Abstract
Natural killer (NK) cells express an array of activating receptors that associate with DAP12 (KARAP), CD3ζ, and/or FcRγ ITAM (immunoreceptor tyrosine-based activation motif)–bearing signaling subunits. In T and mast cells, ITAM-dependent signals are integrated by critical scaffolding elements such as LAT (linker for activation of T cells) and NTAL (non–T-cell activation linker). Using mice that are deficient for ITAM-bearing molecules, LAT or NTAL, we show that NK cell cytotoxicity and interferon-γ secretion are initiated by ITAM-dependent and -independent as well as LAT/NTAL-dependent and -independent pathways. The role of these various signaling circuits depends on the target cell as well as on the activation status of the NK cell. The multiplicity and the plasticity of the pathways that initiate NK cell effector functions contrast with the situation in T cells and B cells and provide an explanation for the resiliency of NK cell effector functions to various pharmacologic inhibitors and genetic mutations in signaling molecules.
Introduction
Natural killer (NK) cells participate in innate immune responses by virtue of their ability to recognize, without prior specific sensitization, microbe-infected, transformed, and allogeneic cells, while sparing most autologous healthy cells.1-4 NK cell activation can elicit 2 different effector functions: cytotoxicity of target cell and/or secretion of a large array of cytokines and chemokines.5 NK cell activation is regulated by the dynamic integration of negative and positive signals.6 Although the ligands, the mode of action, and the function of inhibitory NK receptors have been widely dissected,7 the biologic function of NK cell–activating receptors as well as their downstream signaling circuits are only partially understood.
Noncovalent association with signaling ITAM-bearing subunits is required for the function and often the stable cell-surface expression of several NK cell–activating receptors. In NK cells, ITAM-dependent activating receptors can be divided in 2 groups, according to their association with DAP12 (also known as KARAP and TYROBP), or with CD3ζ or FcRγ ITAM-bearing polypeptides.4,8 In humans, activating killer cell Ig-like receptors (KIR-S), CD94/NKG2C-E, and the NKp44 NCR (natural cytotoxicity receptor) associate with DAP12, whereas the NKp46 and NKp30 NCRs as well as the CD16 pair with CD3ζ and FcRγ. In mice, DAP12-dependent NK cell receptors include the activating Ly49 molecules, CD94/NKG2C-E, NKG2D-S, CD200R4, and PIRL-β, whereas mouse NCR NKp46 (MAR-1), as well as CD16 and NKRP1-C, associates with FcRγ. Among these molecules, human NCRs are involved in natural cytotoxicity against several tumor cell lines9 and have also been reported to interact with viral products.10-12 NKG2D is an activating receptor expressed on both human and mouse NK and T cells, which recognizes nonclassic MHC class I molecules. The cell-surface expression of these ligands is often the consequence of cellular stress, such as DNA damage.13,14 NKG2D ligands include MIC (MHC class I chain related) and ULBP (UL-16 binding protein) molecules in humans as well as Rae (retinoic acid early inducible 1), H60, and MULT-1 (murine UL16-binding protein-like transcript) proteins in mice. Alternative splicing of mouse Nkg2d, also known as Klkr1, generates 2 different transcripts: a long isoform, NKG2D-L, which associates only with the DAP10 subunit, as previously reported for human NKG2D, and a short one, NKG2D-S, which can couple with both DAP10 and DAP12.15,16 Analysis of DAP12-deficient mice has pointed out that 2 DAP12-dependent mouse NK cell receptors, NKG2D-S and Ly49H, are involved in antitumor and antiviral NK cell activity, respectively.15,17
Engagement of ITAM-dependent receptors initiates in NK cells a signal transduction cascade recapitulating some of the early steps that have been dissected in T cells.6,18,19 First, members of src protein tyrosine kinase (PTK) family induces phosphorylation of both ITAM tyrosine residues, allowing the consequent recruitment and activation of Syk and/or ZAP70 PTK.20,21 ZAP70 and Syk are both expressed by NK cells and function redundantly in activating NK cell effector functions.22 In T cells, B cells, and mast cells, activated ZAP70/Syk phosphorylate several key signaling substrates, including transmembrane adaptor proteins (TRAP), such as LAT (linker for activation of T cells) and NTAL (non–T-cell activation linker; also known as LAB, linker for activation of B cells).23-26 TRAPs do not harbor any enzymatic activity per se but act as membrane-associated scaffolding proteins that recruit to the cell membrane several cytosolic-transducing elements, thereby allowing their activation on phosphorylation by PTKs. TRAPs thus integrate and propagate ITAM-dependent signals that culminate in cellular activation. Besides TCR, LAT is involved in cell signaling mediated by activating receptors, such as CD16, CD2, and 2B4 in human NK cells19 ; FcϵRI in mast cells25 ; and GPVI (glycoprotein VI) in platelets,27 whereas NTAL has been reported to couple with B-cell antigen receptor (BCR) in B cells23 and FcϵRI in mast cells.25
Both LAT and NTAL are expressed in human and mouse NK cells,23,24,28 and no alteration of NK cell cytolytic function has been reported in LAT-deficient mice.28 However, it is possible that LAT and NTAL function redundantly in activating NK cell effector function. Along these lines, mast cells with a combined LAT and NTAL deficiency are impaired in signals emanating from FcRγ-dependent FcϵRI engagement, although LAT and NTAL can play, respectively, a positive and a negative role on ITAM-dependent pathways.29,30
Studies using ZAP70/SykKO hematopoietic chimeras have shown that NK cell development and cytotoxicity against various cell targets is unchanged in the absence of these PTKs, in contrast to their mandatory role in T and B cells.22 Using mice that are deficient for DAP12, FcRγ, and CD3ζ alone or in combination, as well as mice that are deficient for LAT and NTAL, we analyzed here the effect in NK cell development and effector function of signaling components that are directly upstream and downstream of ZAP70/Syk.
Materials and methods
Mice
DAP12 knock-in (DAP12KI) mice, generated as previously described, were backcrossed 9 times with C57BL/6 mice.31 DAP12KO mice were kindly provided by Dr T. Takai.32 C57BL/6 mice were purchased from Charles River Laboratories, L'Arbesle, France, and were used as controls for both DAP12KI and DAP12KO mice. C57BL/6 RAG-2/γc double KO mice were obtained from CDTA (Centre de Développement des Techniques Avancées pour l'expérimentation animale, Orleans, France). CD3ζ/FcRγ/RAG (ZGR) triple KO mice have been obtained by breeding CD3ζ/FcRγKO, previously backcrossed 8 times into C57BL/6 mice, with C57BL/6 RAG-1KO (RAGKO) mice.31 ZGKR mice have been obtained by breeding ZGR and DAP12KI mice. RAGKO mice were used as controls for both ZGR and ZGKR mice. LATKO and NTALKO, as well as wild-type (+/+) control mice, derive from interbreeding of +/– mutant mice backcrossed twice with C57BL/6 mice. Double LAT/NTALKO mice have been generated by interbreeding of single LATKO and NTALKO mice.23,29,33,34 Wild-type littermates were used as controls (CTRs). In all the experiments reported mutant mice as well as their respective controls were age- and sex-matched. All experiments have been performed in accordance with protocols approved by national regulation and the European Directives.
MAb, reagents, and cytometric analysis
Unless indicated, all antibodies were purchased from BD Pharmingen, Le Pont de Claix, France. FITC-conjugated anti-CD11b mAb was from Beckman Coulter, Villepinte, France. Biotin-conjugated anti-NKG2D was from e-Bioscience, Montrouge, France. Before staining, cells were preincubated for 10 minutes at 4°C with supernatant of anti-CD16, 2.4G.2, hybridoma. For intracellular IFN-γ staining, indicated extracellular staining was performed after 4 hours of cell incubation with Golgi Stop provided by BD Pharmingen. Samples were then fixed with 4% paraformaldehyde, and PE-conjugated anti–IFN-γ antibody was added in the presence of Perm/Wash 1 × solution (BD Pharmingen). Anti-NTAL NAP-07 mAbs were from Alexis Biochemical, Paris, France, whereas anti-LAT mAbs used for intracellular staining (B32.8.1) were generated by B. Malissen (unpublished data, January 2005).
NK cell preparation and target cells
For polyIC effectors, mice were injected intraperitoneally 18 hours before killing with 200 μg polyIC (polyinosinic-polycytidylic acid)/mouse. Once harvested, spleens of polyIC-stimulated mice were treated for 20 to 30 minutes at 37°C with liberase-collagenase cocktail (Roche Diagnostics, Meylan, France), then depleted of splenocytes expressing CD3, CD4, or CD8, as previously described.15 NK cells were then enriched by positive DX5 selection using autoMACS (magnetic-activated cell sorting) system (Miltenyi, Paris, France). The resulting population contained 70% to 80% CD3– NK1.1+ cells. In the case of RAGKO, ZGR, and ZGKR mice, cells were subjected to only the DX5 positive selection step, yielding an 85% to 95% pure CD3–NK1.1+ population. For lymphokine-activated killer (LAK) cell generation, total splenocytes were cultured in complete RPMI 1640 medium supplemented with NaPyruvate, nonessential MEM amino acids (1 mM; Gibco, Cergy Pontoise, France), β-mercaptoethanol (50 μM) and 4000 UI/mL rhIL-2 Proleukin (Chiron, Emeryville, CA). Medium was changed at day 3 and at day 5 to 6; adherent cells were collected. When necessary, LAK cells were then depleted of cells expressing CD3, CD4, or CD8, yielding a 70% to 80% pure CD3–NK1.1+ population. In the case of RAGKO, ZGR, and ZGKR mice, LAK cells obtained after culture were already 85% to 95% CD3–NK1.1+.
Tumor cell lines were all cultured in RPMI 1640 medium supplemented with antibiotics, glutamine, and β-mercaptoethanol (50 μM). YAC-1 (H-2a, T-cell lymphoma), RMA, and RMA-S (H-2b, T-cell lymphomas, respectively, MHC+ or MHClow), IC-21 (H-2b, macrophage cell line), as well as B16 and B16-expressing Rae-1β (H-2b, melanoma), were previously described.15,31 MCA-205 (H-2b, fibrosarcoma) and MC38 (H-2b, colon carcinoma) and C1498 (H-2b, T-cell lymphoma) were kind gifts from L. Zitvogel and F. Takei, respectively. IL-3–dependent BaF3 and BaF3-m157 murine pro B-cell lines were a kind gift of W. Yokoyama. All tumor cell lines used were tested as mycoplasma free (Mycoplasma detection kit; Roche, Mannheim, Germany).
Cytotoxicity assay
PolyIC-stimulated NK cells or LAK cells obtained as previously described were used as effectors and incubated at the indicated effector-target ratio for 4 hours at 37°C with different tumor cell lines, previously labeled with 51Cr, in a final volume of 200 μL complete RPMI. For redirected killing experiments, NK cell effectors were incubated with the FcR+ human tumor B-cell line, Daudi, in the presence of anti-Ly49D (SED85, mIgG1), kindly provided by D. Raulet, or of respective isotype control mAbs at a final concentration of 20 μg/mL. After incubation, cells were centrifuged, and 100 μL each culture supernatant was collected. Released 51Cr was measured in a Packard γ-counter (Perkin Elmer, Boston, MA). Data are expressed as mean plus or minus SD of triplicate samples.
Generation of mixed bone marrow chimeras
Sublethally irradiated (300 rad [3 Gy]) alymphoid mice RAG-2/γc double KO mice were reconstituted by intravenous injection under anesthetic with a mixture 1:1 of bone marrow cell precursors isolated from RAGKO and ZGR mice and analyzed at least 4 weeks after reconstitution.
In vivo experiments
Groups of 4 to 6 mice were used for the metastasis assay. All mice were between 8 and 16 weeks of age. B16, B16-H60, or B16-Rae1β melanoma cells (3 × 105) were injected intravenously in the retro-orbital sinus of anesthetized mice.35 Lung metastases were evaluated 15 days after injection.
Quantitative RT-PCR and immunoblotting
Total RNA was isolated from indicated cells using RNAeasy Minikit complemented by DNAse Rnase-free treatment (Qiagen, Courtaboeuf, France). cDNAs were generated using 0.1 μg corresponding RNA according to the Superscript II Reverse Transcriptase (RT) protocol and real-time polymerase chain reaction (PCR) was performed, as described.34
For immunoblotting, NK cells were purified, as described under “NK cell preparation and target cells,” from spleens of unstimulated C57BL/6 mice. CD4+ T cells as well as B220+ B cells were obtained by positive selection after incubation with CD4 beads (Miltenyi) or biotinylated anti-B220 mAb plus streptavidin beads (Miltenyi), respectively. Cells were lysed in 80 μL boiling 2 × Laemmli lysis buffer (SDS 4%, Tris 120 mM, pH 6.8) for 5 minutes. Lysates were resolved by 12% acrylamide sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions. Western blots were probed with anti-LAT mAb, 1D1, kindly provided by P. Draber, or anti-NTAL NAP-07 mAb. Detection was performed using enhanced chemiluminescence (ECL) plus detection system (Amersham, Buckinghamshire, United Kingdom).
LAK stimulation for cytokine secretion
Ninety-six–well U-bottom plates were coated overnight with the following mAbs; anti-Ly49D (4E5, 50 μg/mL) or irrelevant control rat IgG2a (50 μg/mL), both diluted in carbonate buffer. Before use, antibody-coated plates were washed 4 times with complete medium. Purified LAK NK cells (1 × 105) cells/well were plated in duplicate samples on antibody-coated wells in the presence of 1000 UI/mL rhIL-2 (Proleukin; Chiron, Emeryville, CA). Cell supernatant cultures were harvested 24 hours after incubation, and mouse IFN-γ contents were then evaluated by enzyme-linked immunosorbent assay (ELISA).
Results
NK cell maturation in DAP12, FcRγ, and CD3ζ polypeptide-deficient mice
All known human and mouse ITAM-dependent NK cell–activating receptors are associated with either DAP12 or with CD3ζ and/or FcRγ.4-6,8 NK cells deficient for these 3 signaling subunits thus represent an adequate model to evaluate the relevance of ITAM-dependent NK cell–activating receptors in NK cell differentiation and activation. We have previously reported a defect in NK cell cytotoxicity in CD3ζ/FcRγKO mice,31 which could be the consequence of intestinal bowel disease (IBD) that develops in these mice.22,36 To prevent the generation of autoreactive T and B lymphocytes responsible of IBD, CD3ζ/FcRγKO mice were thus bred with RAGKO mice to generate ZGR mice (Figure S1A; see the Supplemental Figures link at the top of the online article, at the Blood website). ZGR mice were crossed to loss-of-function DAP12KI mice, to obtain ZGKR mice.
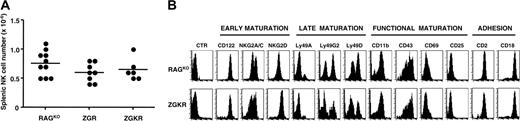
Analysis of splenic NK cells in ZGKR mice. (A) The number of NK cells per spleen isolated from individual mice of the indicated genotype is presented. The numbers were calculated based on the number of splenocytes and the percentage of CD3–MHC class II–NK1.1+ cells present in the lymphocyte gate. Dots indicate individual mice; horizontal bars, mean values. No statistically significant differences were observed with analysis by unpaired Student t test (data not shown). (B) Expression of the indicated NK cell markers was analyzed on gated CD3–MHC class II–NK1.1+ splenocytes isolated from indicated mice. Represented mice also express comparable levels of Ly49 C/I receptors (data not shown). Data are representative of 3 independent experiments (CTR indicates negative control mAb).
Analysis of splenic NK cells in ZGKR mice. (A) The number of NK cells per spleen isolated from individual mice of the indicated genotype is presented. The numbers were calculated based on the number of splenocytes and the percentage of CD3–MHC class II–NK1.1+ cells present in the lymphocyte gate. Dots indicate individual mice; horizontal bars, mean values. No statistically significant differences were observed with analysis by unpaired Student t test (data not shown). (B) Expression of the indicated NK cell markers was analyzed on gated CD3–MHC class II–NK1.1+ splenocytes isolated from indicated mice. Represented mice also express comparable levels of Ly49 C/I receptors (data not shown). Data are representative of 3 independent experiments (CTR indicates negative control mAb).
Splenic absolute NK cell numbers were not significantly altered in either ZGR or ZGKR mice, as compared with controls (Figure 1A), despite reduced NK cell percentages (Figure S1B). Mixed bone marrow experiments unambiguously show that altered NK cell percentages are an indirect consequence of CD3ζ/FcRγ deficiency on the microenvironment in which NK cells develop (Figure S1C). However, the phenotype of NK cells in ZGKR mice, which corresponds to a fully mature NK cell phenotype,37 was not significantly modified in comparison to control mice (Figure 1B). Yet, NKG2D expression is up-regulated in ZGKR mice, as previously reported for DAP12KI mice.15 Taken together, these findings indicate that CD3ζ, FcRγ, and DAP12 are dispensable, alone and in combination, for the differentiation of mature mouse splenic NK cells.
Multiplicity of NK cell–activating pathways
NK cells isolated from ZGKR mice were used as cytolytic effectors against a wide panel of tumor cell lines. Because of altered NK cell percentages detected in ZGR and ZGKR mice (Figure S1B), only purified splenic NK cells from polyIC-stimulated control and mutant mice were used (polyIC NK cells). Lysis of YAC-1 lymphoma cells by NK cells from ZGKR mice was only marginally reduced compared with NK cells from control mice (Figure 2A, top). However, the lysis of B16 melanoma cells expressing the NKG2D ligand Rae1β (B16-Rae1β) was substantially reduced when NK cells were derived from ZGKR mice (Figure 2A, middle). RMA (MHC class I+) and RMA-S (MHC class Ilow) are also less efficiently killed by NK cells isolated from ZGKR mice, although ZGKR NK cells, like wild-type NK cells, killed RMA-S better than RMA cells (Figure 2A, bottom). “Missing self-recognition” is thus operative in NK cells isolated from ZGKR mice, despite the likely involvement of an ITAM-dependent receptor in the recognition of both RMA and RMA-S.
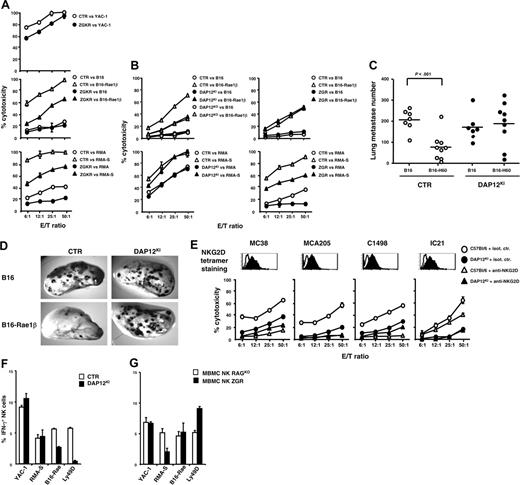
The evidence that ITAM-dependent receptors play roles in cytotoxicity of RMA/RMA-S and B16-Rae1β cells prompted us to further dissect the involvement of individual ITAM-bearing subunits in NK cell cytotoxicity by examining the activity of polyIC NK cells generated from DAP12-deficient and ZGR mice. ZGR NK cells killed B16-Rae1β melanoma cells normally, but NK cells lacking the DAP12 ITAM (from DAP12KI mice) or the DAP12 molecule (from DAP12KO mice) exhibited reduced capacity to lyse B16-Rae1β melanoma cells (Figure 2B). Consistently with the involvement of DAP12 in the killing of NKG2D-ligand+ tumor cells, DAP12KI mice present a severe in vivo defect in their ability to reject in vivo B16 melanoma cells expressing H60 or Rae-1β NKG2D ligands, as compared with untransduced B16 cells (Figure 2C-D). Similarly, compared with wild-type NK cells, DAP12-deficient NK cells exhibited reduced capacity to kill several tumor cell lines that naturally express various NKG2D ligands at different levels (Figure 2E), such as MC38, MCA205, C1498, and IC-21 (Figure 2E). In 3 of 4 cases (MC38, MCA205, C1498), anti-NKG2D mAb severely blocked the lysis mediated by control NK cells, confirming the role of this receptor in the lytic process. But anti-NKG2D only minimally inhibited the lysis of the macrophage tumor cell line IC-21, indicating the involvement of a distinct DAP12-dependent receptor in lysis of these cells (Figure 2E). In contrast, NK cell cytotoxicity of all these tumor cells was not affected by the absence of CD3ζ and FcRγ (data not shown). Reciprocally, lysis of RMA and RMA-S target cells was partially dependent on CD3ζ and FcRγ signaling but was not dependent on DAP12 (Figure 2B). In summary, depending on the tumor cell tested, cytotoxicity is either largely independent of ITAM (eg, YAC-1), requires DAP12 (eg, tumor cell lines expressing NKG2D ligands and IC21), or requires CD3ζ and/or FcRγ (eg, RMA and RMA-S). These results emphasize the multiplicity of NK cell–surface stimulatory receptors that NK cells can use to recognize tumor cells, and the coupling of these receptors to distinct ITAM-dependent or -independent signaling pathways.
Role of ITAM-bearing subunits in NK cell effector functions. (A-B) polyIC NK cells were purified from control (CTR) and mutant mice and used as effectors against indicated tumor cells. Data shown are representative of at least 4 independent experiments. (C-D) Mice were injected intravenously with 3 × 105 cells of B16, B16-H60, or B16-Rae1β melanoma cells, and total lung metastases were counted 2 weeks after tumor challenge. Dots indicate data from individual mice: ○, control mice (CTR); •, DAP12KI mice. Horizontal bars indicate mean values. Statistical analysis was performed using unpaired Student t test. (E) NKG2D tetramer staining (filled histograms) is represented in comparison with staining with an irrelevant KIR2DL3 tetramer (empty histograms).63 Cytotoxicity experiments were performed as described in panels A and B. Blocking experiments were performed by adding anti-NKG2D (MI-6, rat IgG2a) or isotype control mAb at a final concentration of 50 μg/mL, during the 4-hour cytotoxicity assay. (F) Percentages of IFN-γ+ cells in gated CD3–NK1.1+ cells; □ indicate polyIC NK cells from C57BL/6 mice; ▪, polyIC NK cells from DAP12KI mice. IFN-γ+ NK cells were not detectable in cells stimulated with B16 melanoma cells. (G) Percentages of IFN-γ+ cells in mixed bone marrow chimera reconstituted with RAGKO and ZGR precursors were analyzed on NK cell subsets phenotypically defined, as described in legend to Figure S1; □ indicate percentages of IFN-γ+ cells gated in CD3–NK1.1bright2B4+ (NK cells from RAGKO); ▪, percentages of IFN-γ+ cells gated in CD3–NK1.1dim2B4– (NK cells from ZGR mice). Stimulation with target cells was performed at an effector-target (E/T) ratio of 1:1. Ly49D triggering was performed on high-affinity 96-well plates (Immulon, Labsytems, VWR, France) precoated with isotype control antibodies or with anti-Ly49D (4E5) at the final concentration of 50 μg/mL. Values obtained with control antibodies were subtracted from values obtained with Ly49D stimulation. Data are representative of at least 2 experiments. Error bars represent SEM.
Role of ITAM-bearing subunits in NK cell effector functions. (A-B) polyIC NK cells were purified from control (CTR) and mutant mice and used as effectors against indicated tumor cells. Data shown are representative of at least 4 independent experiments. (C-D) Mice were injected intravenously with 3 × 105 cells of B16, B16-H60, or B16-Rae1β melanoma cells, and total lung metastases were counted 2 weeks after tumor challenge. Dots indicate data from individual mice: ○, control mice (CTR); •, DAP12KI mice. Horizontal bars indicate mean values. Statistical analysis was performed using unpaired Student t test. (E) NKG2D tetramer staining (filled histograms) is represented in comparison with staining with an irrelevant KIR2DL3 tetramer (empty histograms).63 Cytotoxicity experiments were performed as described in panels A and B. Blocking experiments were performed by adding anti-NKG2D (MI-6, rat IgG2a) or isotype control mAb at a final concentration of 50 μg/mL, during the 4-hour cytotoxicity assay. (F) Percentages of IFN-γ+ cells in gated CD3–NK1.1+ cells; □ indicate polyIC NK cells from C57BL/6 mice; ▪, polyIC NK cells from DAP12KI mice. IFN-γ+ NK cells were not detectable in cells stimulated with B16 melanoma cells. (G) Percentages of IFN-γ+ cells in mixed bone marrow chimera reconstituted with RAGKO and ZGR precursors were analyzed on NK cell subsets phenotypically defined, as described in legend to Figure S1; □ indicate percentages of IFN-γ+ cells gated in CD3–NK1.1bright2B4+ (NK cells from RAGKO); ▪, percentages of IFN-γ+ cells gated in CD3–NK1.1dim2B4– (NK cells from ZGR mice). Stimulation with target cells was performed at an effector-target (E/T) ratio of 1:1. Ly49D triggering was performed on high-affinity 96-well plates (Immulon, Labsytems, VWR, France) precoated with isotype control antibodies or with anti-Ly49D (4E5) at the final concentration of 50 μg/mL. Values obtained with control antibodies were subtracted from values obtained with Ly49D stimulation. Data are representative of at least 2 experiments. Error bars represent SEM.
We analyzed in parallel the involvement of ITAM-bearing polypeptides in NK cell production of IFN-γ, another hallmark of NK cell effector functions. IFN-γ secretion by NK cells on stimulation with B16-Rae1β was significantly reduced for DAP12KI NK cells as compared with controls, whereas no alteration was observed on stimulation with YAC-1 and RMA-S (Figure 2F). As expected, IFN-γ secretion induced by mAb triggering of the DAP12-associated Ly49D receptor was also abolished in DAP12KI NK cells (Figure 2F). To extend this analysis to intrinsic NK cell CD3ζ/FcRγ deficiency, we took advantage of mixed bone marrow chimeras reconstituted with bone marrow cells derived from RAGKO and ZGR mice. NK cells derived from both RAGKO and ZGR precursors were similarly activated by YAC-1, B16-Rae1β, and plate-bound anti-Ly49D mAb, but the IFN-γ response to ZGR NK cells was impaired in response to RMA-S cells (Figure 2G). Thus, when NK cell cytotoxicity against a tumor target depends on DAP12, CD3ζ,orFcRγ, NK cell IFN-γ secretion induced by the same target is also dependent on the same signaling subunit. Reciprocally, tumor targets that are killed via ITAM-independent pathways (eg, YAC-1) also induce IFN-γ secretion via ITAM-independent pathways.
Plasticity of NK cell activating pathways
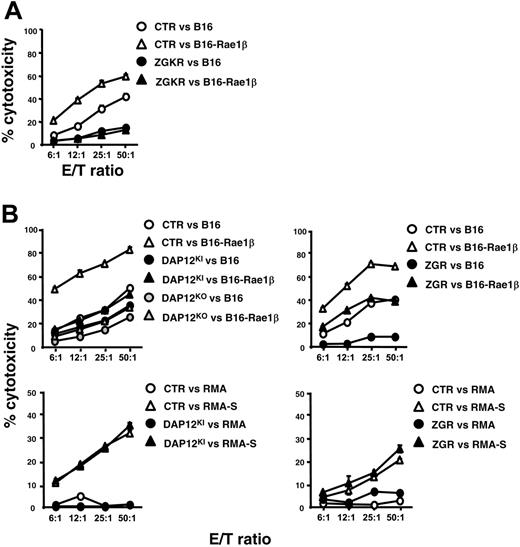
Cytokines are well-known modulators of NK cell effector functions.38 In particular, IL-2–stimulated NK cells (LAK cells) are characterized by an increased ability to kill target cells. We thus investigated whether the signaling circuits involved in NK cell effector function are modified between polyIC NK cells (Figure 2) and LAK cells (Figure 3). LAK cells prepared from ZGKR mice had a profound defect in their ability to kill both B16 and B16-Rae1β tumor cell lines, as compared with control LAK cells derived from RAGKO mice (Figure 3A). Further analysis showed that lysis of B16 cells was reduced only modestly from control levels using LAK cells from 2 distinct DAP12 mutant models (DAP12KI and DAP12KO mice), but lysis of B16-Rae1β cells was greatly reduced using either type of DAP12-mutant LAK cells (Figure 3B). By contrast, lysis of B16 cells was severely reduced using LAK cells from ZGR mice, but the degree of enhanced killing resulting from Rae1β expression in B16 cells was similar for ZGR and control LAK cells (Figure 3B). These findings indicate that B16-Rae1β killing by LAK effectors results from NK cell activation by 2 distinct ITAM-dependent pathways: a CD3ζ/FcRγ-dependent pathway for the lysis of B16 and a DAP12-dependent pathway for lysis dependent on NKG2D ligand (Rae1β) recognition. Remarkably, the killing of both RMA and RMA-S was not affected in any ITAM-mutant LAK cells analyzed, including ZGKR mice (Figure 3B; data not shown), contrasting with the CD3ζ/FcRγ dependency observed for polyIC NK cells (Figure 2A-B). Thus, NK cells use a distinct combination of receptor-ligand pairs to kill the same tumor cells according to their status of activation. In these experimental conditions, stimulation of LAK cells obtained from control mice with RMA-S or B16-Rae1β did not induce any detectable IFN-γ secretion, preventing a comparison with data obtained using polyIC NK cells (Figure 2F-G).
Role of ITAM-bearing subunits in LAK cytotoxicity. (A-B) LAK cells were purified from control and mutant mice and used as effectors against indicated tumor cells. Data are representative of 3 independent experiments. LAK effectors were consistently found to kill RMA tumor cells less well than polyIC-stimulated NK cells did.
Role of ITAM-bearing subunits in LAK cytotoxicity. (A-B) LAK cells were purified from control and mutant mice and used as effectors against indicated tumor cells. Data are representative of 3 independent experiments. LAK effectors were consistently found to kill RMA tumor cells less well than polyIC-stimulated NK cells did.
Expression pattern and role of LAT and NTAL during mouse NK cell maturation
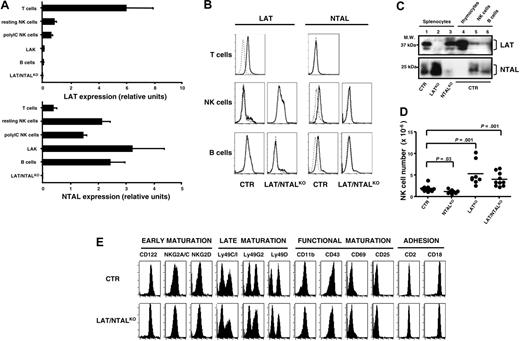
We next investigated the expression and the role of TRAPs in NK cell effector function, because TRAPs integrate ITAM-dependent signals. Quantitative RT-PCR analysis shows that LAT transcripts are present in NK cells, although at considerably reduced levels as compared with T lymphocytes (Figure 4A, top). Interestingly, LAT expression was down-regulated in LAK cells, as compared with resting and polyIC effectors. In contrast, NTAL is well expressed in all NK cell populations analyzed and at levels comparable to those detected in mature B lymphocytes (Figure 4A, bottom). These results were confirmed by intracellular staining and immunoblot analysis (Figure 4B-C). LAT and NTAL are thus both expressed in mouse NK cells as reported earlier.23,24,28 However, their level of expression is different, and IL-2 stimulation down-modulates LAT expression in NK cells.
NK cells were present in all lymphoid organs and peripheral tissues that we examined from single LATKO,NTALKO, and double LAT/NTALKO mice (data not shown), as shown for the spleen in Figure 4D. Moreover, LATKO and LAT/NTALKO mice exhibited an increased number of their splenic NK cell numbers, as compared to their respective controls (Figure 4D). This augmentation may be the consequence of NK cell homeostatic proliferation because of the defective T-cell development in LATKO mice.28,39,40 Consistent with this hypothesis, a similar increase in the size of the NK cell compartment was detected in CD3ϵKO mice (data not shown), consistent with earlier findings.41 No defect in NK cell-surface phenotype was detected in LATKO, NTALKO (data not shown), and LAT/NTALKO mice (Figure 4E), indicating that deficiency in these transmembrane adaptors does not affect splenic NK cell development and maturation.
Role of LAT and NTAL in NK cell effector functions
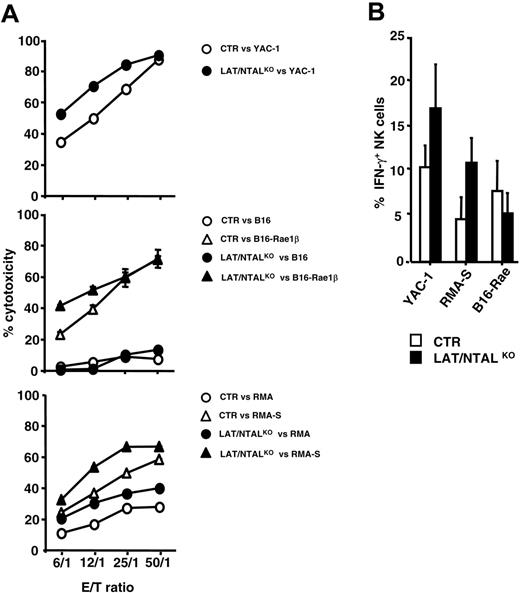
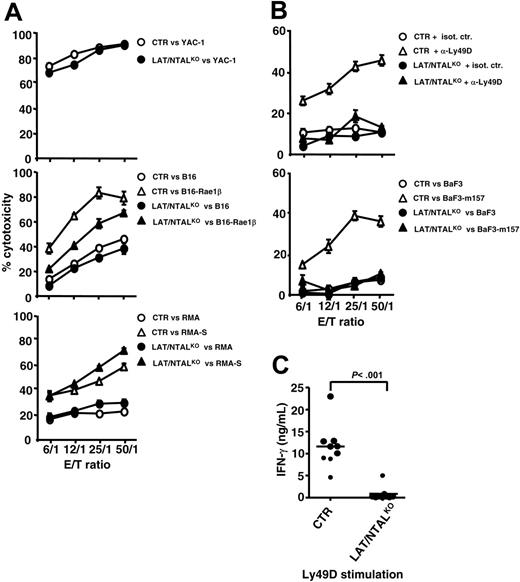
Purified polyIC NK cells obtained from LAT/NTALKO mice kill YAC-1 target cells, consistent with the ITAM-independence of natural cytotoxicity against this tumor cell line (Figure 5A). PolyIC NK cells obtained from LAT/NTALKO mice also kill B16-Rae1β, RMA, and RMA-S as efficiently as control effectors (Figure 5A), despite the partial involvement of ITAMs in that process (Figure 2). If anything, natural cytotoxicity against RMA and RMA-S was slightly more efficient when NK cells were prepared from LAT/NTALKO mice as compared with wild-type control mice. Cytokine production induced by YAC-1 and RMA-S tumor cell lines was not significantly affected by LAT/NTAL deficiency (Figure 5B), whereas a slight decrease of IFN-γ+ NK cells was detected for LAT/NTALKO effectors when incubated with B16-Rae1β. LAK cells obtained from LAT/NTALKO mice also kill YAC-1, B16, and RMA-S (Figure 6A). These data suggest that LAT and NTAL do not play a mandatory role for NK cell cytotoxicity and cytokine secretion induced by any of these tumor cell lines.
LAT and NTAL are expressed in mouse NK cells but are dispensable for splenic NK cell maturation. (A) Lat and Ntal expression levels are expressed as relative units of indicated mRNA normalized using HPRT transcripts. Samples included purified CD4+ T cells (T), sorted (CD3–CD4–CD8–DX5+) NK cells isolated from unstimulated (NK resting) and polyIC stimulated (NK polyIC) mice, sorted (CD3–NK1.1+) LAK, enriched B220+ B cells (B), all derived from C57BL/6 mice, or total splenocytes isolated from double LAT/NTALKO mice. Data (mean ± SD) are representative of 3 independent experiments. (B) LAT and NTAL expression was analyzed on C57BL/6 (CTR) and LAT/NTALKO spleen lymphocytes by intracellular staining gating on T cells (CD3+NK1.1–), NK cells (CD3–NK1.1+), and B cells (CD3–NK1.1–). Staining performed with isotype control mAb (dotted line) has been overlaid to staining obtained using specific anti-LAT or anti-NTAL mAb (bold line). Data shown are representative of 3 independent experiments. (C) LAT and NTAL expression was analyzed by immunoblotting on lysates prepared from (1) 5 × 106 total C57BL/6 splenocytes, (2) 5 × 106 total splenocytes derived from LATKO mice, (3) 5 × 106 total splenocytes derived from NTALKO mice, (4) wild-type thymocytes (1 × 106), (5) purified (CD3–CD4–CD8–DX5+) NK cells (1 × 106), and (6) enriched (B220+) B cells (1 × 106). (D) The numbers of splenic NK cells isolated from indicated mice are represented. Dots indicate individual mice. Horizontal bars indicate mean values. Statistical analysis was performed using unpaired Student t test. (E) The cell-surface expression of indicated NK cell markers was analyzed on CD3–MHC class II–DX5+ splenocytes isolated from represented mice. Indicated mice also express comparable levels of Ly49A marker (data not shown). Data are representative of 4 independent experiments.
LAT and NTAL are expressed in mouse NK cells but are dispensable for splenic NK cell maturation. (A) Lat and Ntal expression levels are expressed as relative units of indicated mRNA normalized using HPRT transcripts. Samples included purified CD4+ T cells (T), sorted (CD3–CD4–CD8–DX5+) NK cells isolated from unstimulated (NK resting) and polyIC stimulated (NK polyIC) mice, sorted (CD3–NK1.1+) LAK, enriched B220+ B cells (B), all derived from C57BL/6 mice, or total splenocytes isolated from double LAT/NTALKO mice. Data (mean ± SD) are representative of 3 independent experiments. (B) LAT and NTAL expression was analyzed on C57BL/6 (CTR) and LAT/NTALKO spleen lymphocytes by intracellular staining gating on T cells (CD3+NK1.1–), NK cells (CD3–NK1.1+), and B cells (CD3–NK1.1–). Staining performed with isotype control mAb (dotted line) has been overlaid to staining obtained using specific anti-LAT or anti-NTAL mAb (bold line). Data shown are representative of 3 independent experiments. (C) LAT and NTAL expression was analyzed by immunoblotting on lysates prepared from (1) 5 × 106 total C57BL/6 splenocytes, (2) 5 × 106 total splenocytes derived from LATKO mice, (3) 5 × 106 total splenocytes derived from NTALKO mice, (4) wild-type thymocytes (1 × 106), (5) purified (CD3–CD4–CD8–DX5+) NK cells (1 × 106), and (6) enriched (B220+) B cells (1 × 106). (D) The numbers of splenic NK cells isolated from indicated mice are represented. Dots indicate individual mice. Horizontal bars indicate mean values. Statistical analysis was performed using unpaired Student t test. (E) The cell-surface expression of indicated NK cell markers was analyzed on CD3–MHC class II–DX5+ splenocytes isolated from represented mice. Indicated mice also express comparable levels of Ly49A marker (data not shown). Data are representative of 4 independent experiments.
Unlike the other tumor cell lines tested, the killing of B16-Rae1β cells was less efficient when LAK cells were isolated from LAT/NTALKO mice (Figure 6A). As the killing of B16-Rae1β cells by LAK cells is partially DAP12 dependent (Figure 3B), we asked whether LAT and NTAL play a role in the function of other DAP12-dependent NK cell–surface receptors, such as Ly49D and Ly49H. Redirected killing mediated by anti-Ly49D mAb was severely impaired with LAK cells derived from double LAT/NTAL mutants (Figure 6B, top). Similarly, LAK cells derived from LAT/NTALKO mice were also impaired in their ability to kill BaF3 cells expressing the Ly49H ligand, m157 (BaF3-m157; Figure 6B, bottom).42,43 IFN-γ secretion induced via Ly49D triggering was also abrogated in LAK cells derived from double LAT/NTALKO, as compared with LAK cells derived from control mice (Figure 6C). Importantly, all mutant LAK cells were fully competent in their ability to secrete cytokines when activated by PMA/ionomycin or IL-12 (data not shown). These data thus suggest that LAT and/or NTAL are involved in DAP12-dependent NK cell effector functions.
Discussion
The receptor/ligand pairs involved in target cell recognition by NK cells, as well as the downstream signaling circuits that initiate NK cell natural cytotoxicity and cytokine secretion, are still incompletely understood. In this report, we have shown that the dependence of NK cell effector functions on ITAM-bearing signaling molecules or LAT/NTAL varies, depending on the tumor target cells and the activation status of the NK cells.
Two different models of DAP12 deficiency (DAP12KO and DAP12KI) revealed that a defect in DAP12 signaling affects the recognition of tumor cell lines expressing NKG2D ligands, consistent with the association of a mouse Nkg2d alternative splice product (NKG2D-S) with DAP12.15,16,44 Yet, the lysis of IC-21 cells is NKG2D independent and DAP12 dependent (Figure 2E), suggesting that other DAP12-dependent NK cell–activating receptors also participate to NK natural cytotoxicity.8 Reciprocally, anti-NKG2D mAb blocks the lysis by control NK cells of several targets to a greater degree than can be accounted for by DAP12 deficiency, and the residual lysis mediated by DAP12-deficient NK cells can be blocked still further by anti-NKG2D mAbs (Figure 2E). Thus, whereas a component of NKG2D-dependent killing is DAP12 dependent, DAP12-independent pathways are also involved in target cell lysis. These results support the involvement of both NKG2D/DAP12 and NKG2D/DAP10 complexes in natural cytotoxicity and help to reconcile previous findings emphasizing the importance of each of these complexes in the process.15,16,45,46
Role of LAT and NTAL in polyIC NK cell effector functions. (A) polyIC NK cells were purified from control (CTR) and mutant mice and used as effectors against indicated tumor cells. Data shown are representative of at least 4 independent experiments. (B) Percentages of IFN-γ+ cells in gated CD3–NK1.1+ cells; □ indicate polyIC NK cells from control mice (CTR); ▪, polyIC NK cells from LAT/NTALKO double KO. Stimulation for IFN-γ analysis was performed as described in the Figure 2 legend. Error bars represent SEM.
Role of LAT and NTAL in polyIC NK cell effector functions. (A) polyIC NK cells were purified from control (CTR) and mutant mice and used as effectors against indicated tumor cells. Data shown are representative of at least 4 independent experiments. (B) Percentages of IFN-γ+ cells in gated CD3–NK1.1+ cells; □ indicate polyIC NK cells from control mice (CTR); ▪, polyIC NK cells from LAT/NTALKO double KO. Stimulation for IFN-γ analysis was performed as described in the Figure 2 legend. Error bars represent SEM.
Although RMA and RMA-S represent widely used models for “missing self”-dependent mouse NK cell cytotoxicity, the identity of the activating receptors/ligands involved in this recognition remains poorly understood. The partially impaired lysis of RMA and RMA-S tumor cell line lysis by polyIC ZGR NK cells is consistent with the reduced lysis of RMA-S by Syk/ZAP70KO NK cells45 and suggests the existence of CD3ζ/FcRγ-dependent receptor(s) that cooperate with at least another ITAM-independent receptor. This latter pathway might be triggered by 2B4, SAP (SLAM-associated protein), and the Fyn PTK. Indeed, it has been recently shown that the adaptor protein SAP and Fyn are required for NK cell–mediated elimination of tumor cells such as RMA-S, and that this effect strongly correlates with the cell-surface expression of CD48, a ligand for 2B4, on tumor targets.47 With the caveat that our panel of tumor cell lines is limited, the effect of CD3ζ/FcRγ deficiency on mouse NK cell activation thus appears more restricted than that of DAP12. This is in marked contrast to observations made in human NK cells, where both CD3ζ/FcRγ-dependent (NKp30 and NKp46) and DAP12-dependent NCRs (NKp44) play a key role in recognition and elimination of several tumor cell lines.48 It is thus tempting to speculate that distinct ITAM-dependent pathways have been selected in NK cells during evolution.49 This hypothesis is consistent with the observation that NKG2D associates with DAP12 in mouse, but not in human, NK cells and thus differently contributes to human and mouse natural cytotoxicity.44,46,50
ITAM-independent natural cytotoxicity is prototypically represented by the killing of YAC-1 tumor cells. Because of the high surface levels of NKG2D ligand on this tumor cell line,51 a DAP12-dependent YAC-1 killing could be predicted. Nevertheless, YAC-1 is efficiently killed by DAP12-deficient NK cells,31,52 ZGKR NK cells (Figure 2), and Syk/ZAP70KO NK cells.22 Vav-1 and Vav-2/Vav-3 guanidine nucleotide exchange factors (GEFs) are respectively involved in DAP10-dependent and ITAM-dependent mouse NK cell cytotoxicity.53 Interestingly, NK cells from Vav1,/2/3KO mice exhibit a greater defect in YAC-1 killing than can be accounted for by blocking NKG2D in the case of wild-type NK cells.53 Furthermore, anti-NKG2D mAbs inhibit YAC-1 lysis mediated by control and DAP12-deficient LAK cells to a similar extent, and a residual lysis is still detectable after NKG2D blocking (Figure S2). Altogether, these findings indicate that natural cytotoxicity of YAC-1 cells consists of a NKG2D-independent component and a NKG2D-dependent component, both of which are ITAM-independent and Vav-dependent. An ITAM-dependent component may also be present and obscured by the larger ITAM-independent component of killing.
Role of LAT and NTAL in LAK effector functions. (A-B) LAK NK cells were purified from control (CTR) and mutant mice and used as effectors against indicated tumor cells. Redirected killing of Daudi cells by purified LAK cells isolated from control (CTR, ○ and ▵) and LAT/NTALKO double KO (• and ▴) has been performed in the presence of isotype control mAbs (isot. ctr.) or anti-Ly49D mAbs (α-Ly49D). Data shown are representative of at least 2 independent experiments. (C) Purified LAK cells isolated from indicated mice were incubated for 24 hours on plates previously coated with anti-Ly49D mAb or isotype control mAb. Cell-culture supernatants were then harvested, and IFN-γ contents were determined by ELISA. Basal values detected in the presence of isotype control mAb were subtracted from values obtained via Ly49D stimulation. Data shown are representative of 5 to 8 independent experiments. Horizontal bars indicate mean values.
Role of LAT and NTAL in LAK effector functions. (A-B) LAK NK cells were purified from control (CTR) and mutant mice and used as effectors against indicated tumor cells. Redirected killing of Daudi cells by purified LAK cells isolated from control (CTR, ○ and ▵) and LAT/NTALKO double KO (• and ▴) has been performed in the presence of isotype control mAbs (isot. ctr.) or anti-Ly49D mAbs (α-Ly49D). Data shown are representative of at least 2 independent experiments. (C) Purified LAK cells isolated from indicated mice were incubated for 24 hours on plates previously coated with anti-Ly49D mAb or isotype control mAb. Cell-culture supernatants were then harvested, and IFN-γ contents were determined by ELISA. Basal values detected in the presence of isotype control mAb were subtracted from values obtained via Ly49D stimulation. Data shown are representative of 5 to 8 independent experiments. Horizontal bars indicate mean values.
Another important view emerging from our observations is that environmental stimuli differentially regulate NK cell ability to be activated by target cells, providing some basis for the apparently conflicting data reported on NK cell cytotoxicity requirement. On one hand, this is illustrated by RMA cells that are killed by polyIC NK cells via CD3ζ/FcRγ-dependent pathways (Figure 2B), but resistant to LAK killing (Figure 3B). On the other hand, B16 melanoma is resistant to killing mediated by polyIC NK but killed by LAK cells. Along these lines, Vav1/2/3 and WASp (Wiskott-Aldrich syndrome protein), are differentially involved in the cytotoxicity mediated by resting NK cells and LAK cells.53,54 This NK cell functional plasticity is reminiscent of T-effector functions, whose requirements vary with T-cell activation status (naive versus memory T cells).55 In addition, our data document the crosstalk that exists between distinct mouse NK cell–signaling pathways involved in natural cytotoxicity, as shown earlier for human NK cells.56,57 Indeed, the complete abrogation of B16-Rae1β lysis by LAK cells derived from ZGKR mice (Figure 3A) results from a NKG2D/DAP12-dependent recognition of Rae1β and a CD3ζ/FcRγ-dependent recognition of still unknown ligands on B16 cells (Figure 3B).
Engagement of ITAM-dependent receptors by their respective ligands constitutes the first event in a multistep signaling process, which involves the recruitment of several transducing elements. Studies of T and mast cells have shown that LAT and/or NTAL are crucial in this process, because they recruit to the proximity of the cell membrane several cytosolic targets of PTK.25,26 In contrast, LAT and NTAL appear dispensable for proper B-cell development and physiology.34 Data reported herein confirm that LAT and NTAL are both expressed in NK cells,23,28 although at different levels. In addition, quantitative RT-PCR experiments demonstrate that LAT expression is reduced in LAK cells as compared with fresh and polyIC NK cells, exemplifying the plasticity of NK cell transduction pathways. Consistent with their role as integrators of ITAM-dependent signals, we show that LAT and/or NTAL are critically involved in the signaling cascade downstream of the DAP12 subunit. The DAP12-associated receptors (NKG2D-S, Ly49D, Ly49H) are thus coupled to a NK cell transduction pathway that recapitulates the “canonical” TCR and FcϵRI signaling cascade, that is, via src-PTK, ZAP70/Syk PTK, and TRAPs (LAT/NTAL) (Figure 6).18,28,58 In contrast, LAT and NTAL are largely dispensable for NK cell effector functions that are initiated by our panel of tumor cells. As expected, ITAM-independent pathways (eg, YAC-1 cytotoxicity) do not require these 2 TRAPs. More surprisingly, LAT and NTAL deficiency does not affect the killing of tumors, such as B16-Rae1β and RMA-S, whose lysis by polyIC NK cells is partially ITAM dependent. Because of the multiplicity of receptor/ligand pairs involved in NK cell effector functions that are elicited by tumor cells, LAT/NTAL deficiency might be compensated by ITAM-dependent or -independent but TRAP-independent pathways. Along these lines, a CD3ζ-dependent/LAT-independent pathway that activates p38 MAPK has been recently identified in T cells.59 Moreover, in mouse B cells the increase in intracellular Ca2+ concentration induced by BCR complex engagement is not significantly affected in the absence of both LAT and NTAL,34 a feature accounted by the role of SLP-65 (SH2 domain-containing leukocyte protein of 65 kDa) adaptor protein.60
The coexistence in NK cells of ITAM-dependent and -independent as well as LAT/NTAL-dependent and -independent signaling pathways that lead to cytotoxicity and IFN-γ secretion provides an explanation for the resiliency of NK cell effector functions to various pharmacologic inhibitors and genetic mutations in signaling molecules. These results also further prompt the dissection of the still unknown NK cell activation pathways that apparently converge with the “canonical pathway” at the level of PLC-γ1 and PLC-γ2 in humans and PLC-γ2 in mice.61,62 Because these signaling molecules regulate intracellular calcium concentration, their deficiency severely affects NK cell effector functions, including cytotoxicity and cytokine secretion mediated by the engagement of ITAM-dependent or DAP10-dependent receptors.
Prepublished online as Blood First Edition Paper, November 15, 2005; DOI 10.1182/blood-2005-08-3504.
Supported by specific grants from European Union (ALLOSTEM), Ligue Nationale contre le Cancer (Equipe labellisée La Ligue) and institutional grants from INSERM, CNRS, and Ministère de l'Enseignement Supérieur et de la Recherche (E.V.); by GIS Maladies Rares (E.V. and E.T.); by the Ligue Nationale contre le Cancer (S.C.); by National Institutes of Health (NIH) (grant RO1CA093678) (D.H.R.); and the LATKO and NTALKO mice have been derived in the frame of Plateforme RIO and supported by a specific grant from Association pour la Recherche contre le Cancer (program ARECA) (B.M.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank T. Takai for DAP12KO mice, P. Love for CD3ζ/FcRγKO mice, L. Zitvogel for tumor cells, W. Yokoyama for BaF3 and BaF3-m157 tumor cells, A. Gillet for excellent management of mouse facility, and C. Beziers for graphical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal