Dickinson). To test function of thymic progenitor-derived pDCs, after 5 to 6 days of culture on OP9 control cells either CpG oligodeoxynucleotide (CpG-ODN) 2216 (ggGGGACGATCGTCgggggG; Sigma-Aldrich, St Louis, MO) or γ-irradiated HSV-1 (KOS strain; gift from DNAX Research Institute, Palo Alto, CA) was added to culture for 18 hours. IFN-α produced in the supernatant was measured by enzyme-linked immunosorbent assay (ELISA; Biosource International, Camarillo, CA).
Proliferation and apoptosis assays
Cell proliferation was measured by CFSE (Molecular Probes, Eugene, OR) dilution. CD34+CD1a- cells were incubated for 10 minutes at 37°C and subsequently labeled with 1 μM CFSE for 10 minutes at 37°C. Cells were cultured as described (see “Retroviral transduction and differentiation assays”). Cycling cells were labeled with Ki-67 (Becton Dickinson) using the Cytofix/Cytoperm Plus Kit (Becton Dickinson). Apoptotic cells were determined by annexin V-FITC (Becton Dickinson) and 7-aminoactinomycin D (7-AAD; Becton Dickinson) labeling. Cells were analyzed by flow cytometry.
Reverse transcription-PCR
Human-specific polymerase chain reaction (PCR) primers were: actin, forward 5′-ATGGAGTTGAAGGTAGTTTCG, reverse 5′-CAAGAGATGGCCACGGCTGCTTCAGC; p0, forward 5′-TCGACAATGGCAGCATCTAC, reverse 5′-ATCCGTCTCCACAGACAAGG; HES-1, forward 5′-CGGACATTCTGGAAATGACA, reverse 5′-GGTACTTCCCCAGCACACTT; Spi-B, forward 5′-GCATACCCCACGGAGAACT, reverse 5′-GGCTGTCCAACGGTAAGTCT; GATA-3, forward 5′-CTCATTAAGCCCAAGCGAAG, reverse 5′-GCATTCCTCCTCCAGAGTGT; Notch1, forward 5′-CGGGGCTAACAAAGATATGC, reverse 5′-CCATATGATCCGTGATGTCC. PCRs for HES-1, GATA-3, and Spi-B were performed on an iCycler PCR (Bio-Rad, Hercules, CA). Expression of Notch1 was determined using a PTC-200 Gradient Cycler (MJ Research, San Francisco, CA).
Western blot
To test for triggering of the Notch1 receptor 0.5 × 106 CD34+CD1a- thymic progenitors were cocultured with 1 × 105 OP9 cells for 3 hours. Precursor cells were recovered from the cultures and analyzed for the presence of non-membrane-bound cytoplasmic intracellular Notch1 by Western blot using a Notch1 antibody recognizing the C-terminus of the receptor (S20; Santa Cruz Biotechnology, Santa Cruz, CA). As loading controls actin levels were measured (I19; Santa Cruz).
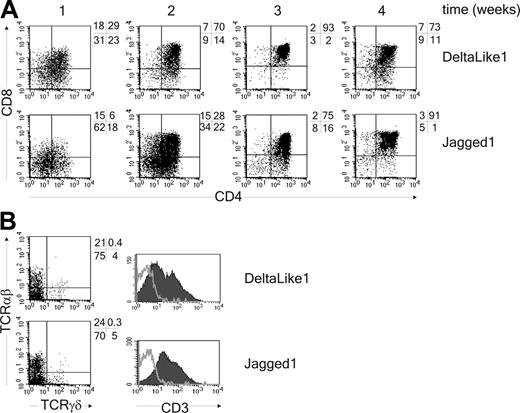
DL1 and Jag1 support T-cell development from thymic progenitor cells in vitro. (A) CD34+CD1a- thymic precursor cells were cocultured with OP9-DL1 or OP9-Jag1 for 4 weeks. Cells were analyzed for expression of CD4 and CD8 every week. (B) Analysis after 4 weeks for expression of TCRαβ, TCRγδ, and CD3 (CD3, filled histogram; isotype control, gray line). One representative experiment of 3 is shown.
DL1 and Jag1 support T-cell development from thymic progenitor cells in vitro. (A) CD34+CD1a- thymic precursor cells were cocultured with OP9-DL1 or OP9-Jag1 for 4 weeks. Cells were analyzed for expression of CD4 and CD8 every week. (B) Analysis after 4 weeks for expression of TCRαβ, TCRγδ, and CD3 (CD3, filled histogram; isotype control, gray line). One representative experiment of 3 is shown.
Results
pDC development is blocked by DL1-Notch interactions
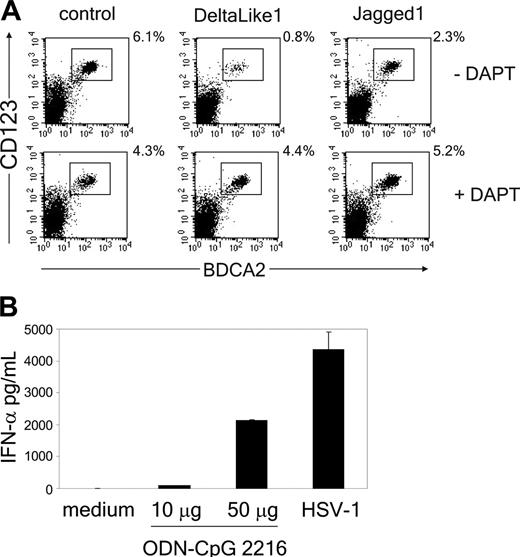
The Notch signaling pathway directs the lineage decision between T and B cells.40 To determine whether Notch also regulates the switch between T cells and pDCs, we adapted the in vitro assay previously described by Schmitt and Zúñiga-Pflücker34 by generating an OP9 cell line expressing human DL1. This novel cell line supported the development of CD4+CD8+TCRαβ+ and TCRγδ+ T cells from uncommitted CD34+CD1a- human thymic precursor cells (Figure 1 and Figure S1, which is available on the Blood website; see the Supplemental Materials link at the top of the online article). In contrast, mature T cells were not generated on OP9 control cells, but this cell line did support development of BDCA2+CD123hi pDCs (Figure 2A). The pDCs derived from thymic progenitor cells were functional because addition of either the TLR9-specific ligand CpG-ODN (2216) or HSV-1, which both have been previously recognized to specifically stimulate pDCs,5,45 induced the secretion of IFN-α in the supernatant (Figure 2B). Interestingly, we found that although development of BDCA2+CD123hi pDCs was supported by the OP9 control line, it was strongly blocked in the presence of DL1 (Figure 2A; Table 1). Addition of the γ-secretase inhibitor IX (DAPT), which inhibits the cleavage and thereby nuclear translocation of the intracellular domain of the Notch receptor,46 blocked DL1-induced T-cell development (Figure S1) and could overcome the inhibition in pDC development (Figure 2A). This confirms that inhibition of pDC development by DL1 results from Notch signaling. Furthermore, because it was reported recently that Jagged1 (Jag1) inhibits development of B cells from mouse thymic precursors,47 we also evaluated the effect of OP9-Jag1 on development of pDCs. Interestingly, Jag1 was also able to support CD4+CD8+TCRαβ+ T-cell development from CD34+CD1a- human thymic precursor cells, albeit with slightly delayed kinetics compared with DL1 (Figure 1; Figure S1). More importantly, equal or higher numbers of pDCs were generated from CD34+CD1a- human thymic precursor cells in coculture with OP9-Jag1 as compared to the OP9 control cultures (Figure 2A; Table 1). This shows that DL1/Notch signaling supports T-cell development on the one hand and specifically blocks the development of thymic precursor cells into pDCs on the other.
DL1, but not Jag1, inhibits pDC development in both percentage and absolute cell number
Experiment no. . | Control . | . | OP9-DL1 . | . | . | OP9-Jag1 . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Percentage . | Absolute cell no. . | Percentage . | Absolute cell no. . | DL1/control ratio . | Percentage . | Absolute cell no. . | Jag1/control ratio . | |||||
| 1 | 4.6 | 32 873 | 0.1 | 12 180 | 0.37 | 1.3 | 29 900 | 0.91 | |||||
| 2 | 6.1 | 50 184 | 0.8 | 29 970 | 0.60 | 2.3 | 108 000 | 2.15 | |||||
| 3 | 22.6 | 119 833 | 2.8 | 47 040 | 0.39 | 14.9 | 143 232 | 1.20 | |||||
| 4 | 4.1 | 20 237 | 0.3 | 7 194 | 0.36 | 2.4 | 30 114 | 1.49 | |||||
| 5 | 7.4 | 109 372 | 0.4 | 14 964 | 0.14 | 3.4 | 55 094 | 0.50 | |||||
| 6 | 11.1 | 497 941 | 1.5 | 165 241 | 0.33 | 7.6 | 657 040 | 1.32 | |||||
| 7 | 33.0 | 726 000 | 1.0 | 258 000 | 0.36 | — | — | — | |||||
| 8 | 19.2 | 951 328 | 0.4 | 41 533 | 0.04 | — | — | — | |||||
| 9 | 13.3 | 29 260 | 0.2 | 1 058 | 0.04 | — | — | — | |||||
Experiment no. . | Control . | . | OP9-DL1 . | . | . | OP9-Jag1 . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Percentage . | Absolute cell no. . | Percentage . | Absolute cell no. . | DL1/control ratio . | Percentage . | Absolute cell no. . | Jag1/control ratio . | |||||
| 1 | 4.6 | 32 873 | 0.1 | 12 180 | 0.37 | 1.3 | 29 900 | 0.91 | |||||
| 2 | 6.1 | 50 184 | 0.8 | 29 970 | 0.60 | 2.3 | 108 000 | 2.15 | |||||
| 3 | 22.6 | 119 833 | 2.8 | 47 040 | 0.39 | 14.9 | 143 232 | 1.20 | |||||
| 4 | 4.1 | 20 237 | 0.3 | 7 194 | 0.36 | 2.4 | 30 114 | 1.49 | |||||
| 5 | 7.4 | 109 372 | 0.4 | 14 964 | 0.14 | 3.4 | 55 094 | 0.50 | |||||
| 6 | 11.1 | 497 941 | 1.5 | 165 241 | 0.33 | 7.6 | 657 040 | 1.32 | |||||
| 7 | 33.0 | 726 000 | 1.0 | 258 000 | 0.36 | — | — | — | |||||
| 8 | 19.2 | 951 328 | 0.4 | 41 533 | 0.04 | — | — | — | |||||
| 9 | 13.3 | 29 260 | 0.2 | 1 058 | 0.04 | — | — | — | |||||
The absolute cell numbers represent numbers of pDC output cells derived from 50 000 input CD34+CD38− progenitor cells. The ratios are representative for the relative increase or decrease of pDCs cocultured with DL1 compared with the control cocultured cells.
Notch triggering by DL1, but not Jag1, blocks pDC development from thymic progenitor cells. (A) CD34+CD1a- cells were cocultured with OP9-DL1, OP9-Jag1, or control cells in the presence or absence of 10 μM γ-secretase inhibitor DAPT. Cultures were analyzed for the presence of CD123hiBDCA2+ pDCs after 1 week of culture. Experiment is representative of 3. (B) At day 5 after culture of CD34+CD1a- cells on OP9 control cells, either CpG-ODN (2216) was added at different concentrations (10 or 50 μg/mL), or HSV-1 was added at 1 pfu/cell for 18 hours. Supernatants were analyzed in duplicate for the presence of IFN-α by ELISA. One representative experiment of 2 is shown.
Notch triggering by DL1, but not Jag1, blocks pDC development from thymic progenitor cells. (A) CD34+CD1a- cells were cocultured with OP9-DL1, OP9-Jag1, or control cells in the presence or absence of 10 μM γ-secretase inhibitor DAPT. Cultures were analyzed for the presence of CD123hiBDCA2+ pDCs after 1 week of culture. Experiment is representative of 3. (B) At day 5 after culture of CD34+CD1a- cells on OP9 control cells, either CpG-ODN (2216) was added at different concentrations (10 or 50 μg/mL), or HSV-1 was added at 1 pfu/cell for 18 hours. Supernatants were analyzed in duplicate for the presence of IFN-α by ELISA. One representative experiment of 2 is shown.
To address whether DL1 would also inhibit development from extrathymic precursors, we examined the effect of DL1 on pDC development from fetal liver CD34+CD38- progenitor cells (Table S1). Although in 4 of 7 experiments a clear inhibition was observed, in one experiment pDCs were not blocked and in 2 experiments a clear augmentation of pDC development was found. Thus a considerable donor-to-donor variability was seen and overall the effects of DL1 on pDC development from CD34+CD38- progenitors cannot be considered to be statistically significant.
Notch1 activation is incompatible with pDC development
Given the essential function of Notch1 in T-cell development,40 we examined the expression of Notch1 in uncommitted human thymic precursors and the downstream, lineage-specified, CD34+CD1a+ pro-T and CD123hiCD45RA+ pDC populations. As expected, Notch1 was expressed in the CD34+CD1a- population and was maintained in CD34+CD1a+ cells, consistent with the fact that these 2 populations have a high T-cell precursor potential. In contrast, development of pDCs from the thymic precursor cells occurred in concert with a down-regulation of the Notch1 receptor (Figure 3A). Moreover, we found that the amount of non-membrane-bound intracellular Notch1 (icNotch1) was increased by DL1 triggering of CD34+CD1a- thymic precursors but not by Jag1. This provides evidence for the activation of the Notch1 pathway by DL1 in this culture system (Figure 3B). To prove the inhibitory effect of Notch1 signaling on pDC development we forced the expression of icNotch1 in CD34+CD1a- cells by retroviral transduction. The icNotch1-transduced progenitor cells were strongly inhibited in their ability to differentiate into pDCs (Figure 3C), similar to effects induced by DL1. In addition, icNotch1 expression was sufficient to drive T-cell differentiation on OP9 control cells (Figure 3D). Taken together these data strongly indicate that the interaction of Notch1 with its ligand DL1 is incompatible with the development of pDCs.
Notch1 activation is incompatible with pDC development. (A) Serial dilutions of CD34+CD1a-, CD34+CD1a+, and CD123hiCD45RA+ cells were analyzed for expression of Notch1 mRNA by RT-PCR. Actin mRNA levels were measured as loading control (n = 3). (B) CD34+CD1a- cells were cocultured for 3 hours with OP9-DL1, OP9-Jag1, or control cells. The presence of non-membrane-bound intracellular Notch1 (icNotch1) was analyzed by Western blot using an antibody recognizing the intracellular part of the Notch1 receptor.An antibody recognizing actin was used as loading control (n = 2). (C) CD34+CD1a- cells were retrovirally transduced with icNotch1 or GFP control virus, cocultured on OP9 control cells, and analyzed for the presence of CD123hiBDCA2+ pDCs after 1 week (n = 4), or (D) analyzed for the presence of CD4, CD8, and CD3 after 3 weeks (n = 2). Experiments representative of at least 2 experiments are shown.
Notch1 activation is incompatible with pDC development. (A) Serial dilutions of CD34+CD1a-, CD34+CD1a+, and CD123hiCD45RA+ cells were analyzed for expression of Notch1 mRNA by RT-PCR. Actin mRNA levels were measured as loading control (n = 3). (B) CD34+CD1a- cells were cocultured for 3 hours with OP9-DL1, OP9-Jag1, or control cells. The presence of non-membrane-bound intracellular Notch1 (icNotch1) was analyzed by Western blot using an antibody recognizing the intracellular part of the Notch1 receptor.An antibody recognizing actin was used as loading control (n = 2). (C) CD34+CD1a- cells were retrovirally transduced with icNotch1 or GFP control virus, cocultured on OP9 control cells, and analyzed for the presence of CD123hiBDCA2+ pDCs after 1 week (n = 4), or (D) analyzed for the presence of CD4, CD8, and CD3 after 3 weeks (n = 2). Experiments representative of at least 2 experiments are shown.
DL1 does not affect proliferation or apoptosis of pDCs
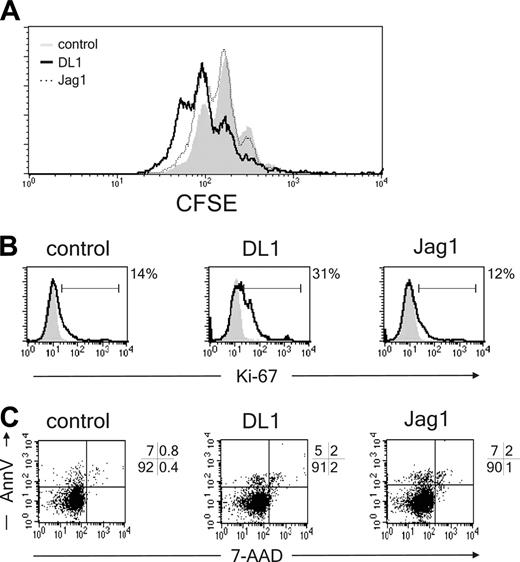
To exclude that DL1/Notch1 signaling affects pDC proliferation or apoptosis rather than differentiation, developing pDCs were analyzed for cell cycle and apoptotic markers. CD34+CD1a- cells were labeled with CFSE and allowed to differentiate in the presence of OP9-DL1, OP9-Jag1, or control cells. After 7 days of coculture, pDC populations were analyzed for CFSE content. Despite a lower percentage and a lower absolute cell count, proliferation of pDCs was not inhibited by DL1 as compared with control cells (Figure 4A). This finding was confirmed in experiments in which cells were labeled with Ki-67 or annexin V, markers for proliferation and apoptosis, respectively. In DL1 cocultures a higher percentage of pDCs are Ki-67+ and thus in cell cycle as compared with control and Jag1 cocultures (Figure 4B). Additionally DL1 did not induce apoptosis in pDCs because the percentage of annexinV+7-AAD- cells was similar compared with the percentages of apoptotic pDCs in control and Jag1 cocultures (Figure 4C). In conclusion, DL1 does not block the development of pDCs by inhibiting proliferation or inducing apoptosis in these cells but instead blocks the differentiation of these cells.
DL1/Notch1 signaling induces up-regulation of GATA-3 and down-regulation of Spi-B
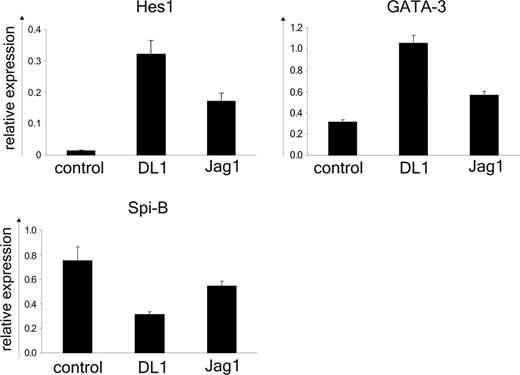
Previously we described an essential role for Spi-B in the development of pDCs.29 Therefore we considered the possibility that Notch1 signaling might negatively regulate the expression of Spi-B in progenitor cells. Following a 24-hour coculture of CD34+CD1a- thymic precursors and OP9 control, OP9-DL1, or OP9-Jag1 cell lines the expression of Spi-B and the Notch1 target gene HES-139 were determined by reverse transcription (RT)-PCR (Figure 5). The mRNA levels were normalized to expression in freshly isolated cells (relative value of 1) using either β-actin or the ribosomal protein p0 as internal controls with similar results. As expected Spi-B was expressed in CD34+CD1a- progenitors cultured on the OP9 control line and, consistent with the absence of Notch1 triggering, low levels of HES-1 mRNA were detected. The pDCs and T cells codeveloped on OP9-Jag1 and in line with this both Spi-B and HES-1 mRNA were present at higher levels in the Jag1-cocultured thymocytes compared with control. However, HES-1 expression was much stronger in the presence of DL1-induced signaling (60-fold versus 10-fold for DL1 and Jag1 cocultures, respectively). This observation confirms reported data by Jaleco et al48 describing a strong induction of HES-1 in DL1, but not Jag1, cocultured CD34+ cord blood progenitor cells and may be a reflection of a weaker Notch signal delivered by Jag1 as compared with DL1. This may explain the delayed kinetics of T-cell development on OP9-Jag1 cells. In agreement with the block of pDC differentiation DL1 reduced the expression of Spi-B about 2.5-fold compared with control cultured cells. In parallel the expression of GATA-3, a factor required for T-cell development28 was determined. Both DL1 and Jag1 maintained the expression of GATA-3 at higher levels compared with control, consistent with the findings presented in Figure 1 showing that both OP9-DL1 and OP9-Jag1 are able to induce T-cell commitment from CD34+CD1a- thymocytes (Figure 5). These data suggest that the DL1/Notch1 pathway regulates the GATA-3/Spi-B balance. This is specific for DL1/Notch1 because the GATA-3/Spi-B mRNA ratio is not altered in the presence of Jag1.
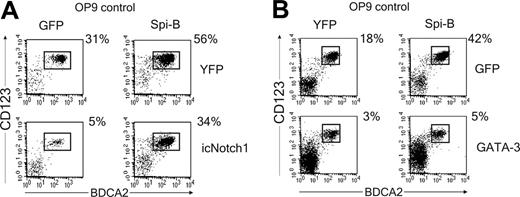
Spi-B rescues the icNotch1-but not GATA-3-induced block in pDC development
Down-modulation of Spi-B by DL1 and the inverse correlation of Notch1 and Spi-B expression patterns suggests that the Notch1 and Spi-B pathways are linked. This may indicate that Spi-B overexpression could rescue DL1/Notch1-induced inhibition of pDC development. To test this hypothesis, icNotch1 and Spi-B were cotransduced in CD34+CD1a- progenitor cells and cultured on OP9 control cells. Consistent with our previously published results, forced expression of Spi-B induced a 2-fold increase in the percentage of pDCs as compared with the control transduced cells7 (Figure 6A). Notably, cotransduction of both Spi-B and icNotch1 completely released the icNotch1-induced block of pDC development, resulting in percentages of pDCs similar to control transduced progenitors (Figure 6A). Given the observation that DL1 induces expression of GATA-3 it is tempting to speculate that the balance between GATA-3 and Spi-B expression determines the developmental outcome of a thymic progenitor cell. To test this hypothesis CD34+CD1a- progenitors were cotransduced with GATA-3 and Spi-B. Similar to the developmental inhibition induced by icNotch1 transduction, GATA-3 expression strongly blocked the development of pDCs (Figure 6B). Unexpectedly Spi-B was unable to overcome the GATA-3-induced block in pDC development (Figure 6B). Interestingly, and in contrast to icNotch1, forced expression of GATA-3 did not induce development of CD4+CD8+ double-positive T cells (data not shown) on OP9 control cells. Taken together, our results indicate that DL1 directs the T/pDC lineage decision by regulation of the downstream lineage-specific factors GATA-3 and Spi-B. Expression of Spi-B may inhibit the DL1/Notch1-induced up-regulation of GATA-3. However, when GATA-3 expression is firmly established, Spi-B is no longer able to redirect precursors toward the pDC lineage.
DL1 does not affect pDC proliferation or apoptosis. (A) CD34+CD1a- cells were labeled with CFSE and cocultured with OP9-DL1, OP9-Jag1, or control cell lines. CFSE content of BDCA2+CD123hi pDCs was analyzed after 1 week of coculture (n = 3). (B) CD34+CD1a- cells were cocultured with OP9-DL1, OP9-Jag1, or control cell lines and analyzed after 1 week of culture for the percentage of Ki-67+ pDCs. Percentages of Ki-67+CD123hiBDCA2+ pDCs above background levels are indicated next to the histograms (n = 2) or (C) percentage apoptotic (annexin V+7-AAD-) pDCs (n = 2). Experiments representative of at least 2 experiments are shown.
DL1 does not affect pDC proliferation or apoptosis. (A) CD34+CD1a- cells were labeled with CFSE and cocultured with OP9-DL1, OP9-Jag1, or control cell lines. CFSE content of BDCA2+CD123hi pDCs was analyzed after 1 week of coculture (n = 3). (B) CD34+CD1a- cells were cocultured with OP9-DL1, OP9-Jag1, or control cell lines and analyzed after 1 week of culture for the percentage of Ki-67+ pDCs. Percentages of Ki-67+CD123hiBDCA2+ pDCs above background levels are indicated next to the histograms (n = 2) or (C) percentage apoptotic (annexin V+7-AAD-) pDCs (n = 2). Experiments representative of at least 2 experiments are shown.
DL1/Notch1 signaling induces up-regulation of GATA-3 and down-regulation of Spi-B. RT-PCR analysis of HES-1, GATA-3, and Spi-B mRNA was performed on CD34+CD1a- cells after 24 hours of coculture with OP9-DL1, OP9-Jag1, or control cell lines. Values were normalized to freshly isolated cells. The ribosomal protein p0 was used as internal control. The error bars represent SDs of triplicate samples. One representative experiment of 4 is shown.
DL1/Notch1 signaling induces up-regulation of GATA-3 and down-regulation of Spi-B. RT-PCR analysis of HES-1, GATA-3, and Spi-B mRNA was performed on CD34+CD1a- cells after 24 hours of coculture with OP9-DL1, OP9-Jag1, or control cell lines. Values were normalized to freshly isolated cells. The ribosomal protein p0 was used as internal control. The error bars represent SDs of triplicate samples. One representative experiment of 4 is shown.
Spi-B rescues pDC development blocked by icNotch1 but not by GATA-3. (A) CD34+CD1a- cells were double transduced with icNotch1 and Spi-B or control virus and cocultured with OP9-control cells (n = 4). (B) CD34+CD1a- cells were double transduced with GATA-3 and Spi-B or control virus and cocultured with OP9-control cells (n = 3). Cultures were analyzed after 1 week for the presence of CD123hiBDCA2+ pDC. Dot plots shown are gated on double transduced GFP+YFP+ cells. Experiments representative of at least 3 experiments are shown.
Spi-B rescues pDC development blocked by icNotch1 but not by GATA-3. (A) CD34+CD1a- cells were double transduced with icNotch1 and Spi-B or control virus and cocultured with OP9-control cells (n = 4). (B) CD34+CD1a- cells were double transduced with GATA-3 and Spi-B or control virus and cocultured with OP9-control cells (n = 3). Cultures were analyzed after 1 week for the presence of CD123hiBDCA2+ pDC. Dot plots shown are gated on double transduced GFP+YFP+ cells. Experiments representative of at least 3 experiments are shown.
Discussion
pDCs can develop within the human thymus from CD34+CD1a- thymic precursors.17 On induction of CD1a expression, which correlates with commitment to the T-cell lineage,49 the thymic precursors lose their capacity to develop into pDCs.16 We recently identified Spi-B as a key regulator of human pDC development. Knocking down Spi-B expression by RNAi impairs the development of pDCs,29 whereas forcing Spi-B expression in hematopoietic progenitor cells blocks the development of non-pDC lineages.7 Here we addressed the question how early T and pDC development are regulated in the thymus, using a murine bone marrow stromal cell line constitutively expressing human DL1, a system previously shown to support murine and human T-cell development.34,50
We show that DL1 blocks the differentiation of human pDCs, consistent with findings in the mouse.51 More importantly, we show that this block is due to triggering of the Notch1 receptor because the γ-secretase inhibitor DAPT rescued pDC development in the presence of DL1. Furthermore, Notch1 is down-regulated when uncommitted CD34+CD1a- thymic precursors differentiate into pDCs. Moreover, mimicking constitutive DL1/Notch1 signaling by forcing the expression of the intracellular part of the Notch1 receptor also strongly blocks development of pDCs and is sufficient to support development of CD4+CD8+ double-positive T cells following coculture with OP9 control cells. This shows that the DL1/Notch1 pathway favors T-cell development at the expense of pDCs. Strikingly, this effect is specific for DL1 because Jag1 supports development of both T cells and pDCs from CD34+CD1a- thymic progenitor cells. This indicates that DL1 has a unique function in the T/pDC lineage decision in the thymus. It is of note that development of human T cells in the presence of Jag1 contrasts with previously reported data in which it was shown that murine T cells are able to develop in the presence of DL1, but not of Jag1.47 The reason for this discrepancy is currently unknown. The DL1-induced block in pDC development was not an exclusive effect on thymic progenitor cells because in 4 of 7 experiments fetal liver precursor cells were also inhibited from developing into pDCs. However, the inhibitory effect of DL1 on fetal liver precursor cells was not statistically significant. This is likely due to the observed donor-to-donor variations because in 2 of 7 experiments we observed stimulation of pDC development. The explanation for this variation (which was never observed with thymic precursors) is unclear, but this may be attributed to differences in up-regulation of GATA-3. Importantly, ectopic expression of GATA-3 in fetal liver CD34+CD38- cells always resulted in a complete inhibition of pDC development (n = 3; R. S. and H. S., unpublished observations, September-October 2000). Because evidence in humans and mice indicates that pDCs can develop from myeloid as well as lymphoid precursors,10,12 it may also be possible that Notch signaling differentially affects development from myeloid or lymphoid precursors, having a much stronger effect on pDC development from lymphoid precursors. The variability in the effects of DL1 on pDC development from fetal liver precursors may be due to variability of myeloid- or lymphoid-biased precursors. It is of note that data in the mouse also indicate that development of pDCs from extrathymic precursors can be inhibited by DL1.51 Consistent with our observations Ferrero et al52 showed that murine Notch1-deficient bone marrow cells are fully capable of normal pDC development, thereby confirming that pDC development is not dependent on Notch1 signaling. Interestingly, the absolute numbers, pDCs that developed from Notch1-deficient precursor cells, were slightly (∼ 2-fold) increased. The authors of this paper speculate that this may be caused by the initial 2:1 (CD45.2/CD45.1) ratio in which the bone marrow chimeras were set up.52 It is, however, also possible that removal of Notch1 resulting in a release of Notch-mediated inhibition contributes to this stimulation.
Within the murine thymus DL1 seems to be expressed on cortical stromal cells but absent on their medullar counterparts.53 The localization of pDCs in the thymus inversely correlates with the expression of DL1 because pDCs are present in the medulla but not in the cortex.8 This supports our finding that Notch1 signaling directs the T/pDC switch in favor of the T cells; early T-cell development occurs in the cortex in the presence of DL1, whereas pDCs develop in the medulla were DL1 is absent. Thus, the different anatomic localization of pDCs and early T cells may be, at least in part, controlled by the distribution of DL1 expression in the thymic microenvironment. The physiologic reason for the physical separation of early T cells and pDCs remains unclear. It has been observed that IFNs induce differentiation and subsequent apoptosis of cortical thymic epithelial.54 A mechanism is proposed in which virally infected cells or activated leukocytes or both secrete IFNs and thereby contribute to the thymic atrophy and impaired T-cell development seen during many infections. On stimulation pDCs are able to produce vast amounts of IFN-α/β,3,9,55 and if present in the thymic cortex, activated pDCs could induce disruption of the cortical epithelium and thereby disturb development of T cells. The spatial segregation of pDCs and developing T cells imposed by DL1/Notch1 signaling may prevent such deleterious effects. This allows for production of IFNs within the thymic medulla, which may be important for preventing viral infections of thymocytes.15
Gene expression analysis revealed the mechanism by which the DL1/Notch1 signaling pathway controls the T/pDC lineage decision. We found that DL1 maintains GATA-3 expression and induced a 2.5-fold reduction in the expression of Spi-B in thymic precursors compared with OP9-control cells. This observation is consistent with our previously published observation that high Spi-B expression is incompatible with T-cell development.7 More importantly, these data strongly suggest that DL1 specifically directs the T/pDC lineage decision in the human thymus by regulating the balance between GATA-3 and Spi-B. Activation of the DL1/Notch1 pathway shifts the GATA-3/Spi-B equilibrium toward GATA-3 and favors T-cell development. In the absence of DL1/Notch1 signaling, the balance shifts toward Spi-B and supports pDC development. In addition, our data show that expression of Spi-B prior to T-cell commitment imposes a pDC developmental program on thymic precursors, perhaps by preventing GATA-3 induction. However, once expression of GATA-3 is established, Spi-B is no longer able to redirect precursors toward the pDC lineage. The identification of a single receptor in control of the T/pDC lineage decision supports the idea that these 2 cell types, at least in the thymus, share a common precursor. In conclusion, we show here that the DL1/Notch1 signaling pathway controls the T/pDC lineage decision in the thymus by regulation of the lineage-specific genes GATA-3 and Spi-B either directly or indirectly. DL1 triggering induces T-cell development, whereas pDC differentiation is impaired through down-modulation of Spi-B.
Prepublished online as Blood First Edition Paper, November 29, 2005; DOI 10.1182/blood-2005-05-2090.
Supported by the Netherlands Organization for Science (NWO), grant ALW 805-17531, and the Landsteiner foundation, grant LSBR 0203.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Berend Hooijbrink for his help with FACS sorting and maintenance of the FACS facility. Dr M. Hazekamp and staff at the Leiden University Medical Center and the Amsterdam Medical Center are acknowledged for providing postnatal thymus tissue. We thank Dr K. Weijer and the staff of the Bloemenhove Clinic in Heemstede, The Netherlands, for providing fetal liver. Drs J. C. Zúñiga-Pflücker and R. La Motte-Mohs (University of Toronto, Toronto, ON, Canada) are thanked for their help with the OP9 coculture assays.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal