Abstract
Constitutive activation of the nuclear factor-kappaB (NF-κB) pathway has been shown to be involved in the resistance of tumor cells to apoptosis in several human malignancies of the hematopoietic lineage. By using electrophoretic mobility shift assay (EMSA) and confocal microscopic analysis, we demonstrate that NF-κB is constitutively activated in cutaneous T-cell lymphoma (CTCL) cell lines HuT-78, MyLa, and SeAx and in peripheral blood lymphocytes (PBLs) from patients with Sézary syndrome (SS) presenting a high ratio of tumor cells, with evidence of p50 and RelA/p65 in DNA-linked complexes. Transfection of SeAx line with a κB/luciferase reporter plasmid showed that translocated NF-κB complexes were functional. Selective inhibition of NF-κB, by transfecting CTCL cell lines with a super-repressor form of IκBα, led to apoptosis. We evidenced down-regulation of NF-κB activation and induction of CTCL cell apoptosis in the presence of proteasome 26S inhibitors ALLN, MG132, and bortezomib. Bortezomib at nanomolar concentrations inhibited constitutive activation of NF-κB and induced apoptosis of CTCL cells, with evidence of an upregulation of Bax expression. These results demonstrate the key role played by NF-κB in the resistance of CTCL to apoptosis and suggest that bortezomib might be useful for the treatment of patients with advanced stages of CTCL refractory to standard antineoplastic chemotherapy.
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a heterogeneous entity, among which mycosis fungoides (MF) and the leukemic variant Sézary syndrome (SS) are the most common subtypes.1,2 Both MF and SS are characterized by the clonal expansion of cells bearing a CD3+ CD4+ CD45RO+ phenotype of memory T lymphocytes.3-7 Even though remission is usually reached in patients with early, skin-localized stages of CTCL, advanced tumor or erythromerma stages of MF and Sézary syndrome are associated with an aggressive course and a poor prognosis, despite the extensive use of different protocols of antineoplastic chemotherapy.8 Indeed, the 3-year median survival of patients with stage IV MF or Sézary syndrome has not been modified by intensive polychemotherapy or autologous bone marrow transplantation.9 More recently, both in vitro and in vivo data showed that CTCL cells from patients with SS were resistant to apoptosis induced by antineoplastic agents including arsenic trioxide, a therapeutic tool exerting its proapoptotic effect through the mitochondrial pathway.10 However, the mechanisms responsible for the resistance of Sézary cells to a wide range of death-inducing agents in vivo remain largely unknown. In the present study, we attempted to elucidate the molecular mechanisms underlying the resistance of CTCL cells to apoptosis.
Nuclear factor-kappaB (NF-κB) is a transcription factor that is central to the generation of immune and inflammatory responses, cell cycle regulation, and protection against apoptosis.11 In mammalian cells, the NF-κB family consists of 5 distinct members: c-rel, p65/RelA, RelB, p50/p105 (NF-κB1), and p52/p100 (NF-κB2), which are able to homodimerize or heterodimerize. Under normal conditions, NF-κB is sequestered in an inactive state by the IκBs inhibitory molecules in the cytoplasm. A variety of different stimuli, such as proinflammatory cytokines tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β), lipopolysaccharide derived from gram-negative bacteria, Tax protein encoded by human T leukemia virus (HTLV)-1, or oxidative stress can activate NF-κB via phosphorylation of the IκBs, triggering their ubiquitination and degradation by the proteasome 26 S.12-14 The kinase responsible for phosphorylating IκBs is IκB kinase (IKK), which is composed of 2 catalytic subunits IKKα and IKKβ, and a third noncatalytic subunit called NEMO/IKKγ. In lymphocytes, activation of NF-κB results in expression of target genes involved in proliferation, cytokine production, and survival. In vivo studies using knock-out mice have confirmed the hypothesis that this family of transcription factors is critically involved in the development, activation, cytokine production, and survival of normal B cells and T cells.15 Thus, both p50–/– and cRel–/– mice show impaired T-cell responses and decreased peripheral pools of effector/memory CD4+ T cells as well as alterations of regulatory T cells.15 In addition to its physiological functions in B- and T-lymphocyte differentiation, growth, and survival, NF-κB has been shown to be constitutively activated in several hematopoietic lymphoid malignancies mostly belonging to the B lineage, among which are large B-cell lymphomas,16 mantle cell lymphomas,17 and multiple myeloma.18 In contrast, the NF-κB status has been ill-defined in human neoplastic T-cell malignancies, including CTCLs, since the only previously published study investigating this issue in patients with MF relied exclusively upon immunohistochemical in situ analysis.19
Recently, the antitumor effects of proteasome inhibitors, mostly bortezomib, have been reported in different types of cancer.20-22 The antiproliferative and/or death-promoting properties of bortezomib have been demonstrated in vitro on a wide range of malignancies including melanoma, breast cancer, and hematological malignancies originating from myeloid or lymphoid lineages.23-26 The therapeutic interest of proteasome inhibitors has been supported by promising results from clinical trials in patients with multiple myeloma showing resistance to classical antineoplastic chemotherapy.27-29 Several lines of evidence indicate that proapoptotic and antiproliferative effects of bortezomib on cancer cells result from an inhibition of the NF-κB pathway.
In the present study, we investigated the NF-κB status in CTCL cell lines and in tumor cells from patients with Sézary syndrome. We demonstrate that NF-κB is constitutively activated in CTCL cells and plays a key role in their survival and resistance to apoptosis. We also show that inhibition of constitutive activation of NF-κB induces apoptosis in CTCL cells. Finally, we show that bortezomib exerts antitumor effects on CTCL cells in vitro.
Patients, materials, and methods
Patients
Patients gave written informed consent after approval by the local ethical committee. Essential characteristics of study patients are listed in Table 1. The diagnosis of Sézary syndrome was established according to clinical and morphological criteria, for instance, a chronic erythroderma, with presence of large-size Sézary cells on cytological examination of a peripheral blood smear, and a dense, bandlike dermal infiltrate with epidermotropism in skin biopsies. A dominant clonal rearrangement of the T-cell receptor γ (TCRγ) locus was found in both lesional skin and in peripheral blood lymphocytes (PBLs) by using polymerase chain reaction (PCR) multiplex-based analysis at the DNA level, as previously described.30 Patients selected to enter the present study exhibited a Sézary cell ratio exceeding 5% of PBLs (range, 5-48; median, 15.5). The quantitative evaluation of peripheral blood tumor cells was assessed by combining CD4/anti-TCRVβ double-immunostaining flow cytometric analysis of PBLs, and TCRVβ complementary determining region 3 (CDR3) length analysis by the immunoscope method as described previously.31,32
Main characteristics of patients
Patient no. . | Age (at diagnosis) . | TNM staging* . | Previous treatments† . | PBLs‡ . | Ly§ . | Percent CD4+/CD3+ . | TCRVβ∥(percent CD4+ cells) . | Percent Sézary cells . |
|---|---|---|---|---|---|---|---|---|
| 1 | 79 (69) | T4N3M0 | PUVA, IFNα, ECPC, chlorambucil + prednisone | 11 900 | 4044 | 96.3 | Vβ3 (97.4) | 5 |
| 2 | 78 (71) | T4N1M0 | PUVA, IFNα, ECPC | 8 300 | 2490 | 95.0 | NA | 10 |
| 6 | 72 (64) | T4N3M0 | PUVA | 5 800 | 1600 | 97.2 | Vβ22 (88.2) | 12 |
| 10 | 84 (84) | T4N1M0 | None | 14 100 | 8460 | 94.5 | Vβ5.1 (94.8) | 40 |
| 11 | 67 (62) | T4N3M0 | ECPC | 3 200 | 480 | 48.5 | NA | 8 |
| 18 | 68 (64) | T4N0M0 | PUVA, ECPC + IFNα, CHOP | 11 300 | 6100 | 96.2 | Vβ11 (97.1) | 11 |
| 20 | 66 (66) | T4N3M0 | None | 17 500 | 5780 | 88.0 | Vβ2 (85.1) | 25 |
| 23 | 20 (20) | T4N3M0 | None | 6 500 | 2060 | 86.7 | Vβ5.1 (78.2) | 6 |
| 24 | 59 (59) | T4N3M0 | IFNα | 16 000 | 1760 | 94.9 | Vβ18 (98.7) | 48 |
| 25 | 79 (79) | T4N3M0 | None | 81 300 | 25200 | 98.1 | Vβ13.1 (98.5) | 20 |
| 26 | 63 (59) | T4N3M0 | IFNα, PUVA | 6 700 | 740 | 82.8 | Vβ2 (38.6) | 19 |
| 27 | 76 (76) | T4N3M0 | MTX, mechlorethamine, bexarotene, ECPC + IFNα | 18 900 | 8520 | 99.4 | Vβ213 (83.2) | 39 |
| 28 | 53 (47) | T4N3M0 | IFNα, PUVA, ECPC, CHOP, bexarotene | 17 800 | 6410 | 97.9 | NA | 20 |
| 29 | 55 (55) | T4N3M0 | ECPC, IFNα, mechlorethamine, PUVA, MTX, bexarotene | 9 900 | 1490 | 85.5 | NA | 5 |
| 30 | 65 (65) | T4N1M0 | IFNα, prednisone | 15 200 | 1670 | 87.0 | NA | 6 |
Patient no. . | Age (at diagnosis) . | TNM staging* . | Previous treatments† . | PBLs‡ . | Ly§ . | Percent CD4+/CD3+ . | TCRVβ∥(percent CD4+ cells) . | Percent Sézary cells . |
|---|---|---|---|---|---|---|---|---|
| 1 | 79 (69) | T4N3M0 | PUVA, IFNα, ECPC, chlorambucil + prednisone | 11 900 | 4044 | 96.3 | Vβ3 (97.4) | 5 |
| 2 | 78 (71) | T4N1M0 | PUVA, IFNα, ECPC | 8 300 | 2490 | 95.0 | NA | 10 |
| 6 | 72 (64) | T4N3M0 | PUVA | 5 800 | 1600 | 97.2 | Vβ22 (88.2) | 12 |
| 10 | 84 (84) | T4N1M0 | None | 14 100 | 8460 | 94.5 | Vβ5.1 (94.8) | 40 |
| 11 | 67 (62) | T4N3M0 | ECPC | 3 200 | 480 | 48.5 | NA | 8 |
| 18 | 68 (64) | T4N0M0 | PUVA, ECPC + IFNα, CHOP | 11 300 | 6100 | 96.2 | Vβ11 (97.1) | 11 |
| 20 | 66 (66) | T4N3M0 | None | 17 500 | 5780 | 88.0 | Vβ2 (85.1) | 25 |
| 23 | 20 (20) | T4N3M0 | None | 6 500 | 2060 | 86.7 | Vβ5.1 (78.2) | 6 |
| 24 | 59 (59) | T4N3M0 | IFNα | 16 000 | 1760 | 94.9 | Vβ18 (98.7) | 48 |
| 25 | 79 (79) | T4N3M0 | None | 81 300 | 25200 | 98.1 | Vβ13.1 (98.5) | 20 |
| 26 | 63 (59) | T4N3M0 | IFNα, PUVA | 6 700 | 740 | 82.8 | Vβ2 (38.6) | 19 |
| 27 | 76 (76) | T4N3M0 | MTX, mechlorethamine, bexarotene, ECPC + IFNα | 18 900 | 8520 | 99.4 | Vβ213 (83.2) | 39 |
| 28 | 53 (47) | T4N3M0 | IFNα, PUVA, ECPC, CHOP, bexarotene | 17 800 | 6410 | 97.9 | NA | 20 |
| 29 | 55 (55) | T4N3M0 | ECPC, IFNα, mechlorethamine, PUVA, MTX, bexarotene | 9 900 | 1490 | 85.5 | NA | 5 |
| 30 | 65 (65) | T4N1M0 | IFNα, prednisone | 15 200 | 1670 | 87.0 | NA | 6 |
NA indicates not available.
According to the TNM (tumors-nodes-metastases) classification.
PUVA indicates psoralen with ultraviolet A radiation therapy; IFNα, interferon alpha; MTX, methotrexate; ECPC, extra-corporeal photochemotherapy; CHOP, polychemotherapy regimen containing cyclophosphamide, doxorubicin, vincristine, and prednisone.
PBL indicates peripheral blood leukocytes.
Ly (lymphocytes) expressed as peripheral blood absolute cell counts per cubic millimeter.
The TCRVβ segment expressed by tumoral cells was identified by TCRVβ/CD4 double-immunostaining flow cytometric analysis of PBLs in 10 of the 15 cases described here, as PBLs from patients 2, 11, 28, 29, and 30 did not predominantly immunoreact with any anti-TCVβ monoclonal antibody. Nevertheless, the clonal nature of the expansion was confirmed in all cases by PCR analysis of TCRVγ-Jγ rearranged DNA segments, by Vβ-family—specific RT-PCR, and by immunoscopy analysis of expanded TCRVβ families.
Reagents
N-acetyl-leucyl-leucyl-norleucinal (ALLN) (Biomol Research Laboratories, Plymouth Meeting, PA) was dissolved as a stock solution at 0.652 mM in dimethylsulfoxide (DMSO). MG132 was purchased from Calbiochem (La Jolla, CA) and diluted in DMSO at 10 mM. The proteasome inhibitor bortezomib (PS-341; Velcade) was kindly supplied by Millenium (Cambridge, MA), dissolved at 0815 M in DMSO, and stored at –20°C.
Cell lines and PBLs from CTCL patients
The cell line HuT-78 (SS) was purchased from European Collection of Animal Cell Cultures (ECACC, Salisbury, England). SeAx (SS) and MyLa 2059 (MF) cell lines were kindly provided by Dr Keld Kaltoft (University of Aarhus, Denmark). CTCL cell lines were grown at 37°C with 5% CO2 at 5 × 104 cells/mL in RPMI 1640 medium (Gibco, Life Technologies, Gaithersburg, MD) supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 1% HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (RPMI complete medium).
PBLs were isolated by Ficoll-Hypaque centrifugation followed by elimination of monocytes by adhesion for 2 hours.
Cell proliferation assay
Cells either transiently transfected or exposed to bortezomib (5-40 nM) were cultured in 96-well microplates (1 × 105/well) at 37°C for 24 to 48 hours and pulsed with 5 μCi (0.185 MBq)/mL 3[H]-thymidine during the last 16 hours of culture. Incorporated tritium was quantified using a MicroBeta1450 counter (Wallac, Turku, Finland).
Analysis of apoptosis, mitochondrial membrane potential, and cell cycle
PBLs from SS patients or CTCL cell lines incubated in the presence of NF-κB inhibitors for 6 to 60 hours or transiently transfected were analyzed for apoptosis according to the following protocols.
Annexin staining. Detection of phosphatidylserine on apoptotic cells was performed by using an annexin V/propidium iodide (PI) detection kit (Beckman Coulter, Immunotech, Marseille, France) according to the manufacturer. Briefly, 5 × 105 cells were incubated in the dark at 4°C with annexin V–fluorescein isothiocyanate (FITC) and 2.5 μg/mL PI in phosphate buffer for 10 minutes before 18 000 ungated events were collected within the hour by flow cytometry (FACScalibur, BD Biosciences, Mansfield, MA) and analyzed with the ProCellQuest software provided by the manufacturer.
Mitochondrial membrane potential. The 3,3′-diethyloxacarbocyanine (DiOC6) fluorescent probe (Molecular Probes, Eugene, OR) was used to quantify mitochondrial transmembrane potential Δψm as previously described.33 In brief, 5 × 105 cells were incubated in RPMI complete medium with 0.1 μM DiOC6 for 30 minutes at 37°C, washed, and resuspended in complete medium with 10 μg/mL PI before immediate analysis by flow cytometry. The percentage of cells exhibiting a low level of DiOC6 uptake, which reflects loss of Δψm, was determined.
Cell cycle analysis. Cells (5 × 105) were washed twice with ice-cold PBS, fixed in cold 70% ethanol for 1 hour at 4°C, washed twice in cold PBS, treated with 200 U per mL DNAse-free RNAse A (Sigma, St Louis, MO) for 30 minutes at 37°C, and stained with 25 μg/mL PI. Distribution of cell-cycle phases was determined within the hour using PI fluorescence emission (15 000 events) on FACScalibur and analyzed with Modfit Software (BD Biosciences).
Immunoblot analysis
Cells (10 × 106) were lysed for 1 hour at 4°C in buffer (TrisHCl 50 mM, NaCl 150 mM, EGTA 1 mM, 1% NP40, NaF 1 mM, Na3VO4 1 mM, 0.25% DOC 10%, PMSF 1 μM, aprotinin 19 μg/mL, pepstatin 10 μM, leupeptin 10 μM). After centrifugation (20 800 g, 15 minutes, 4°C), protein extracts (50 μg) were loaded onto a 10% polyacrylamide gel containing sodium dodecyl sulfate, subjected to electrophoresis, and transferred to nitrocellulose membrane. The blot was blocked with 5% nonfat milk and incubated for 24 hours at 4°C with mouse anti–poly(ADP-ribose) polymerase (anti-PARP) (1/250, Pharmingen, San Diego, CA), anti-Bcl2 (1/40, Dako, Carpinteria, CA), or anti-p53 (1/400, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti–caspase3 (1/500, Euromedex, Souffleweyersheim, France), anti–Bcl-XL, or anti-Bax (1/40, Santa Cruz Biotechnology), goat anti-Bid (2 μg/mL, Santa Cruz Biotechnology), and subsequently with antimouse (1/2000), antirabbit, or antigoat (1/10 000) antibody coupled to horseradish peroxidase (Amersham Pharmacia Biotech, Piscataway, NJ). An enhanced chemiluminescent system (Amersham Pharmacia Biotech) was used for detection. Equal protein loading was confirmed by the use of mouse monoclonal anti-GAPDH (2 μg/mL, Sigma). Densitometry was performed using Imager Fx System and analyzed using Image J software.
Electrophoretic mobility shift assay
Cells (5-10 × 106) were incubated overnight in RPMI 1640 supplemented with 2% FCS and either lysed for determination of basal NF-κB status or treated with NF-κB inhibitors. Cells were lysed at 4°C in 400 μL of hypotonic buffer (HEPES pH 7.8 10 mM, MgCl2 15 mM, KCl 10 mM, DTT 0.5 mM, PMSF 0.2 mM), centrifuged to recover the nuclei that were resuspended in 30 μL of a higher salt buffer (HEPES pH 7.8 20 mM, glycerol 25%, NaCl 420 mM, MgCl2 15 mM, EDTA 0.2 mM, DTT 0.5 mM, PMSF 0.2 mM) to extract the nuclear proteins. To prepare the radiolabeled probe, 2.5 pmol of double-stranded DNA κB element 5′-AGTTGAGGGGACTTTCCCAGGC-3′ was incubated 30 minutes at 37°C with T4 polynucleotide kinase in the presence of 5 pmol of γ32pATP. The probe was purified on Sephadex column G25. Three micrograms of nuclear proteins were incubated in binding buffer (HEPES pH 7.9 8 mM, glycerol 8%, KCl 12 mM, EDTA pH 8.5 40 μM, MgCl2 1.6 mM, spermidin 1.6 mM, DTT 0.4 mM) containing 3 μg poly (dI-dC) for 20 minutes on ice. Binding reactions were performed by adding 105 cpm of 32P-labeled oligonucleotide for 20 minutes at 4°C. Samples were analyzed by electrophoresis in a 4% polyacrylamide gel. Gel was fixed, vacuum dried, and exposed to autoradiographic films for 24 hours before being scanned using ScanQuant. The specificity of binding was examined by competition with the unlabeled oligonucleotide (50×). For supershift assays, following the first 20 minutes of incubation in binding buffer supplemented with 3 μg poly (dI-dC), 200 ng antibodies against p50, p65, p52, RelB, and cRel subunits of NF-κB (kindly provided by Nancy Rice, National Cancer Institute, Bethesda, MD) were added for an additional incubation of 1 hour at 4°C before the binding reaction was performed.
Confocal microscopy
Cells were cytospun on slides (5.104 cells/slide) during 2 minutes at 180 g, fixed in acetone (1 vol)/methanol (1 vol) for 10 minutes, blocked in a solution of 30% bovine serum albumin in PBS for 20 minutes, and permeabilized using 0.2% Tween-PBS for 30 minutes. Slides were incubated with rabbit polyclonal anti-p65 or goat polyclonal anti-p50 (both at 4 μg/mL, Santa Cruz Biotechnology) for 1 hour, washed twice with PBS, and incubated with the secondary antibody labeled with FITC, Texas Red, or Alexa 568 (1/50) for 1 hour. Mounting was performed using Vectashield medium (Vector Laboratories, Burlingame, CA) including DAPI (4′, 6-diamino-2-phenyindole) fluorochrome for nuclei staining. Slides were examined using a laser confocal microscope (Carl Zeiss, Oberkochen, Germany).
Gene transfer by nucleofection
Cutaneous T-cell lymphoma cell lines (2 × 106 cells/100 μL electroporation buffer) were transiently transfected using the Human T Cell Nucleofector Kit, according to the manufacturer (Amaxa Biosystems, Cologne, Germany). Four hundred nanograms of a κB-dependent reporter plasmid (Promega, Lyon, France) were cotransfected with 0.4 to 2.4μg of empty vector (pcDNA3) or plasmid vector expressing a mutant form of IκBα (pIκBα S32/36A).34 Transfection efficiency was determined by electroporating 0.8 μg of vector containing green fluorescence protein (GFP) gene driven by the cytomegalovirus (CMV) promoter. Two-hundred ng of plasmid containing the β-galactosidase gene was used to normalize the activity of luciferase of the samples. After electroporation, cells were immediately cultured in RPMI complete medium at 37°C for 18 to 48 hours. Cells (1 × 106) were harvested by centrifugation, washed with PBS, and lysed with Reporter Lysis Buffer for luciferase assay detection, performed in duplicate (Luciferase Assay System, Promega). Measurement of β-galactosidase activity (βgal Reporter GeneAssay, Roche, Meylan, France) was performed according to the manufacturer. Viability was determined by trypan blue exclusion. Apoptosis was assessed by annexin V/PI staining and by cell cycle assay for subG1 determination using a FACSCalibur. Determination of GFP expression in transfected cells by flow cytometry analysis indicated that transfection efficiency ranged between 15% and 30%.
Statistical analysis
For analysis of apoptosis and mitochondrial Δψm, values represent the means ± SD for at least 3 separate experiments performed in triplicate, unless otherwise noted. The significance of differences between experimental variables was determined by the use of the Student t test, and P less than .05 was considered as significant.
Results
Constitutive NF-κB activation in CTCL cell lines and peripheral blood tumor cells derived from patients with CTCL
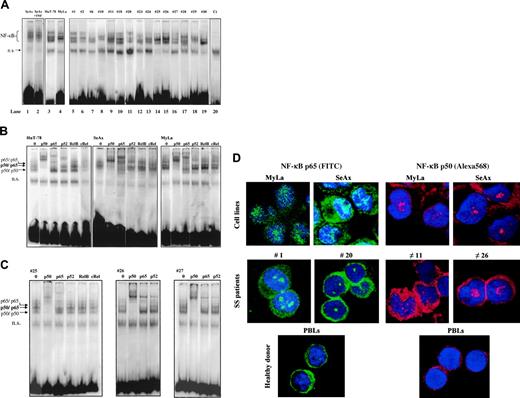
We first aimed at determining the status of NF-κB in CTCL. For this, we examined the sequence-specific DNA-binding activity of NF-κB by using electrophoretic mobility shift assay (EMSA). As shown in Figure 1, NF-κB is constitutively activated in CTCL cell lines SeAx, HuT-78, and MyLa (lanes 1 to 4). Gel shift analysis also showed evidence for NF-κB constitutive activation in PBLs from 15 patients with SS (Figure 1A, lanes 5 to 19 and Table 1). All 30 SS patients examined so far exhibit a constitutive activation of NF-κB, while in contrast, no evidence of NF-κB nuclear translocation could be detected in PBLs from 6 healthy donors (Figure 1A and data not shown). In all EMSA experiments, normalization of constitutive nuclear translocation of NF-κB was obtained by comparison with a nonspecific binding band (designated as ns in Figure 1A) as well as by using OCT-1 and SP1 probes (data not shown). Interestingly, the incubation of CTCL cell lines with 100 U/mL TNFα did not further enhance NF-κB binding activity, as shown for SeAx in Figure 1A.
To identify the NF-κB family members activated in CTCL cells, we performed a supershift EMSA assay on CTCL lines (Figure 1B) and PBLs from SS patients (Figure 1C). Both anti-p50 and anti-p65 antibodies induced a supershift in extracts from HuT-78, SeAx, and MyLa cell lines as well as in those from SS PBLs, while antibodies reacting with RelB and c-Rel had no effect on DNA-binding complexes. However, anti-p52 antibody induced a slight super-shift in HuT-78 cell line and in 2 of 3 SS samples, shown in Figure 1C. These data clearly demonstrated that DNA-binding complexes in CTCL cells essentially consist of p50/p65 heterodimers as well as p50/p50 and p65/p65 homodimers, in accordance with an activation of the canonical NF-κB pathway. These results were independently confirmed by an ELISA-based transcription factor assay (BD Bioscience) (data not shown). Finally, confocal microscopic analysis also confirmed that p50 and p65 proteins were both constitutively expressed in the nucleus of HuT-78 and SeAx cell lines, as well as of PBLs from SS patients (Figure 1D).
Constitutive NF-κB activation in CTCL cells. (A) CTCL cells constitutively express activated NF-κB. Nuclear extracts from CTCL cell lines (SeAx, HuT-78, MyLa) and from PBLs from 15 patients with Sézary syndrome were analyzed by EMSA. Analysis of SeAx (lane 1), HuT-78 (lane 3), and MyLa (lane 4) CTCL lines, and of PBLs from 15 patients with SS (lanes 5-19), and from one healthy donor as control (lane 20) is presented. N.S. represents a nonspecific unidentified band. Treatment with TNFα (10 ng/mL for 30 minutes) did not increase the constitutive nuclear translocation of NF-κB in SeAx cells (lane 2), and no translocation of NF-κB was detected in nuclear extracts of PBLs from a healthy donor (lane 20). The data shown are representative of 3 independent experiments. (B) Supershift assay of CTCL cell lines. Nuclear extracts from HuT-78, SeAx, and MyLa cells were subjected to supershift assays with specific NF-κB antibodies directed against p50, p65, p52, RelB, and c-Rel subunits. The positions of the retarded NF-κB species are indicated by arrows on the left. Similar results were obtained in 2 independent experiments. (C) Supershift assay of CTCL cells from 3 patients with Sézary syndrome. The assay was performed as described in panel B. (D) Evidence of NF-κB nuclear translocation in CTCL by confocal microscopic analysis. CTCL cells were fixed, permeabilized, and stained with DAPI (nucleus marker, blue); with anti-p65 (1/25); and visualized with anti–rabbit FITC–(green) or Texas Red (red)–labeled secondary Ab. Images were acquired on a Zeiss LSM-510 META laser scanning confocal microscope equipped with a Zeiss Plan Apochromat 63 ×/1.4 NA oil objective, using LSM510 software (version 3.2). Results obtained with CTCL cell lines SeAx and MyLa, PBLs from 2 patients with SS (cases 1 and 20), and from one healthy donor are presented. p50 and p65 proteins are detected in intranuclear and cytoplasmic localizations in CTCL cells only. The proportion of cells showing a positive nuclear staining reached 90% in CTCL lines and 80% in PBLs from SS patients. In contrast, resting PBLs from healthy donors exhibit a cytoplasmic pattern of p50/p65 staining.
Constitutive NF-κB activation in CTCL cells. (A) CTCL cells constitutively express activated NF-κB. Nuclear extracts from CTCL cell lines (SeAx, HuT-78, MyLa) and from PBLs from 15 patients with Sézary syndrome were analyzed by EMSA. Analysis of SeAx (lane 1), HuT-78 (lane 3), and MyLa (lane 4) CTCL lines, and of PBLs from 15 patients with SS (lanes 5-19), and from one healthy donor as control (lane 20) is presented. N.S. represents a nonspecific unidentified band. Treatment with TNFα (10 ng/mL for 30 minutes) did not increase the constitutive nuclear translocation of NF-κB in SeAx cells (lane 2), and no translocation of NF-κB was detected in nuclear extracts of PBLs from a healthy donor (lane 20). The data shown are representative of 3 independent experiments. (B) Supershift assay of CTCL cell lines. Nuclear extracts from HuT-78, SeAx, and MyLa cells were subjected to supershift assays with specific NF-κB antibodies directed against p50, p65, p52, RelB, and c-Rel subunits. The positions of the retarded NF-κB species are indicated by arrows on the left. Similar results were obtained in 2 independent experiments. (C) Supershift assay of CTCL cells from 3 patients with Sézary syndrome. The assay was performed as described in panel B. (D) Evidence of NF-κB nuclear translocation in CTCL by confocal microscopic analysis. CTCL cells were fixed, permeabilized, and stained with DAPI (nucleus marker, blue); with anti-p65 (1/25); and visualized with anti–rabbit FITC–(green) or Texas Red (red)–labeled secondary Ab. Images were acquired on a Zeiss LSM-510 META laser scanning confocal microscope equipped with a Zeiss Plan Apochromat 63 ×/1.4 NA oil objective, using LSM510 software (version 3.2). Results obtained with CTCL cell lines SeAx and MyLa, PBLs from 2 patients with SS (cases 1 and 20), and from one healthy donor are presented. p50 and p65 proteins are detected in intranuclear and cytoplasmic localizations in CTCL cells only. The proportion of cells showing a positive nuclear staining reached 90% in CTCL lines and 80% in PBLs from SS patients. In contrast, resting PBLs from healthy donors exhibit a cytoplasmic pattern of p50/p65 staining.
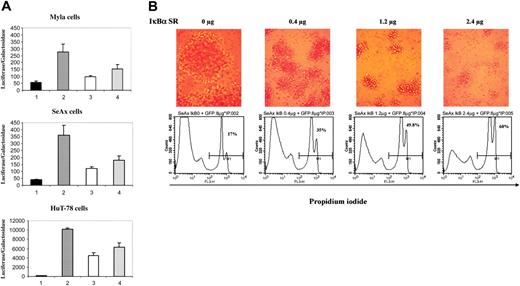
Transfection of the super-repressor in CTCL lines inhibits NF-κB activation and induces apoptosis. (A) Dose-dependent inhibition of NF-κB transcriptional activation in CTCL cell lines. CTCL cell lines (MyLa, SeAx, and HuT-78 cells) were transiently transfected with a control luciferase vector lacking κB sequences (lanes 1) or with a κB reporter plasmid without (lane 2) or with a plasmid vector expressing the IκBα super-repressor (pIκBα S32/36A) at 2 different concentrations 0.4 μg (lane 3) and 1.2 μg (lane 4). Results are expressed as the ratio of luciferase activity normalized on the basis of β-galactosidase expression. The data are representative of 3 independent experiments performed in duplicate. (B) Dose-dependent increase in apoptosis of SeAx cell line. The numbers in each bottom panel indicate the percentages of propidium iodide–positive cells 24 hours after transfection with IκBα super-repressor. Above each flow cytometry panel is shown the morphological status of cells, as observed by phase-contrast microscopy (Leitz, 25 ×/2.6 NA objective). Numeric pictures were taken at the same magnification just before harvesting transfected cells. IκBα SR = IκBα super-repressor. Similar results were obtained in 3 independent experiments.
Transfection of the super-repressor in CTCL lines inhibits NF-κB activation and induces apoptosis. (A) Dose-dependent inhibition of NF-κB transcriptional activation in CTCL cell lines. CTCL cell lines (MyLa, SeAx, and HuT-78 cells) were transiently transfected with a control luciferase vector lacking κB sequences (lanes 1) or with a κB reporter plasmid without (lane 2) or with a plasmid vector expressing the IκBα super-repressor (pIκBα S32/36A) at 2 different concentrations 0.4 μg (lane 3) and 1.2 μg (lane 4). Results are expressed as the ratio of luciferase activity normalized on the basis of β-galactosidase expression. The data are representative of 3 independent experiments performed in duplicate. (B) Dose-dependent increase in apoptosis of SeAx cell line. The numbers in each bottom panel indicate the percentages of propidium iodide–positive cells 24 hours after transfection with IκBα super-repressor. Above each flow cytometry panel is shown the morphological status of cells, as observed by phase-contrast microscopy (Leitz, 25 ×/2.6 NA objective). Numeric pictures were taken at the same magnification just before harvesting transfected cells. IκBα SR = IκBα super-repressor. Similar results were obtained in 3 independent experiments.
Selective inhibition of constitutive activation of NF-κBbythe dominant-negative IκBα induces apoptosis of CTCL cell lines
To ensure that the NF-κB complex translocated into the nucleus of CTCL cells was able to induce expression of NF-κB–related genes and to analyze the consequences of a specific down-regulation of NF-κB in CTCL cells, we transfected CTCL cell lines with an NF-κB responsive vector together with either the super-repressor form of IκBα (pCMV-IκBα S32/36A) or an empty vector. When overexpressed, the IκBα S32/36A protein binds to NF-κB but, unlike wild-type IκBα, cannot be phosphorylated because of substitutions of serine 32 and 36 by alanine and thus behaves as a dominant-negative molecule, blocking the NF-κB pathway. Results showed that κB-luciferase reporter vector had a high basal expression in the CTCL cell lines SeAx, HuT-78, and MyLa as compared with a control luciferase vector lacking κB sequences (Figure 2A, compare lanes 2 to 1). Selective inhibition of NF-κB activity was achieved in the 3 CTCL cell lines transfected with the plasmid vector expressing pCMV-IκBα S32/36A, with a dose-dependent decrease in luciferase activity (Figure 2, compare lanes 3 and 4 to lanes 2). For instance, transfection with 1.2 μg of the super-repressor induced a mean inhibition of luciferase activity of 67%, 56%, and 65% in SeAx, HuT-78, and MyLa cells, respectively (n = 4) (Figure 2A).
Because NF-κB has been reported to protect cells from apoptosis, we examined the consequences on cell viability of a down-regulation of the NF-κB pathway by the IκBα super-repressor in CTCL lines. For this purpose, we investigated cell proliferation, sub-G1 peak induction, propidium iodide (PI) uptake, and annexin-V cell-surface expression in CTCL lines transfected with the IκBα S32/36A repressor. Results show that IκBα dominant-negative mutant induces a dose-dependent apoptosis in CTCL cell lines in comparison with cells transfected with control empty vector, as shown by flow cytometry detection of PI-positive SeAx cells at 24 hours (Figure 2B and data not shown). In accordance, loss of cell viability also was observed through microscopic observation of CTCL cell morphology 24 hours after transfection (Figure 2B). Moreover, flow cytometry analysis of DNA content 24 hours after transfection with the super-repressor showed a dose-dependent increase in sub-G1 population. The data obtained with the SeAx cell line showed an increase of the proportion of sub-G1 cells in the population transfected with IκBα super-repressor (Table 2). The alterations of cell viability were confirmed by measuring cell proliferation 24 hours after cell transfection. Thus, as compared to mock-transfected cells, a dose-dependent decrease in thymidine incorporation was detected after transfection of SeAx cells with super-repressor (Table 2).
Effect of IκBα super-repressor on sub-G1 content and cell proliferation index
. | Super-repressor . | . | . | ||
|---|---|---|---|---|---|
. | 0.4 μg . | 1.2 μg . | 2.4 μg . | ||
| Sub-G1 cells | 5.1 ± 3.9 | 9.4 ± 3.6 | 31.1 ± 7.8 | ||
| Proliferation index | 49.6 ± 3.6 | 44.3 ± 3.2 | 10.1 ± 8.2 | ||
. | Super-repressor . | . | . | ||
|---|---|---|---|---|---|
. | 0.4 μg . | 1.2 μg . | 2.4 μg . | ||
| Sub-G1 cells | 5.1 ± 3.9 | 9.4 ± 3.6 | 31.1 ± 7.8 | ||
| Proliferation index | 49.6 ± 3.6 | 44.3 ± 3.2 | 10.1 ± 8.2 | ||
Results are presented as fold increase in percentage of sub-G1 cells, in comparison with control mock-transfected cells, arbitrarily set as 1, and as percentage of cell proliferation, in comparison with control mock-transfected cells, arbitrarily set as 100. Results are expressed as the mean ± SD, n = 3.
Effects of the proteasome inhibitors ALLN and MG-132 on the constitutive activation of NF-κB in CTCL cells and their survival
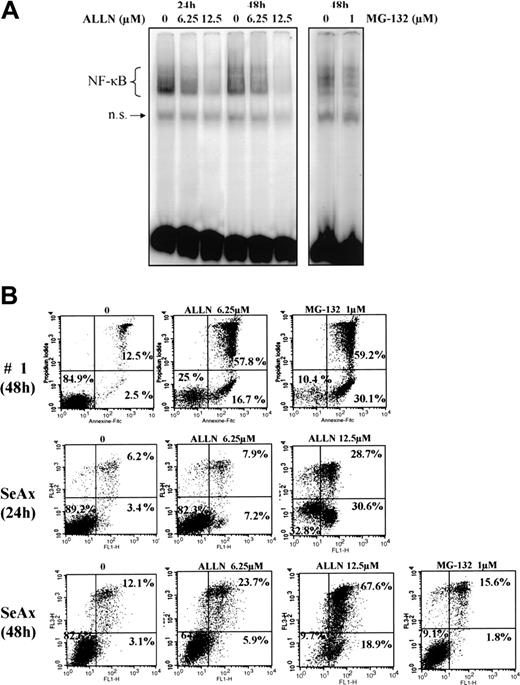
We next investigated the effects of several proteasome inhibitors on NF-κB activation in CTCL. Inhibitors of the 26S proteasome prevent degradation of the negative regulator IκBα, thereby keeping NF-κB inactive due to its sequestration in the cytoplasm. In this set of experiments, EMSAs were performed with CTCL cell lines and with PBLs from SS patients after treatment with proteasome 26S inhibitors ALLN and MG-132. We observed that ALLN and MG-132 both were able to inhibit the constitutive translocation of NF-κB in CTCL cell lines SeAx and MyLa, and in SS PBLs (Figure 3A and data not shown).
The proteasome inhibitors ALLN and MG132 down-regulate the constitutive activation of NF-κB and induce apoptosis of CTCL cells. (A) Inhibition of NF-κB DNA-binding activity by ALLN and MG-132 in SeAx cells. Nuclear extracts of SeAx cells were prepared and subjected to EMSA as described in “Patients, materials, and methods.” In one case, cells were either not treated (lanes 1 and 4) or treated with 6.25 μM (lanes 2 and 5) or 12.5 μM (lanes 3 and 6) ALLN during 24 hours or 48 hours, respectively. In the other, cells were not treated (lane 7) or treated with 1 μM MG-132 (lane 8) during the last 5 hours of a culture lasting 48 hours. The data shown here are representative of 2 independent experiments. (B) Increase in annexin-V binding in SeAx cells and CTCL cells from one patient with Sézary syndrome. Cells were treated with or without ALLN (6.25 and 12.5 μM) for 24 and 48 hours or with MG-132 (1 μM during the 5 last hours of culture), as indicated above each panel. Annexin-V binding was carried out with an annexin V–FITC detection kit and analyzed by flow cytometry. Abscissa and vertical axis represent the fluorescent intensities of annexin V–FITC and PI, respectively. For each panel, the numbers in the bottom left, bottom right, and top right quadrants indicate the percentages of annexin V–/PI– cells (viable cells), annexin V+/PI– cells (apoptotic, viable cells), and annexin V+/PI+ cells (dead cells by apoptosis), respectively. Results are representative of 1 of 3 independent experiments.
The proteasome inhibitors ALLN and MG132 down-regulate the constitutive activation of NF-κB and induce apoptosis of CTCL cells. (A) Inhibition of NF-κB DNA-binding activity by ALLN and MG-132 in SeAx cells. Nuclear extracts of SeAx cells were prepared and subjected to EMSA as described in “Patients, materials, and methods.” In one case, cells were either not treated (lanes 1 and 4) or treated with 6.25 μM (lanes 2 and 5) or 12.5 μM (lanes 3 and 6) ALLN during 24 hours or 48 hours, respectively. In the other, cells were not treated (lane 7) or treated with 1 μM MG-132 (lane 8) during the last 5 hours of a culture lasting 48 hours. The data shown here are representative of 2 independent experiments. (B) Increase in annexin-V binding in SeAx cells and CTCL cells from one patient with Sézary syndrome. Cells were treated with or without ALLN (6.25 and 12.5 μM) for 24 and 48 hours or with MG-132 (1 μM during the 5 last hours of culture), as indicated above each panel. Annexin-V binding was carried out with an annexin V–FITC detection kit and analyzed by flow cytometry. Abscissa and vertical axis represent the fluorescent intensities of annexin V–FITC and PI, respectively. For each panel, the numbers in the bottom left, bottom right, and top right quadrants indicate the percentages of annexin V–/PI– cells (viable cells), annexin V+/PI– cells (apoptotic, viable cells), and annexin V+/PI+ cells (dead cells by apoptosis), respectively. Results are representative of 1 of 3 independent experiments.
To examine the effect of NF-κB down-regulation on CTCL cell survival, we next evaluated CTCL cell viability following treatment with proteasome inhibitors ALLN and MG-132 by flow cytometric analysis of annexin-V and propidium iodide staining. Results shown in Figure 3B demonstrated that ALLN and MG-132 were able to induce apoptosis of SeAx cell line, and SS PBLs, with alterations of mitochondrial transmembrane potential (data not shown). Similar results were observed with MyLa and HuT-78 cell lines (data not shown).
The proteasome inhibitor bortezomib down-regulates the constitutive activation of NF-κB, inhibits proliferation, and induces apoptosis in CTCL cells
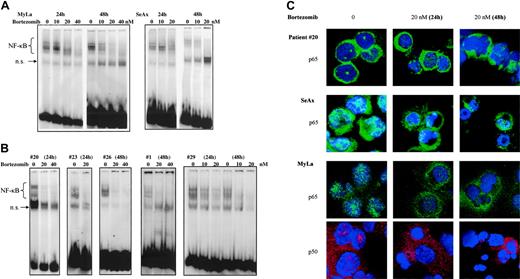
To further investigate specific effects of NF-κB down-regulation on CTCL cell survival, we used bortezomib, a proteasome inhibitor. When EMSA was performed using nuclear extracts from bortezomib-treated CTCL cell lines and patients' cells, NF-κB DNA-binding activity gradually declined at 24 hours and was barely detectable at 48 hours after treatment with 20 and 40 nM bortezomib (Figure 4A,B).
Results from EMSA were confirmed by studies of nuclear NF-κB localization using confocal microscopy, which revealed a decrease of p65 and p50 nuclear staining following treatment of CTCL cell lines and SS PBLs with bortezomib (Figure 4C).
We next investigated the effects of bortezomib on CTCL proliferation in vitro. The [3H]-thymidine uptake by MyLa and SeAx cell lines was measured at the end of 24 and 48 hours of culture in the presence of 5 to 40 nM of bortezomib. Results showed that a 24-hour treatment with bortezomib induced a dramatic decrease in the proliferation of CTCL cell lines MyLa and SeAx (Figure 5). This effect was even more striking after 48 hours of treatment, with minimal residual proliferation in the presence of 10 nM of bortezomib (Figure 5).
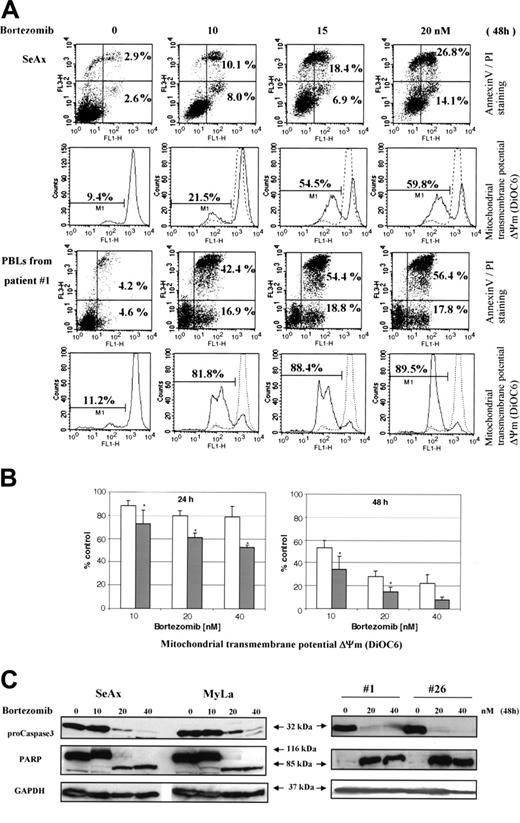
In accordance with its inhibitory effect on NF-κB translocation, bortezomib induced a dose- and time-dependent apoptosis of CTCL cell lines and PBLs from 12 SS patients (Figure 6A,B and data not shown). The mean (± SD) increase of annexin V–positive tumor cells as compared to untreated cells reached 22.9 ± 8.2 and 67.6 ± 6.9-fold in the presence of 10 nM bortezomib, 30.2 ± 7.8 and 78 ± 4.7-fold with 20 nM, and 34.8 ± 10.1 and 89 ± 2.3-fold with 40 nM after incubation during 24 and 48 hours, respectively. Apoptosis of CTCL cells induced by bortezomib was also characterized by a loss of mitochondrial transmembrane potential (Δψm), as shown for 12 SS patients in Figure 6B. The data obtained with control PBLs from 8 healthy volunteers revealed that bortezomib-induced Δψm loss was significantly less pronounced than in tumor cells (Figure 6B).
We also confirmed by Western blot that bortezomib was able to induce specific markers of apoptosis in SeAx cell line and tumor cells from patients with SS. As shown in Figure 6C, an activation of caspase 3, revealed by the decrease in 32-kDa procaspase 3 and a cleavage of PARP to generate a proteolytic fragment of 85 kDa, was observed following treatment with bortezomib for 48 hours.
Bortezomib-induced apoptosis of CTCL cells is associated with enhanced expression of Bax
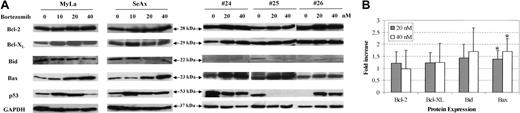
We finally investigated changes in the expression of Bcl-2 family oncoproteins following treatment of CTCL cells with bortezomib. Apoptosis antagonist proteins such as Bcl-2, Bcl-XL, and proapoptotic proteins Bax and Bid were analyzed by immunoblot in CTCL cell lines and in PBLs from patients with SS, in comparison with PBLs from healthy donors. Results showed that treatment of MyLa and SeAx cell lines with bortezomib for 48 hours induced a strong increase in the expression of Bax in CTCL cell line, while a weak increase of Bcl-2 or Bcl-XL levels could be detected (Figure 7). Similar results were observed with extracts from tumor cells from 12 patients with SS, showing a significant increase of 1.39 ± 0.35 and 1.71 ± 0.53 in Bax expression following a 48-hour incubation with 20 and 40 nM of bortezomib, respectively, as compared with untreated cells (Figure 7) (P < .01). However, the fold increase of Bcl-2 (1.21 ± 0.48 and 0.98 ± 0.77) Bcl-XL (1.23 ± 0.43 and 1.24 ± 0.79) and Bid (1.44 ± 0.56 and 1.71 ± 0.98) after 20 and 40 nM bortezomib, respectively, were not significant. We also investigated p53 protein expression in CTCL cell lines and PBLs from 3 SS patients. Although p53 expression was enhanced following bortezomib exposure in CTCL cell lines and in one patient, as expected, no alteration was detected in a second patient, and a decrease was observed in the third one.
The proteasome inhibitor bortezomib inhibits the constitutive activation of NF-κB in CTCL cells in a dose- and time-dependent fashion. CTCL cell lines SeAx and MyLa (A) and CTCL cells from 5 patients with Sézary syndrome (B) were cultured in the presence of bortezomib (10, 20, and 40 nM) during 24 and 48 hours as indicated above each lane or group of lanes. Nuclear extracts were prepared and subjected to EMSA. The data are representative of 2 independent sets of experiments. (C) Confocal microscopy analysis. Confocal images of PBLs from one patient with Sézary syndrome (top panels), SeAx cells (middle panels), and MyLa cells (bottom panels) show the localization of p65 (green) and p50 (red) in untreated cells (left column), cells treated with 20 nM bortezomib for 24 (middle column) or 48 hours (right column). DAPI was used for nuclear staining. Images were acquired as described in Figure 1D.
The proteasome inhibitor bortezomib inhibits the constitutive activation of NF-κB in CTCL cells in a dose- and time-dependent fashion. CTCL cell lines SeAx and MyLa (A) and CTCL cells from 5 patients with Sézary syndrome (B) were cultured in the presence of bortezomib (10, 20, and 40 nM) during 24 and 48 hours as indicated above each lane or group of lanes. Nuclear extracts were prepared and subjected to EMSA. The data are representative of 2 independent sets of experiments. (C) Confocal microscopy analysis. Confocal images of PBLs from one patient with Sézary syndrome (top panels), SeAx cells (middle panels), and MyLa cells (bottom panels) show the localization of p65 (green) and p50 (red) in untreated cells (left column), cells treated with 20 nM bortezomib for 24 (middle column) or 48 hours (right column). DAPI was used for nuclear staining. Images were acquired as described in Figure 1D.
Discussion
Results from both in vitro studies and from therapeutic trials in CTCL patients have provided evidence for a resistance of CTCL to apoptosis triggered by growth factor deprivation, activation-induced cell death (AICD), and antineoplastic chemotherapeutic agents including mitochondrial drugs.10 Since constitutive activation of NF-κB has been demonstrated in several human hematological malignancies including multiple myeloma, large B-cell nonHodgkin lymphoma (NHL), mantle cell lymphoma, and HTLV-I–related acute T-cell leukemia lymphoma,16-18,35 and since several NF-κB–dependent biological activities, such as Bcl-2, have been shown to be up regulated in CTCL cells,36 we investigated the NF-κB status in CTCL and its contribution to survival of tumor T cells. Until now, only one study, based on immunostaining analysis of CTCL skin biopsies with anti-p65 antibodies, suggested a constitutive nuclear translocation of NF-κB complex in patients with MF.19 However, conclusions from these in situ studies were hampered by the lack of any specific assignment of the NF-κB status in neoplastic cells versus nonmalignant lymphocytes. To address the NF-κB status in the malignant subset, we focused our analysis on 3 human CTCL lines: SeAx, HuT-78 (SS), and MyLa (MF), and on peripheral blood lymphocytes derived from patients with Sézary syndrome showing a high ratio of tumor cells. This latter definition was based not only on cytological examination and on analysis of the clonality status by PCR-based analysis of TCRγ V-J DNA sequences, but also by combining TCRVβ/CD4 double-immunostaining analysis. This ensured that all patients presented with a massively predominant clonal population among their peripheral CD4 T-cell repertoire.
Bortezomib inhibits proliferation of MyLa and SeAx cell lines. Cells were cultured in the presence of increasing concentrations of bortezomib (5, 10, 20, and 40 nM) for 24 and 48 hours. DNA synthesis was assessed by [3H] thymidine uptake during the last 16 hours of culture. The results expressed in cpm are the mean ± SEM of 6-way culture wells from 3 independent experiments.
Bortezomib inhibits proliferation of MyLa and SeAx cell lines. Cells were cultured in the presence of increasing concentrations of bortezomib (5, 10, 20, and 40 nM) for 24 and 48 hours. DNA synthesis was assessed by [3H] thymidine uptake during the last 16 hours of culture. The results expressed in cpm are the mean ± SEM of 6-way culture wells from 3 independent experiments.
We show here that in CTCL cell lines and in all SS cases analyzed so far, translocated NF-κB complexes are characteristic of an activation of the so-called canonical pathway, predominantly involving p50/p65 dimers. This pattern is reminiscent of the one observed in T lymphocytes following activation through the TCR, leading to activation of IKK, and mostly dependent upon the kinase activity of IKKβ subunit. Moreover, we also show evidence for the nuclear translocation of p52 in the HuT-78 cell line and in some SS cases, indicating that, in addition to the classical pathway, the alternative pathway also is constitutively activated in a subset of CTCLs. This latter pathway is activated following the functional engagement of IKKα homodimers by the NIK kinase and the subsequent processing of p100 to produce the p52 subunit. Of interest, the alternative pathway has been shown to be activated in HTLV-1–infected T cells through the induction of p100 by oncoprotein Tax37 and in adult T-cell leukemia.38 Further studies are currently carried out to investigate the selective contribution of the alternative pathway to the survival of CTCL cells and to their resistance to apoptosis induced by several agents, including chemotherapeutic agents.
Bortezomib induces mitochondrial transmembrane potential loss, apoptosis, and caspase 3 activation in CTCL cells. SeAx, MyLa, and PBLs from patients with Sézary syndrome were treated with increasing concentrations (5-40 nM) of bortezomib for 24 to 48 hours. (A) Annexin-V binding was carried out with an Annexin V–FITC detection kit and measured by flow cytometry. Abscissa and vertical axis represent the fluorescent intensities of annexin V–FITC and PI, respectively. For each panel, the numbers in bottom right and top right quadrants indicate the percentages of annexin V+/PI– cells (apoptotic, viable cells) and annexin V+/PI+ cells (dead cells by apoptosis), respectively. The mitochondrial transmembrane potential Δψm was detected using the fluorescent probe DiOC6 by flow cytometry. The numbers indicate the percentages of cells exhibiting a decrease in Δψm. The overlaying ashed curve represents the control untreated cells. The results presented for SeAx cells (top panel) are representative of 1 of 6 independent experiments. Results obtained with PBLs from one patient with Sézary syndrome (bottom panel) are representative of data derived from 12 patients (panel B). (B) PBLs from 12 patients with Sézary syndrome and from 8 healthy donors were treated with increasing concentrations (10-40 nM) of bortezomib for 24 and 48 hours (left and right panels, respectively) and assayed for apoptosis by detection of Δψm alterations. The percentages of low Δψm cells were determined in duplicate and normalized using control untreated cells, which were arbitrarily set as 100. Results obtained with PBLs from healthy donors and from SS patients are represented by □ and ▪, respectively. Each value represents the mean ± SEM (n = 12 and n = 8, SS and healthy PBLs, respectively). *Statistically significant difference between healthy and SS PBLs. (C) CTCL cell lines (SeAx and MyLa) and PBLs from 2 patients with Sézary syndrome were treated with increasing doses of bortezomib (10-40 nM) for 48 hours. Cells were analyzed by Western blotting for expression of pro-caspase 3 (32 kDa) and PARP as an intact (116-kDa) or cleaved (85-kDa) protein. GAPDH signal was used as an internal control for loading. Data represent 1 of 2 independent experiments giving similar results.
Bortezomib induces mitochondrial transmembrane potential loss, apoptosis, and caspase 3 activation in CTCL cells. SeAx, MyLa, and PBLs from patients with Sézary syndrome were treated with increasing concentrations (5-40 nM) of bortezomib for 24 to 48 hours. (A) Annexin-V binding was carried out with an Annexin V–FITC detection kit and measured by flow cytometry. Abscissa and vertical axis represent the fluorescent intensities of annexin V–FITC and PI, respectively. For each panel, the numbers in bottom right and top right quadrants indicate the percentages of annexin V+/PI– cells (apoptotic, viable cells) and annexin V+/PI+ cells (dead cells by apoptosis), respectively. The mitochondrial transmembrane potential Δψm was detected using the fluorescent probe DiOC6 by flow cytometry. The numbers indicate the percentages of cells exhibiting a decrease in Δψm. The overlaying ashed curve represents the control untreated cells. The results presented for SeAx cells (top panel) are representative of 1 of 6 independent experiments. Results obtained with PBLs from one patient with Sézary syndrome (bottom panel) are representative of data derived from 12 patients (panel B). (B) PBLs from 12 patients with Sézary syndrome and from 8 healthy donors were treated with increasing concentrations (10-40 nM) of bortezomib for 24 and 48 hours (left and right panels, respectively) and assayed for apoptosis by detection of Δψm alterations. The percentages of low Δψm cells were determined in duplicate and normalized using control untreated cells, which were arbitrarily set as 100. Results obtained with PBLs from healthy donors and from SS patients are represented by □ and ▪, respectively. Each value represents the mean ± SEM (n = 12 and n = 8, SS and healthy PBLs, respectively). *Statistically significant difference between healthy and SS PBLs. (C) CTCL cell lines (SeAx and MyLa) and PBLs from 2 patients with Sézary syndrome were treated with increasing doses of bortezomib (10-40 nM) for 48 hours. Cells were analyzed by Western blotting for expression of pro-caspase 3 (32 kDa) and PARP as an intact (116-kDa) or cleaved (85-kDa) protein. GAPDH signal was used as an internal control for loading. Data represent 1 of 2 independent experiments giving similar results.
Results from transfection experiments of CTCL lines with the IκBα super-repressor demonstrate that inhibition of NF-κB pathway leads to major alterations of CTCL viability and proliferative capacities, in a way similar to other malignancies, such as large activated B-cell lymphoma.16 Although selective inhibition of NF-κB could not be achieved in primary CTCL cells from patients, due to the low level of transfection (data not shown), it is likely that constitutive activation of this pathway is involved in the survival and/or in the proliferation of tumor cells in vivo. This latter hypothesis is further supported by cell death induced by proteasome inhibitors, which inhibit the nuclear translocation of NF-κB complexes, even though this latter set of drugs induces a broad range of changes due to the high number of molecules that are regulated by proteasome 26S activity.20 Thus, although the results presented herein do not rule out the role of other signaling pathways in the survival of CTCL, it appears that NF-κB is a key player in the CTCL resistance to cell death, including apoptosis induced by mitochondrial drugs (data not shown).
The mechanism(s) underlying the constitutive activation of NF-κB in CTCL cells remain(s) unknown and might be due to genetic alterations of NF-κB or IκB genes or to an uncontrolled activation of IKK. Among potential epigenetic factors, we studied the role of TNF, since this cytokine is a widely known activator of NF-κB and since both an up-regulation of the TNF pathway and an autocrine TNF feedback loop have recently been reported in a subset of CTCL.39 From our preliminary observations, it is unlikely that the autocrine production of TNF provides a major contribution to CTCL survival, as anti-TNF neutralizing antibodies did not show any effect either on NF-κB nuclear translocation or on CTCL survival in vitro (data not shown). Studies investigating IKK activity and the phosphorylation status of IκBα are currently in progress, in order to test at which level the NF-κB pathway is activated and which factor/mechanism is involved.
The molecular mechanism of CTCL cell death induced by proteasome inhibitors, mostly bortezomib, remains elusive so far. In mantle cell lymphoma, it has been shown that bortezomib induces a cleavage of Bcl-2 and an inhibition of Bfl-1/A1 and of Bcl-XL protein expression, for instance, a down-regulation of 3 oncoproteins with antiapoptotic properties.17 In contrast, recent works indicate that bortezomib induces apoptosis of multiple myeloma cells without any detectable change in Bcl-2 expression.24 In this latter study, no evidence for change in Bax expression could be evidenced either, supporting the existence of other mechanisms triggered by bortezomib, which remain to be elucidated. In the context of CTCL, even though increased expression of antiapoptotic proteins such as Bcl-2 has been observed in our patients in accordance with results from previous studies,36,40 no evidence for any cleavage or significant down-regulation of Bcl-2 could be found following exposure to bortezomib. Indeed, it is likely that bortezomib exerts its death-inducing effects in CTCL at least partly through an induction of the Bax, which has shown to be capable of forming pores into mitochondrial membrane.41 Of note, NF-κB has been shown to inhibit apoptosis in several experimental systems, and recent findings have demonstrated that NF-κB regulates, through an indirect pathway, the bax gene expression in tumor cell lines.42 In addition, recent data demonstrate that p50/p65 dimers inhibit bax promoter activity, while p50/p50 dimers may enhance it.43 Altogether, these data suggest that translocated p50/p65 complex is involved in the down-regulation of Bax expression in CTCL. Nevertheless, other mechanisms might contribute to bortezomib-induced apoptosis of CTCL, such as induction of p53. Indeed, up-regulation of this protein has been reported to play a key role in the antitumor effects of bortezomib in the context of multiple myeloma.44
Bortezomib alters expression of Bax in CTCL cell lines and tumoral cells from patients with Sézary syndrome. SeAx cells, HuT-78 cells, and PBLs from patients with Sézary syndrome were treated with or without increasing concentrations of bortezomib (10-40 nM) for 48 hours. Expression of Bcl-2 family members was analyzed after Western blotting, using the indicated antibodies (Bcl-2, Bcl-xl, Bid Bax, and p53). The membrane was stripped and reprobed for expression of GAPDH to control for loading (A). The graph (B) shows the relative modifications of protein expression after 20 and 40 nM bortezomib, in comparison with control untreated cells, which were arbitrarily set as 1 (mean increase ± SD, n = 12). *Statistically significant increase as compared to untreated samples.
Bortezomib alters expression of Bax in CTCL cell lines and tumoral cells from patients with Sézary syndrome. SeAx cells, HuT-78 cells, and PBLs from patients with Sézary syndrome were treated with or without increasing concentrations of bortezomib (10-40 nM) for 48 hours. Expression of Bcl-2 family members was analyzed after Western blotting, using the indicated antibodies (Bcl-2, Bcl-xl, Bid Bax, and p53). The membrane was stripped and reprobed for expression of GAPDH to control for loading (A). The graph (B) shows the relative modifications of protein expression after 20 and 40 nM bortezomib, in comparison with control untreated cells, which were arbitrarily set as 1 (mean increase ± SD, n = 12). *Statistically significant increase as compared to untreated samples.
The present set of data from in vitro studies also suggest that bortezomib might be useful in vivo for the treatment of advanced stages of CTCL showing resistance to classical antineoplastic polychemotherapy. Indeed, the low response rate of SS to systemic therapies and the promising results of bortezomib use for treatment of hematological malignancies with NF-κB activation, such as multiple myeloma, raise the interest of clinical trials investigating the efficacy of bortezomib in patients with refractory forms of CTCL.
Prepublished online as Blood First Edition Paper, October 11, 2005; DOI 10.1182/blood-2005-06-2536.
Supported by grants from Association pour la Recherche contre le Cancer, Fondation pour la Recherche Médicale, Canceropôle Ile-de-France, and Société Française de Dermatologie.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Dr Nathalie Parquet for referring patients, to Pascale Paine, Laurence Grollet, and Dr Jean-Michel Cayuela for providing results of molecular analysis of clonality, to Michel Schmidt for confocal microscopy, to Franck Verrechia for his help in transduction experiments, to Alain Mauriel for helpful discussion, and to Millenium Pharmaceutical Inc, Cambridge, MA, for providing Velcade.

![Figure 5. Bortezomib inhibits proliferation of MyLa and SeAx cell lines. Cells were cultured in the presence of increasing concentrations of bortezomib (5, 10, 20, and 40 nM) for 24 and 48 hours. DNA synthesis was assessed by [3H] thymidine uptake during the last 16 hours of culture. The results expressed in cpm are the mean ± SEM of 6-way culture wells from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/6/10.1182_blood-2005-06-2536/4/m_zh80060692870005.jpeg?Expires=1769136772&Signature=iQHDl-2mrw~Hvhx-IZPsPsGyjjiahZ~FxpCiME5gYxWE4IKkfdXzlKV7crmJEE6bvOTLOVDumdakUatcwU6FsbAjKM~bbiMLFL1wXTJOoH1CqlmghqmBKhTwZwGsq-SKC5SiHH~2atLI7FUZCAxfgLpmwufKeRaZyls2Hydsm9y35K4ydPQOisfSCGTH3G~PtB-o5jdR4t9i7g2NprYi~za-kult9NNJrZzBpAxN6A5mVr9XT9dE85N0Ncs9aRnQie6qpfWmliYoCOifQ6V5lf2d43q6ruabCyQvwsa6iiql8B~~7tNhAXZTpo5Yv6wbhq-LUUpDCe3Tr-8g3uf25g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal