Abstract
T-cell receptor (TCR) diversity of virus-specific CD8+ T cells likely helps prevent escape mutations in chronic viral infections. To understand the dynamics of the virus-specific T cells in more detail, we followed the evolution of the TCR repertoire specific for a dominant HLA-B*08–restricted epitope in Nef (FLKEKGGL) in a cohort of subjects infected with HIV. Epitope-specific CD8+ T cells used structurally diverse TCR repertoires, with different TCRβ variable regions and with high amino acid diversity within antigen recognition sites. In a longitudinal study, distinct Vβ populations within the HIV-specific TCR repertoire expanded simultaneously with changes in plasma viremia, whereas other Vβ populations remained stable or even decreased. Despite antigenic variation in some subjects, all subjects had the consensus sequence present during the study period. Functional analysis of distinct Vβ populations revealed differences in HIV-specific IFN-γ secretion ex vivo as well as differences in tetramer binding, indicating functional heterogeneity among these populations. This contrasts with findings in a subject on antiretroviral therapy with suppression of viremia to less than 50 copies/mL, where we observed long-term persistence of a single clonotype. Our findings illustrate the flexibility of a heterogeneous HIV-1–specific CD8+ TCR repertoire in subjects with partial control of viremia.
Introduction
Increasing evidence suggests that pathogen-specific CD8+ T cells with diverse T-cell receptor (TCR) repertoires are beneficial for optimal suppression of rapidly evolving viruses such as hepatitis C,1 LCMV,2 and HIV/SIV.3-7 Virus-specific CD8+ T cells are an important effector arm of the immune defense against HIV-1/SIV infection.8-14 After acute infection partial viral control is associated with persistent and often strong CD8+ T-cell responses directed against several HIV epitopes.15,16 One such immunodominant epitope lies within HIV Nef (“FLKEKGGL” [residues 90-97]) and is restricted by HLA-B*080112,17-20 (“B8-FL8”). Epitope-specific responses are the sum of individually responding T-cell clones with discrete T-cell receptors and a structurally diverse TCR repertoire is likely to be necessary for optimal suppression of viremia in infections with the ability to rapidly evolve escape mutations.1,3,21-24 A diverse array of T-cell receptors might decrease the chance of cytotoxic T lymphocyte (CTL) escape due to cross-recognition of mutated epitopes25,26 and is also more likely to contain high-affinity T-cell clones able to efficiently recognize targeted epitopes and therefore mediate better control of virus replication.23
In HIV infection, perturbations of the peripheral T-cell receptor repertoire have been described during primary infections,27-30 and it has been hypothesized they are due to the clonal expansion and exhaustion of HIV-specific T-cell clones during the initial viral burst of viremia after acute infection.31-33 However, long-term persistence of HIV-1–specific CD8+ T-cell clones has been observed in individuals with chronic or progressive HIV-1 infection.20,34 The possible effect of TCR diversity on disease outcome emphasizes the need to evaluate in more detail the dynamics of HIV epitope-specific T cells in vivo and to investigate pathogen-specific effector functions of structurally diverse T-cell populations at the HIV epitope level.
In this study, we longitudinally followed the TCR repertoire of HIV-1–specific CD8+ T-cell responses in vivo in subjects with partial control of viremia. Most of the individuals were treated during acute infection and temporarily controlled plasma viremia after one or more supervised treatment interruptions (STIs).11,35 In these subjects, the T-cell receptor repertoire of HIV-1–specific CD8+ T cells was oligoclonal with high structural diversity. Longitudinal analysis showed discordant expansions and contractions of distinct Vβ populations in response to changing levels of viremia. Functional analysis of distinct Vβ populations revealed differences in antigen-specific IFN-γ secretion ex vivo. Our study illustrates the functional diversity and kinetics of HIV-1–specific CD8+ T cells directed against an immunodominant HLA-class I–restricted epitope.
Materials and methods
Participants studied
Participants were enrolled at Boston Massachusetts General Hospital, Vanderbilt University Medical center, and Medizinische Hochschule Hannover. The study was approved by the Partners Human Research committee at Massachusetts General Hospital, and each participant gave informed consent for study participation.
HLA class I tissue typing
The HLA type of the individuals was determined by sequence-specific primer–polymerase chain reaction (SSP-PCR)36 performed at the Massachusetts General Hospital (MGH) Tissue Typing Laboratory or at DCI Laboratory (Nashville, TN). Only subjects with HLA-B*08 were included in the study.
Peptides
Peripheral blood mononuclear cells (PBMCs) from each subject were screened for recognition of overlapping peptides from p17 Gag, p24 Gag, Nef, RT, gp41, gp120, Tat, and Rev and peptides representing optimal epitopes20 presented by HLA class I molecules expressed by each subject (median, 24 peptides) in enzyme-linked immunospot (ELISpot) assays or intracellular cytokine staining (ICS). The sequences for the peptides corresponded to the B clade SF2 sequence. Peptides were synthesized at the Massachusetts General Hospital peptide synthesis core facility.
ELISpot assays
Fresh PBMCs were plated at 100 000 cells/well in 96-well polyvinylidene plates (Millipore, Bedford, MA) precoated with 0.5 μg/mL anti–IFN-γ mAb, 1-DIK (Mabtech, Stockholm, Sweden). The end concentration of peptides was 10 μM. The plates were incubated overnight at 37°C, 5% CO2, and developed as described previously.37,38 Background was less than 20/106 PBMCs (2 spots/well at 100 000 PBMCs/well) in all cases. Responses of more than 60 IFN-γ spot-forming cells/106 PBMCs were therefore considered as significant positive responses.
Flow cytometric evaluation of surface antigens and tetramer staining
Antibodies specific for human CD3, CD4, and CD8 were purchased from Becton Dickinson (BD Biosciences, San Jose, CA). For staining of T-cell receptor variable regions the following antibodies were used: BL37.2 (Vβ1), MPB2D5 (Vβ2), CH92 (Vβ3), WJF24 (Vβ4), IMMU157 (Vβ5.1), 36213 (Vβ5.2), 3D11 (Vβ5.3), ZOE (Vβ7.1), ZIZOU4 (Vβ7.2), 56C5.2 (Vβ8), FIN9 (Vβ9), C21 (Vβ11), VER2.32 (Vβ12), IMMU222 (Vβ13.1), H132 (Vβ13.2), JU74.3 (Vβ13.6), CAS1.1.3 (Vβ14), TAMAYA1.2 (Vβ16), E17.5F3 (Vβ17), BA62.6 (Vβ18), ELL1.4 (Vβ20), IG125 (Vβ21.3), IMMU546 (Vβ22), and AF23 (Vβ23) (all derived from Beckman Coulter, San Diego, CA). Tetramers were routinely titrated for estimation of optimal concentration and tested for nonspecific binding as well. Antibodies and tetramers were added to freshly isolated PBMCs at room temperature for 30 minutes. Stained samples were analyzed on a FACSCalibur flow cytometer using CELLQuest software (Becton Dickinson). There was no significant effect of Vβ antibody staining on tetramer staining of T-cell clones or PBMCs. For some experiments CD8+ T cells were purified using monoclonal antibodies directed against CD8 coupled to magnetic beads (Invitrogen, Carlsbad, CA) according to the instructions of the manufacturer.
Peptide–MHC tetramer assays
Peptide–MHC tetramers (HLA-B*0801–FLKEKGGL) were synthesized as described previously.39 Stainings were performed by incubating 0.5 to 2 million PBMCs for 30 minutes at room temperature with the appropriate tetramer. Control samples were PBMCs from HLA-mismatched HIV-infected persons.
Intracellular IFN-γ staining
Intracellular cytokine staining assays were performed as previously described38 with 4 μM peptide, anti-CD28 and anti-CD49d MAbs (1 μg/mL each; Becton Dickinson) and brefeldin A (Sigma, St Louis, Missouri)/mL. After a 6-hour incubation cells were washed and stained with the surface antibodies anti-CD8 and anti-CD4 (Becton Dickinson). Cells were fixed and permeabilized according to the manufacturer's instructions (Caltag, Burlingame, CA) and anti–IFN-γ mAb (Becton Dickinson) was added at 4°C for 30 minutes. Cells were washed and analyzed on a FACScalibur flow cytometer (Becton Dickinson). Control conditions were established by use of autologous PBMCs, which were treated identically but without peptide stimulation.
Purification of tetramer-positive cells by magnetic beads
Tetramer-positive CD8+ T cells were purified by using a commercial assay (Miltenyi Biotec, Auburn, CA) as described previously.1,4 T cells (30-40 × 106) were incubated with PE-conjugated tetramers. After washing, anti-PE antibodies conjugated with magnetic beads were added and separated on magnetic columns according to the manufacturer's protocol (Miltenyi Biotec). In all experiments, the purity of the enriched populations was greater than 95% (range, 96%-99%).
cDNA synthesis and TCR sequencing
RNA was extracted from purified T cells using STAT-60 (Tel-Test B, Friendswood, TX). A modified anchored reverse transcriptase (RT)–PCR was performed with Powerscript Reverse transcriptase (Clontech, Palo Alto, CA) from total RNA as previously described1,4,40 using a gene-specific primer for the TCR β-constant region with a modified cDNA anchor primer. Negative controls were included at all amplification steps. Amplification of the cDNA by PCR was performed using TCR constant region–based primers and an anchor-specific primer 5′-AAT CCT TTC TCT TGA CCA TG-3′ (Clontech, Palo Alto, CA). PCR products of 500 to 600 base pairs were gel purified and cloned using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA). Selected colonies were sequenced using the Taq DyeDeoxy Terminator cycle sequencing Kit (PE Applied Biosystems, Norwalk, CT) and capillary electrophoresis on an ABI 3700 PRISM automated sequencer (PE Applied Biosystems, Foster City, CA).
Isolation of HIV-1–specific CTL clones
CTL clones were isolated as previously described3 and maintained in R10 with 100 U/mL rlL-2. Clones were stimulated with irradiated feeder cells and 1 μg/mL monoclonal antibody anti-CD3 antibody 12F6 (a generous gift from Johnson Wong, Massachusetts General Hospital). Clones were screened for HIV peptide–specific activity against autologous and/or HLA class I–matched B-LCL pulsed with the respective peptides.
Sequencing of autologous virus
Autologous virus was sequenced from plasma RNA using population sequencing, as described.41 Viral RNA was isolated from plasma, and nested PCR was conducted by using a set of described primers specific for HIV-1.41 First-round PCR cycling conditions were as follows: 94 °C for 2 minutes, 35 to 50 cycles of 30 seconds at 94 °C, 30 seconds at 56 °C, 2 minutes at 72 °C, and a final extension of 68 °C for 20 minutes, and nested PCR reactions were shortened to a 1-minute extension time. PCR fragments were then gel purified and sequenced bidirectionally on an ABI 3100 PRISM automated sequencer. Sequencher (Gene Codes Corp, Ann Arbor, MI) and MacVector 4.1 (Oxford Molecular, Oxford, United Kingdom) were used to edit and align sequences.
Statistical analysis
Values are expressed as mean plus or minus standard deviation (SD). Comparisons between groups were performed using Mann-Whitney test or Student t test, where appropriate. Comparison analyses were performed with SPSS version 11.01 (SPSS Inc, Chicago, IL). All reported P values are 2-tailed, and a P value less than .05 was considered significant.
Results
B8-FL8 is a frequently targeted immunodominant HIV epitope in HIV-1–infected HLA-B*08+ individuals
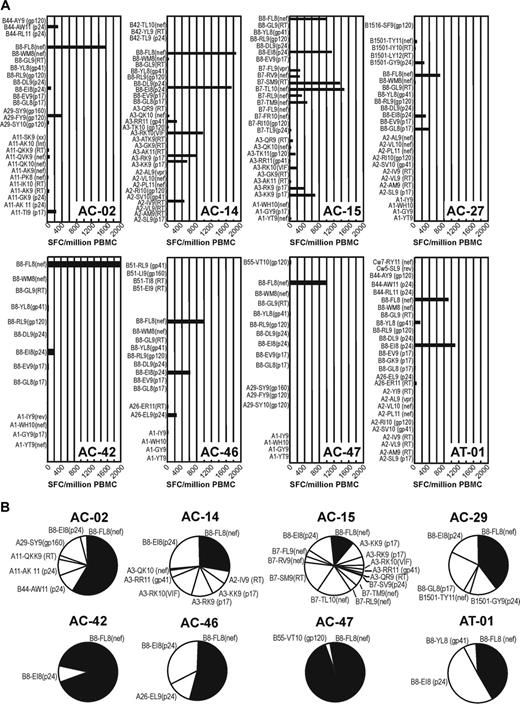
This study population was composed of subjects expressing the HLA-B*08 allele, of which 7 were diagnosed during acute or early HIV-1 infection.11,12 The HLA alleles of 8 study subjects and their clinical characteristics are displayed in Table 1. HIV-1–specific T-cell responses were measured with intracellular cytokine stainings and ELISpot after stimulation with overlapping peptides representing the entire HIV genome as described previously12 and by ELISpot with a panel of known HIV epitopes determined by the individual's HLA class I type (Figure 1). CD8+ T-cell responses against B8-FL8 were the strongest CD8+ T-cell responses in 6 of 8 individuals (Figure 1). The frequency of B8-FL8–specific CD8+ T cells ranged between 0.92% and 3.11% of CD8+ T cells as determined by B8-FL8 tetramer staining. Six of 8 subjects also recognized the HLA-B*08–restricted HIV-epitope B8-EI8 (HIVp24 residues 128-135/“EIYKRWII”). In addition, 11 HLA-B*08–positive subjects with chronic HIV disease predominantly recognized B8-F8 and B8-EI8 (data not shown). These data confirm earlier observations12,18,42 that the B8-FL8 is a frequently targeted and immunodominant HIV epitope in HIV-1–infected individuals positive for the HLA-B*08 allele.
Demographics of subjects studied
Patient ID . | Year of birth . | HLA . | Cohort . | Treatment status . | Log HIV RNA copies/mL plasma* . | CD4+ T-cell count/μL* . |
|---|---|---|---|---|---|---|
| AC-02 | 1955 | A11, 29; B8, 44; Cw7, 4 | Acute (STI) | Off | 2.13 | 740 |
| AC-14 | 1953 | A2, 3; B8, 62; Cw7, 10 | Acute (STI) | Off | 3.06 | 774 |
| AC-15 | 1957 | A1, 3; B7, 8; Cw7,- | Acute (STI) | Off | 3.21 | 506 |
| AC-27 | 1939 | A23, 30; B8, 35; Cw4, 7 | Acute | Off | 4.40 | 395 |
| AC-42 | 1956 | A1,-; B8, 18, Cw5, 7 | Acute | On | 1.70 | 818 |
| AC-46 | 1951 | A1, 26; B8, 51; Cw7, 15 | Acute (STI) | On/off | 1.70 | 986 |
| AC-47 | 1974 | A1, 29; B8, 55; Cw3, 7 | Acute | Off | 4.05 | 360 |
| AT-01 | 1966 | A0201, 26; B8, 44; Cw5, 7 | Chronic | Off | 5.47 | 129 |
Patient ID . | Year of birth . | HLA . | Cohort . | Treatment status . | Log HIV RNA copies/mL plasma* . | CD4+ T-cell count/μL* . |
|---|---|---|---|---|---|---|
| AC-02 | 1955 | A11, 29; B8, 44; Cw7, 4 | Acute (STI) | Off | 2.13 | 740 |
| AC-14 | 1953 | A2, 3; B8, 62; Cw7, 10 | Acute (STI) | Off | 3.06 | 774 |
| AC-15 | 1957 | A1, 3; B7, 8; Cw7,- | Acute (STI) | Off | 3.21 | 506 |
| AC-27 | 1939 | A23, 30; B8, 35; Cw4, 7 | Acute | Off | 4.40 | 395 |
| AC-42 | 1956 | A1,-; B8, 18, Cw5, 7 | Acute | On | 1.70 | 818 |
| AC-46 | 1951 | A1, 26; B8, 51; Cw7, 15 | Acute (STI) | On/off | 1.70 | 986 |
| AC-47 | 1974 | A1, 29; B8, 55; Cw3, 7 | Acute | Off | 4.05 | 360 |
| AT-01 | 1966 | A0201, 26; B8, 44; Cw5, 7 | Chronic | Off | 5.47 | 129 |
All subjects were men. Off indicates without antiretroviral treatment during study; on, antiretroviral treatment during study; on/off, treatment interruption; and STI, individual participating in the structural treatment interruption trial.11
At study entry.
Structural diversity of immunodominant HIV-specific CD8+ T cells
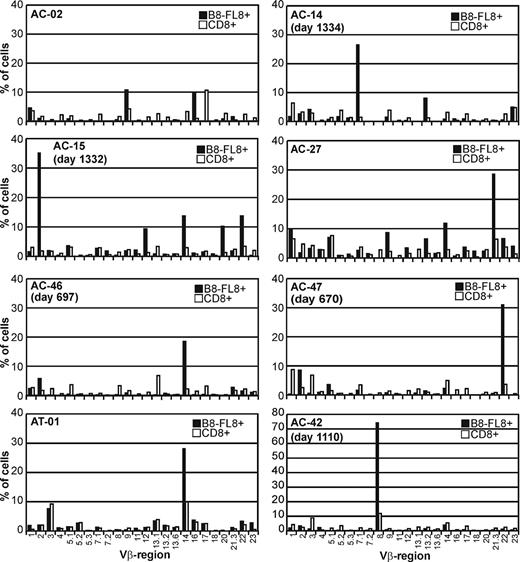
We next evaluated the TCR diversity of CTLs recognizing the B8-FL8 epitope by staining with a panel of Vβ-specific antibodies4 and by sequencing the TCRβ chains of purified tetramer-positive CD8+ T cells. These antibodies detect 24 Vβ regions that belong to 19 different Vβ families. Figure 2 summarizes Vβ repertoires of unselected CD8+ T cells (white bars) in comparison with the Vβ repertoire of B8-FL8 tetramer–positive CD8+ T cells (black bars) in 8 study subjects. Within the total CD8+ T-cell repertoire, we observed the utilization of a broad range of different Vβ genes with occasional expansions of single Vβ regions that comprised more than 10% of the entire CD8+ TCR repertoire. In B8-FL8 tetramer–positive CD8 cells (black bars), we found a more restricted utilization of different Vβ regions as compared with the total T-cell repertoire. In 6 of 8 individuals we observed distinct expansions of B8-FL8–specific T cells in which a single Vβ chain was expressed by greater than 25% of the tetramer-positive CD8+ T cells. Although some Vβ regions, such as Vβ9, Vβ14, or Vβ21.3, were frequently used within this cohort, we observed a broad range of 3 to 11 different Vβ regions used per individual. More importantly, there was no evidence for a close phylogenetic relation or structural similarities within the CDR1 and CDR2 regions between the Vβ regions that were preferentially used by B8-FL8 tetramer–positive CD8 cells (data not shown). These data demonstrate the ability to identify subpopulations of tetramer-positive T cells by their TCR usage and provide evidence for structurally diverse epitope-specific Vβ utilization.
To analyze the amino acid diversity of the antigen-binding site in more detail1,4 and to confirm the results from the Vβ antibody staining, we analyzed the TCR repertoire of B8-FL8–specific T cells in 3 subjects. Two individuals (AC-14 and AC-15) maintained relative control of viremia, below 10 000 HIV-1 RNA copies/mL plasma, for longer than 1 year before entering the study. The third individual (AC-42) experienced a rapid rebound of viremia while interrupting antiretroviral treatment (data not shown) and was maintained on highly active antiretrovial therapy (HAART) with optimal viral suppression for longer than 2 years before entering our study.
We isolated HIV-specific CD8+ T cells based on their B8-FL8 tetramer binding (95%-99% purity after enrichment; data not shown) and sequenced the TCRβ clonotypes as previously described.1,4,6,40 In subjects AC-14 and AC-15, 6 and 7 different clonotypes, respectively, could be detected within the B8-FL8–specific CD8+ TCR repertoire. Most CDR3 amino acid sequences were heterogenous, and there was no common motif detectable throughout the sequences. This was in sharp contrast to AC-42 in whom the TCR repertoire was dominated by a single clonotype representing the vast majority of the sequences (Table 2).
TCR sequencing of B8-FL8–specific CD8+ T cells
Patient ID (IMGT nomenclature for TCRβ-variable regions) . | Vβ region . | Dβ region . | Jβ region . | . | Clonotypes, no./no. total . | Vβ staining, % . |
|---|---|---|---|---|---|---|
| AC-42 | ||||||
| BV2S1 (20-1) | C S A | Q G V A G G P | Y E Q Y | 2.7 | 1/82 | 3.42 |
| BV2S1 (20-1) | C S A | R D D L G R V G | N Q P Q H | 1.5 | 1/82 | 3.42 |
| BV4S1 (29-1) | C S V | E D R G T G V V | N E Q F | 2.1 | 2/82 | NA |
| BV6S5 (7-2) | C A S S | L E R D | Y E Q Y | 2.7 | 1/82 | NA |
| BV8S1 (12-3) | C A S S | F F S G T A S | Q P Q H | 1.5 | 77/82 | 74.36 |
| AC-14 | ||||||
| BV7S1 (4-1) | C A S S | Q E Q G R V V G | N E Q F | 2.1 | 4/54 | 13.31 |
| BV5S5 (5-4) | C A S S | L A A L D R V G W | D T Q Y | 2.3 | 2/54 | NA |
| BV5S2 (5-6) | C A S S | L A A L D R V G W | D T Q Y | 2.3 | 3/54 | 1.92 |
| BV5S5 (5-4) | C A S | I L A R G G V W T | D T Q Y | 2.3 | 36/54 | NA |
| BV5S1 (5-1) | C A S S | F T G A T D | Y D Y T | 1.2 | 8/54 | 10.66 |
| BV3S1 (28) | C A | T Q T D R P V S D | Q P Q H | 1.5 | 1/54 | 2.53 |
| AC-15 | ||||||
| BV12S1 (10-3) | C A I S | E T G E | E T Q Y | 2.5 | 2/21 | 9.35 |
| BV14S1 (27) | C A S S | L G Q G L A | N Y G Y T | 1.2 | 3/21 | 13.73 |
| BV20S1 (30) | C A W | D V K D R R I G | N E Q F | 2.1 | 5/21 | 10.18 |
| BV22S1 (2) | C A S S | E F P G L | S Y E Q Y | 2.7 | 1/21 | 12.38 |
| BV2S1 (20-1) | C S A R | A Q R T | Y G Y T | 1.2 | 1/21 | 35.23 |
| BV2S1 (20-1) | C S A | T I L A G V P Y G | E Q Y | 2.7 | 6/21 | 35.23 |
| BV2S1 (20-1) | C S A | S S Q R G G I | Y E Q Y | 2.7 | 3/21 | 35.23 |
Patient ID (IMGT nomenclature for TCRβ-variable regions) . | Vβ region . | Dβ region . | Jβ region . | . | Clonotypes, no./no. total . | Vβ staining, % . |
|---|---|---|---|---|---|---|
| AC-42 | ||||||
| BV2S1 (20-1) | C S A | Q G V A G G P | Y E Q Y | 2.7 | 1/82 | 3.42 |
| BV2S1 (20-1) | C S A | R D D L G R V G | N Q P Q H | 1.5 | 1/82 | 3.42 |
| BV4S1 (29-1) | C S V | E D R G T G V V | N E Q F | 2.1 | 2/82 | NA |
| BV6S5 (7-2) | C A S S | L E R D | Y E Q Y | 2.7 | 1/82 | NA |
| BV8S1 (12-3) | C A S S | F F S G T A S | Q P Q H | 1.5 | 77/82 | 74.36 |
| AC-14 | ||||||
| BV7S1 (4-1) | C A S S | Q E Q G R V V G | N E Q F | 2.1 | 4/54 | 13.31 |
| BV5S5 (5-4) | C A S S | L A A L D R V G W | D T Q Y | 2.3 | 2/54 | NA |
| BV5S2 (5-6) | C A S S | L A A L D R V G W | D T Q Y | 2.3 | 3/54 | 1.92 |
| BV5S5 (5-4) | C A S | I L A R G G V W T | D T Q Y | 2.3 | 36/54 | NA |
| BV5S1 (5-1) | C A S S | F T G A T D | Y D Y T | 1.2 | 8/54 | 10.66 |
| BV3S1 (28) | C A | T Q T D R P V S D | Q P Q H | 1.5 | 1/54 | 2.53 |
| AC-15 | ||||||
| BV12S1 (10-3) | C A I S | E T G E | E T Q Y | 2.5 | 2/21 | 9.35 |
| BV14S1 (27) | C A S S | L G Q G L A | N Y G Y T | 1.2 | 3/21 | 13.73 |
| BV20S1 (30) | C A W | D V K D R R I G | N E Q F | 2.1 | 5/21 | 10.18 |
| BV22S1 (2) | C A S S | E F P G L | S Y E Q Y | 2.7 | 1/21 | 12.38 |
| BV2S1 (20-1) | C S A R | A Q R T | Y G Y T | 1.2 | 1/21 | 35.23 |
| BV2S1 (20-1) | C S A | T I L A G V P Y G | E Q Y | 2.7 | 6/21 | 35.23 |
| BV2S1 (20-1) | C S A | S S Q R G G I | Y E Q Y | 2.7 | 3/21 | 35.23 |
TCR nucleotide sequences and predicted amino acid sequences of the purified T-cell populations. The number of sequences and the total number of molecular clones with rearranged TCR sequences are indicated as well as the frequency of Vβ regions determined at a separate study visit. Data for AC-42 clonotypes are presented for day 1026, and those for Vβ staining are from day 1110. Data for AC-14 clonotypes are from day 1440, and those for Vβ staining are from day 1500. Data for AC-15 clonotypes are from day 1437, and those for Vβ staining are from day 1332.
NA indicates that no commercial antibody was available against this Vβ region.
In 2 subjects we were also able to isolate CTL clones specific for this epitope. We independently isolated B8-FL8–specific CD8+ T-cell clones at 2 different time points from subject AC-42 (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). Peptide-specific IFN-γ secretion and cytotoxicity to peptide-pulsed autologous target cells confirmed the specificity for B8-FL8 of these clones (data not shown). These clones used the identical TCRβ sequence that was identified as the majority population in this individual ex vivo (Table 2; Figure S1). Subject AC-46 showed predominant usage of Vβ2 and Vβ14 by staining with monoclonal Vβ-specific antibodies in combination with tetramer staining (Figure 2). Five different B8-FL8–specific CTL clones were isolated via limiting dilution cloning of peripheral CD8+ T cells, and TCR analysis demonstrated Vβ2 or Vβ14 usage for all 5 isolated CTL clones (Figure S1). Although TCR sequencing data provides more information regarding the diversity of the TCR repertoire, these results were concordant with the TCR variable region usage as assessed by antibody staining. In summary, we observed a heterogeneous oligoclonal TCR repertoire in subjects controlling viremia characterized by structural diversity within the antigen recognition site and by the utilization of a variety of TCR variable regions.
HIV-specific CD8+T-cell repertoire in HIV-infected HLA-B*08+individuals. (A) HIV-specific CD8+ T-cell repertoire tested by IFN-γ ELISpot in 8 HLA-B*08+ HIV-infected individuals as described in “Materials and methods.” CTL magnitudes are expressed as spot-forming cells per million PBMCs (SFC/million PBMCs). (B) Pie charts display the hierarchy of IFN-γ+ CD8+ T-cell responses in the respective individual.
HIV-specific CD8+T-cell repertoire in HIV-infected HLA-B*08+individuals. (A) HIV-specific CD8+ T-cell repertoire tested by IFN-γ ELISpot in 8 HLA-B*08+ HIV-infected individuals as described in “Materials and methods.” CTL magnitudes are expressed as spot-forming cells per million PBMCs (SFC/million PBMCs). (B) Pie charts display the hierarchy of IFN-γ+ CD8+ T-cell responses in the respective individual.
Vβ repertoire of B8-FL8 tetramer–positive CD8+T cells. Vβ usage of unselected peripheral CD8+ T cells (□) and B8-FL8 tetramer–positive CD8+ T cells (▪) in 8 HIV-infected individuals. Percentages indicate the frequency of Vβ+ cells within the respective T-cell population.
Vβ repertoire of B8-FL8 tetramer–positive CD8+T cells. Vβ usage of unselected peripheral CD8+ T cells (□) and B8-FL8 tetramer–positive CD8+ T cells (▪) in 8 HIV-infected individuals. Percentages indicate the frequency of Vβ+ cells within the respective T-cell population.
Longitudinal analysis of HIV-1–specific CD8+ T cells
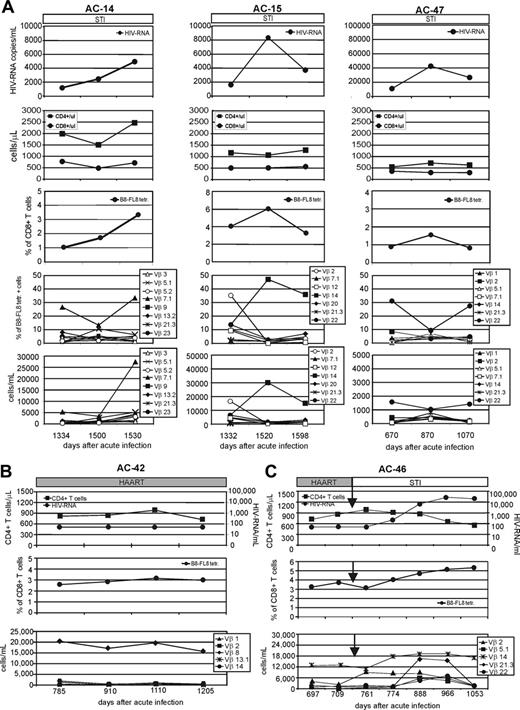
HIV-1–specific immune responses need to respond to high levels of viral replication and a high degree of genetic variability. To understand the relation between viral dynamics and the HIV-1–specific immune response in more detail at the epitope level, we next analyzed the kinetics of B8-FL8–specific CD8+ T cells in HIV-1–infected individuals. It has been previously shown that the breath and magnitude of HIV-specific T-cell responses may be substantially altered in response to changes in viral load that occur during STI.12,18,42 We were able to follow the magnitude and breadth of a dominant CD8+ T-cell response in subjects with varying levels of viremia. In 3 subjects who suppressed plasma viremia after treatment discontinuation, we followed the evolution of the B8-FL8–specific CD8+ T cells for longer than 1 year (Figure 3A). Two of the individuals (AC-14 and AC-15) maintained control of viremia below 10 000 HIV-1 RNA copies/mL plasma for more than 1 year before entering the study. The third individual (AC-47), also off therapy for more than 1 year, had a higher HIV-1 viral load, ranging between 10 000 and 50 000 copies/mL. All 3 subjects had B8-FL8 tetramer–positive populations within their CD8+ T cells ranging from 1% to more than 5%. Changes in levels of HIV-1 plasma viremia were paralleled by synchronous changes in the magnitude of the tetramer-positive population over time (Figure 3A). For AC-42, who was on HAART during the entire study period, we observed a strong B8-FL8 tetramer–positive population that remained stable (ranging between 2.5% and 3.5% of CD8+ T cells) during the entire study period with more than 1 year of follow-up (Figure 3B). In a fifth individual (AC-46) the frequency of tetramer-positive cells was stable while this subject was on HAART; however, we observed an increase in the frequency of B8-FL8 tetramer-positive CD8+ T cells after initiation of STI, which was synchronous with the increase in the level of viremia (Figure 3C). These data give evidence for an adaptive process of HIV-1–specific CD8+ T cells directed against immunodominant epitopes in response to changes in viremia.
Longitudinal analysis of viral load, B8-FL8 tetramer expression, and B8-FL8–specific Vβ repertoire. (A) Longitudinal analysis of HIV-1 RNA copies per milliliter of plasma, absolute numbers of CD4+ and CD8+ T cells, percentage of B8-FL8 tetramer–positive cells per peripheral CD8+ T cells, as well as the relative and absolute number of B8-FL8–specific Vβ populations in 3 untreated HIV-infected individuals (AC-14, AC-15, and AC-47). Days indicate time after diagnosis of acute HIV infection. (B) Longitudinal analysis of HIV-1 RNA copies per milliliter of plasma, percentage of B8-FL8 tetramer–positive cells per peripheral CD8+ T cells, and absolute number of B8-FL8–specific Vβ populations for an HIV-infected patient (AC-42) under antiretroviral treatment. (C) Longitudinal analysis as in panel A for an HIV-infected patient (AC-46) undergoing STI after a phase of optimal viral suppression under antiretroviral treatment.
Longitudinal analysis of viral load, B8-FL8 tetramer expression, and B8-FL8–specific Vβ repertoire. (A) Longitudinal analysis of HIV-1 RNA copies per milliliter of plasma, absolute numbers of CD4+ and CD8+ T cells, percentage of B8-FL8 tetramer–positive cells per peripheral CD8+ T cells, as well as the relative and absolute number of B8-FL8–specific Vβ populations in 3 untreated HIV-infected individuals (AC-14, AC-15, and AC-47). Days indicate time after diagnosis of acute HIV infection. (B) Longitudinal analysis of HIV-1 RNA copies per milliliter of plasma, percentage of B8-FL8 tetramer–positive cells per peripheral CD8+ T cells, and absolute number of B8-FL8–specific Vβ populations for an HIV-infected patient (AC-42) under antiretroviral treatment. (C) Longitudinal analysis as in panel A for an HIV-infected patient (AC-46) undergoing STI after a phase of optimal viral suppression under antiretroviral treatment.
Discordant variations within the antigen-specific Vβ repertoire in individuals with replicating virus
Although it has been shown that different T-cell clones can vary in their responsiveness to a range of antigen concentrations, or to variations within the recognized epitopes, evidence that this might influence the dynamics of the HIV-1–specific TCR repertoire in vivo is still lacking. With most B8-FL8–specific CD8+ T cells using more than one Vβ region in most subjects, we analyzed the kinetics of different Vβ populations over time. In our longitudinal analysis, we observed expansions or contractions of distinct Vβ populations in association with the HIV-1–specific CD8+ T-cell population (Figure 3). Importantly, substantial changes were only observed within subsets of Vβ populations, whereas the majority of Vβ populations in each response remained stable. We observed up to 10-fold expansions of single Vβ populations over time in untreated individuals. Occasionally, these expansions changed the composition of the Vβ repertoire and resulted in the dominance of a previously subdominant population. To analyze whether these dynamic changes could also be observed during antiretroviral treatment with optimal viral suppression, we followed the Vβ repertoire of a dominant B8-FL8 tetramer–positive CD8+ T-cell population in subject AC-42 for more than 1 year (Figure 3B). This B8-FL8 tetramer–positive CD8+ T-cell population was dominated by a single Vβ8+ subpopulation that remained stable during 1 year of follow-up.
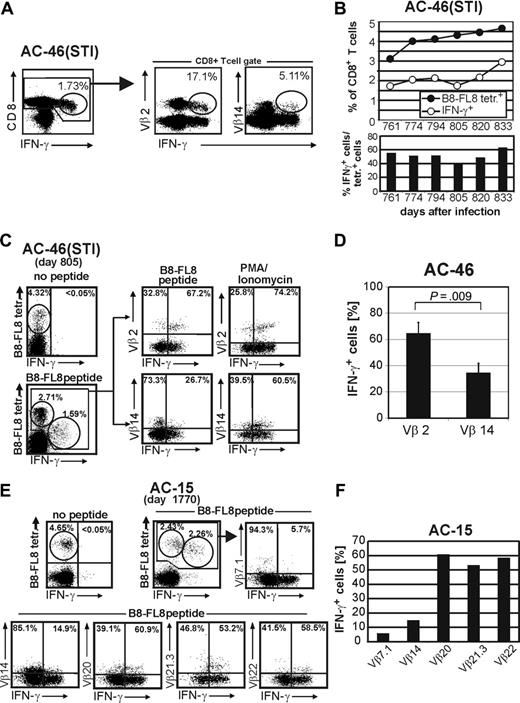
Subject AC-46 was followed during a period of antiretroviral treatment and subsequent treatment interruption (Figure 3C). During antiretroviral treatment we identified 2 codominant Vβ populations, namely Vβ2 and Vβ14, which remained stable during antiretroviral treatment. After treatment was stopped we subsequently (within 2 weeks) observed a rise of the Vβ2+ population alone, whereas the Vβ14+ population remained stable. This 5-fold increase took place before a rise in plasma viremia was detectable. As viremia increased, we observed overall expansion of B8-FL8 tetramer-positive CD8+ T cells, together with expansions and contractions of distinct Vβ populations within the tetramer-positive cells over time (Figure 3). These data provide evidence that, although changes in the overall frequency of HIV-1–specific CD8+ T cells may parallel changes in levels of viremia, within the epitope-specific T-cell populations there may be discordant expansions and contractions of distinct subpopulations of T cells that can be identified by their Vβ TCR expression.
Individual Vβ populations differ in their tetramer expression patterns and their antigen specific IFN-γ secretion ex vivo
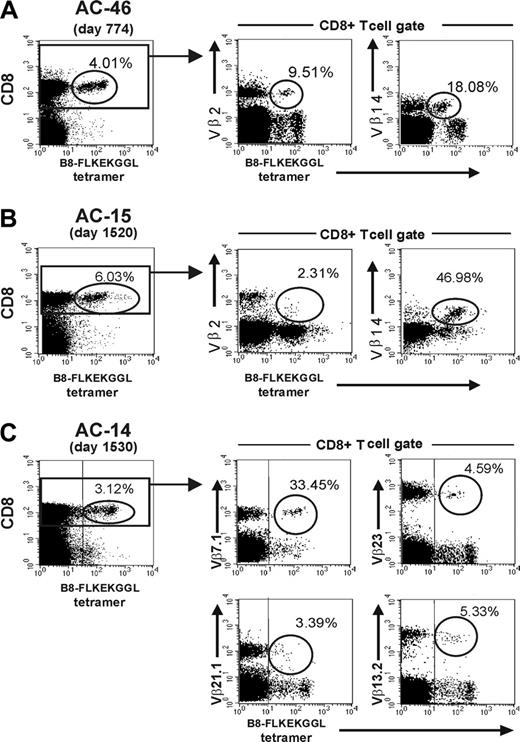
The finding of different kinetics of distinct B8-FL8–specific Vβ populations in vivo in patients with detectable viremia prompted us to investigate the functional role of HIV epitope–specific Vβ populations in more detail. Analysis by flow cytometry revealed subpopulations of cells with differences in the level of tetramer staining. Further analysis of these epitope-specific cells revealed differences in Vβ utilization between these cell populations with different staining characteristics (Figure 4). In subject AC-46 we analyzed the tetramer expression pattern of Vβ2+ and the Vβ14+ CD8+ T-cell populations. Here, we observed that almost the entire Vβ14+ population (representing 18% of the tetramer-positive cells) was in the tetramer-low population, whereas the less frequent Vβ2+ population (9.5% of all tetramer-positive cells) was located in the tetramer-intermediate/high population (Figure 4A). In subject AC15 who also used the Vβ14 gene, the Vβ 14+ population was localized within the B8-FL8 tetramer-high population (Figure 4B). Substantial heterogeneity in tetramer binding was present in subject AC-14 as well (Figure 4C). The dominantly used Vβ7.1 population (33.45% of B8-FL8 tetramer-positive cells on day 15).30 was located in the tetramer-high population, whereas Vβ21.1 was located in the tetramer-low–positive population. Two other Vβ populations at that time point (Vβ13.2+ and Vβ23+) were located in the tetramer-intermediate population (Figure 4C). The observation of heterogenous tetramer staining prompted us to perform a more detailed functional analysis of tetramer-positive cells.
Tetramer expression patterns of distinct Vβ populations. Frequency and staining patterns of B8-FL8 tetramer–positive CD8+ T cells in subjects AC-46 (A), AC-15 (B), and AC-14 (C). Percentages indicate the frequency of tetramer-positive cells per CD8+ T cells (left) or the frequency of Vβ+ cells within the B8-FL8 tetramer–positive population (middle and top).
Tetramer expression patterns of distinct Vβ populations. Frequency and staining patterns of B8-FL8 tetramer–positive CD8+ T cells in subjects AC-46 (A), AC-15 (B), and AC-14 (C). Percentages indicate the frequency of tetramer-positive cells per CD8+ T cells (left) or the frequency of Vβ+ cells within the B8-FL8 tetramer–positive population (middle and top).
We next quantified HIV peptide–specific IFN-γ secretion within HIV tetramer–positive CD8+ T-cell populations. In AC-46, B8-FL8-specific IFN-γ secretion of 2 dominant Vβ populations (Vβ2 and Vβ14) revealed a relative underrepresentation of the Vβ14+ population (5.11% of all IFN-γ+ cells) as compared with the Vβ2+ population (17.1% of all IFN-γ+ cells) (Figure 5A). These results indicated a much lower level of IFN-γ secretion in response to peptide within the Vβ14+ population. Interestingly, Vβ14+ cells showed a lower level of tetramer binding as compared with the Vβ2+ population, in which most cells demonstrated HIV-specific production of IFN-γ in response to B8-FL8 peptide stimulation.
Longitudinal follow-up of B8-FL8 tetramer staining together with intracellular cytokine staining after stimulation with B8-FL8 peptides revealed a constant increase of both B8-FL8 tetramer–positive and IFN-γ+ CD8+ T cells specific for the B8-FL8 epitope as shown in this subject after initiation of STI (Figure 5B). Over a period of frequent sampling between day 761 and 833 after treatment interruption, the frequency of IFN-γ+ cells ranged between 40% and 60% of the tetramer–positive cells (Figure 5B). These observations suggested that changes in IFN-γ production of distinct HIV-specific CD8+ T-cell subpopulations (as defined by their Vβ expression) over time might reflect the dynamics of individual Vβ populations with different functional capacities. To explore this hypothesis we performed costaining for intracellular IFN-γ production after B8-FL8 peptide stimulation, together with surface staining for B8-FL8 tetramers in association with antibody staining specific for individual Vβ populations (Figure 5C). We observed a significantly higher fraction of IFN-γ+ cells after B8-FL8 peptide stimulation in the Vβ2+ population as compared with Vβ14+/B8-FL8 tetramer–positive cells, which was confirmed at 4 independent time points (64.72% ± 8.2% versus 34.59% ± 7.2%; P = .009) (Figure 5D). However, after stimulation with PMA/ionomycin or PHA the proportion of IFN-γ+ cells was comparable within both populations (Figure 5C). Similarly, subject AC-15 demonstrated substantial differences in HIV-specific IFN-γ secretion between different Vβ populations within the B8-FL8 tetramer–positive CD8+ T-cell population, ranging from 5.7% (for Vβ 7.1) to 60.9% (for Vβ 20) (Figure 5E-F). In no subject was there a correlation between the frequency of a particular Vβ population within the B8-FL8 tetramer–positive population and the fraction of that Vβ population capable of secreting IFN-γ in response to B8-FL8 peptide stimulation. These data indicate substantial differences in peptide responsiveness between individual HIV-1–specific CD8+ T-cell Vβ populations.
Functional analysis of HIV tetramer–positive cells and their Vβ populations ex vivo. (A) IFN-γ secretion of Vβ2 and Vβ14+ CD8+ T cells in AC-46 ex vivo. Percentages indicate the frequency of IFN-γ+ cells per CD8+ T cells (left) or the frequency of Vβ+ cells within the IFN-γ+ population (right). (B) Kinetics of B8-FL8 tetramer–positive cells and IFN-γ+ cells after stimulation with B8-FL8 peptide in AC-46 during STI. Bars indicate relative frequency (%) of IFN-γ+ cells within the B8-FL8 tetramer+ population. (C) Costaining of B8-FL8 tetramer and intracellular IFN-γ secretion in Vβ2 and Vβ14+ CD8+ T-cell populations in AC-46 ex vivo after stimulation with B8-FL8 peptide or PMA/ionomycin. (D) Summary of the IFN-γ secretion of B8-FL8 tetramer+ Vβ2+ and Vβ14+ CD8+ T cells after stimulation with B8-FL8 peptide in 3 independent experiments. (E) Costaining of B8-FL8 tetramer and intracellular IFN-γ secretion in 4 Vβ+ populations in AC-15 ex vivo after stimulation with B8-FL8 peptide. (F) Bar graph indicates the frequency of IFN-γ+ cells per Vβ population.
Functional analysis of HIV tetramer–positive cells and their Vβ populations ex vivo. (A) IFN-γ secretion of Vβ2 and Vβ14+ CD8+ T cells in AC-46 ex vivo. Percentages indicate the frequency of IFN-γ+ cells per CD8+ T cells (left) or the frequency of Vβ+ cells within the IFN-γ+ population (right). (B) Kinetics of B8-FL8 tetramer–positive cells and IFN-γ+ cells after stimulation with B8-FL8 peptide in AC-46 during STI. Bars indicate relative frequency (%) of IFN-γ+ cells within the B8-FL8 tetramer+ population. (C) Costaining of B8-FL8 tetramer and intracellular IFN-γ secretion in Vβ2 and Vβ14+ CD8+ T-cell populations in AC-46 ex vivo after stimulation with B8-FL8 peptide or PMA/ionomycin. (D) Summary of the IFN-γ secretion of B8-FL8 tetramer+ Vβ2+ and Vβ14+ CD8+ T cells after stimulation with B8-FL8 peptide in 3 independent experiments. (E) Costaining of B8-FL8 tetramer and intracellular IFN-γ secretion in 4 Vβ+ populations in AC-15 ex vivo after stimulation with B8-FL8 peptide. (F) Bar graph indicates the frequency of IFN-γ+ cells per Vβ population.
Sequencing of autologous virus in subjects at viral set point
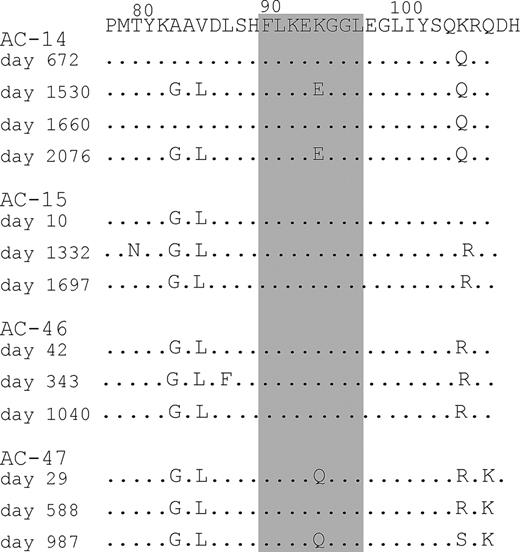
To understand the evolution of viral sequence variation in subjects that recognize the B8-FL8 epitope, we analyzed the autologous virus sequence of the B8-FL8 epitope, including the flanking regions, in 4 study subjects (Figure 6). In 2 subjects (AC-14 and AC-47) fluctuating amino acid changes were observed. For the other 2 subjects (AC-15 and AC-46) we did not observe sequence variation within the B8-FL8 epitope, yet these latter 2 subjects had evidence of discordant expansions and contractions within their epitope-specific Vβ populations. Our findings indicate that in subjects at viral set point, even if sequence variants are present, they do not necessarily remained fixed in the population of viral quasispecies, and even when stable can be associated with fluctuations in the epitope-specific T-cell repertoire.
Sequencing of autologous virus. Bulk sequencing of autologous viral sequences derived from AC-14, AC-15, AC-46, and AC-47 covering the B8-FL8 epitope and its flanking regions derived from different time points over the course of the study period.
Sequencing of autologous virus. Bulk sequencing of autologous viral sequences derived from AC-14, AC-15, AC-46, and AC-47 covering the B8-FL8 epitope and its flanking regions derived from different time points over the course of the study period.
Discussion
In this study, we performed a detailed longitudinal analysis of the T-cell receptor repertoire of immunodominant HIV-specific HLA class I–restricted CD8+ T-cell responses in individuals with relatively stable levels of viremia and stable CD4+ T-cell counts during the chronic stage of disease. During longitudinal analysis increasing viremia was associated with increased frequencies of B8-FL8 tetramer–positive CD8+ T cells, indicating the reactive nature of an immunodominant adaptive immune response in individuals with relative control of viremia and is consistent with earlier studies.12,18,43,44 However, cross-sectional analysis did not show a significant correlation between HIV epitope–specific CD8+ T cells and level of viremia in our cohort, as has also been observed in other studies.14
In chronic viral infections with high rates of viral mutations such as HCV or HIV infection a diverse TCR repertoire used by virus-specific CD8+ T-cell responses has been hypothesized to be beneficial for control of viremia because of cross-recognition of epitope variations.1,3,25,45 The objectives of this study were to characterize the TCR repertoires of immunodominant epitope-specific CD8+ T cells and to evaluate the kinetics and function of structurally diverse epitope-specific T-cell receptor repertoires. We determined the structure of the epitope-specific TCR repertoire by sequencing ex vivo–purified HIV-specific CD8+ T cells and by staining with a panel of monoclonal antibodies specific for 24 Vβ regions as previously described.1,4,6,40 A recent study reported preferential Vβ 13.2 gene usage by B8-FL8–specific CD8+ T cells in subjects maintaining CD4 counts more than 500 for longer than 8 years.46 Although we observed an overrepresentation of certain Vβ regions within the epitope-specific TCR repertoire, most of our subjects used structurally diverse TCRs with different Vβ regions and with high amino acid diversity within the antigen recognition site of TCRβ chains. Furthermore, only 2 of our subjects had significant populations of tetramer-positive cells expressing the Vβ 13.2 TCR, and in neither case was it the dominantly expressed TCR-β chain. Possible reasons for these apparently discordant results include the different populations studied. Our cohort of subjects was at an early stage of HIV infection and was promptly started on antiretroviral therapy, which may have preserved a more diverse TCR repertoire. Dong et al46 studied subjects with chronic infection and uncontrolled viremia, which may limit TCR diversity over the course of disease. In ongoing studies, we are evaluating subjects at later stages of infection and with varying levels of control of viremia to determine the association between the breadth of the TCR repertoire and control of viremia.
Because of the limitations in the number of cells available in our study, we were not able to perform a comparison of TCR sequencing with the corresponding Vβ staining at the same study visit. However, a comprehensive analysis of the reproducibility of this method has been published earlier by our group.1,4 Moreover, Vβ-region usage analyzed by sequencing and staining with Vβ-specific antibodies at different visits only a few weeks apart was similar, and all Vβ-populations detected by sequencing were detectable by Vβ staining. Our findings are consistent with earlier observations from our group and others, demonstrating a polyclonal or diverse TCR repertoire within epitope-specific responses in HIV and SIV infection.4,6,29,45,47-49 Interestingly, the subject in this study with the narrowest TCR usage was on HAART with an undetectable viral load.
To determine whether increased frequencies of HIV epitope–specific CD8+ T cells resulted from simultaneous increases of all T-cell subpopulations or from expansion of a fraction of TCR clonotypes, we performed a longitudinal follow-up of the HIV epitope–specific Vβ repertoire. We observed that increased frequencies of HIV-1–specific CD8+ T cells were caused by expansions of single Vβ populations, representing only a few TCR clonotypes within the HIV epitope–specific Vβ repertoire. Importantly, in some subjects dominant Vβ populations substantially decreased as the total magnitude of HIV-specific CD8+ T cells increased. Thus, during the course of infection-subdominant HIV-specific clonotypes within the TCR repertoire may become dominant, and dominant clones may become subdominant or even undetectable for extended periods of time in the setting of partially controlled viral replication. In contrast, the Vβ repertoire of HIV-1–specific CD8+ T cells was remarkably stable during treatment intervals with optimal viral suppression. In one individual, we observed changes of the Vβ repertoire within 2 weeks after initiation of STI, even before an increase of plasma viremia was detectable. These findings indicate that constant viral replication induces changes in the composition of structurally distinct subpopulations within the pathogen-specific TCR repertoire over time and that partial control of viremia by HIV-specific CD8+ T cells is associated with discordant expansions and contractions of individual Vβ populations within the repertoire of HIV-1–specific CD8+ T cells.
Sequencing of this epitope in 4 subjects with relatively stable viremia demonstrated that the 2 subjects without evidence of sequence variation (AC-15 and AC-46) had discordant expansions and contractions of Vβ subpopulations, and 2 other subjects with evidence of sequence variations (AC14 and AC-47) maintained the hierarchy of Vβ expression within tetramer-positive cells. However, whether these differential Vβ expansions represent subpopulations of T cells with varying cross-recognition of epitope variants that maintain relative control of viremia or whether the observed epitope variants have reduced viral fitness is speculative.
We also wanted to determine whether distinct HIV epitope–specific CD8+ T-cell subpopulations showed differences in response to peptide stimulation ex vivo. Differences in tetramer expression in HIV-infected subjects have been previously observed50 and have been associated with different levels of T-cell activation and apoptosis. We extended these findings by demonstrating different levels of tetramer expression within distinct Vβ-expressing T-cell populations. Tetramer-low and -high populations could be reproducibly observed with or without Vβ staining, and we did not observe any distinct staining pattern to suggest the observed differences were due to Vβ antibodies used for staining. Analysis of dominant Vβ populations (Vβ2 and Vβ14 in AC-46), which showed different kinetics in vivo after treatment interruption, revealed differences in tetramer binding as well as differences in the ability to secrete IFN-γ after stimulation with saturating amounts of B8-FL8 peptide ex vivo. Levels of tetramer binding correlated with the frequency of IFN-γ+ cells, because Vβ2-expressing T cells stained more brightly with tetramer, and a higher proportion of these cells were able to produce IFN-γ on peptide stimulation. This may reflect a higher expression level of TCR on the cell surface, or a higher binding affinity for the peptide/MHC complex. Further studies are needed to understand the role of different activation thresholds of T cells at the clonal level and the influence of antigenic variation within the HLA class I–restricted epitope for shaping the pathogen-specific TCR repertoire.
In summary, we provide evidence that the magnitude of an epitope-specific CD8+ T-cell response is a reactive process driven by the extent of replicating virus and involves subpopulations of functionally distinct epitope-specific CD8+ T cells, which could be defined by their expressed T-cell receptors. Our results provide evidence of rapid changes within the TCR repertoire of HIV epitope–specific CD8+ T cells and suggest that the ability to maintain a diverse and, more importantly, a dynamic TCR repertoire directed against viral epitopes might be a crucial factor for control of viral replication.
Prepublished online as Blood First Edition Paper, December 1, 2005; DOI 10.1182/blood-2005-04-1636.
Supported by National Institute of Health (grant R01 AI39966) (S.A.K.), and the Vanderbilt-Meharry Center for AIDS Research (CFAR).
D.M.O. designed and performed the research, analyzed the data, and wrote the manuscript. K.B. and M.B. performed many of the immunologic assays on acutely infected subjects. M. Altfeld, M. Addo, and R.D. provided ELISPot screening data on immune responses of acutely infected subjects. E.R. helped identify study subjects for inclusion in this cohort. B.C.S., J.A.C., C.B.D., A.S., and S.L. performed supporting immunologic analyses on chronically infected subjects. T.A. and K.M.O. performed HIV-1 sequence analyses on longitudinally followed subjects. B.D.W. helped in experimental designs. S.A.K. designed experiments in the study, analyzed the data, and prepared the manuscript.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
S.A.K. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal