Abstract
The CXC chemokines platelet factor 4 (PF-4/CXCL4) and connective tissue-activating peptide III (CTAP-III) are released by activated human platelets in micromolar concentrations. So far, neutrophils have been recognized to cleave the precursor CTAP-III to form the active chemokine neutrophil-activating peptide 2 (NAP-2/CXCL7) through limited proteolysis by membrane-associated cathepsin G. Here we show for the first time that activated human skin mast cells (MCs) convert CTAP-III into biologically active NAP-2 through proteolytic cleavage by released chymase. A direct comparison on a cell number basis revealed that unstimulated MCs exceed the CTAP-III–processing potency of neutrophils about 30-fold, whereas MCs activated by IgE cross-linking exhibit even 1000-fold higher CTAP-III–processing capacity than fMLP-stimulated neutrophils. Intriguingly, PF-4 counteracted MC- as well as neutrophil-mediated NAP-2 generation at physiologically relevant concentrations. Addressing the underlying mechanism, we obtained evidence that PF-4 acts as an inhibitor of the CTAP-III–processing enzymes cathepsin G and chymase without becoming cleaved itself as a competitive substrate. Because cleavage of the CTAP-III–unrelated substrate substance P was also affected by PF-4, our results suggest a regulatory role for PF-4 not only in NAP-2 generation but also in neutrophil- and MC-mediated processing of other physiologically relevant inflammatory mediators.
Introduction
Connective tissue-activating peptide III (CTAP-III) constitutes one of several molecular variants of β-thromboglobulin-antigen (βTG-Ag), a group of chemokines that differ by their degree of N-terminal truncation. Along with platelet factor 4 (PF-4/CXCL4) CTAP-III represents one of the major chemokines stored as preformed proteins in the alpha-granules of blood platelets. Both molecules become released into the environment immediately upon platelet activation and may arise to rather high local concentrations, as indicated by normal serum levels (PF-4, 0.4-1.9 μM; CTAP-III, 1.6-4.8 μM).1 As suggested by our previous work, CTAP-III and PF-4 appear to have complementary functions in the early recruitment and activation of neutrophil granulocytes (neutrophils) (recently reviewed by Brandt et al1,2 ). In contrast to ELR-positive chemokines such as IL-8/CXCL8, the ELR-negative chemokine PF-4 lacks the ability to induce chemotaxis and degranulation of primary granules in neutrophils3 but very efficiently stimulates their firm adhesion to endothelial cells as well as the selective exocytosis of secondary granule markers.3,4 Notably, the receptor for PF-4 on neutrophils is not a G protein–coupled receptor but a cell surface–expressed chondroitin sulfate proteoglycan.5,6 In contrast, CTAP-III represents an inactive precursor of the neutrophil-activating peptide 2 (NAP-2/CXCL7), a typical ELR-positive chemokine that potently stimulates chemotaxis as well as degranulation in neutrophils7-9 through interaction with its G protein–coupled receptors CXCR-2 and CXCR-1.10-12 To convert CTAP-III into its active derivative, limited truncation by enzymatic proteolysis is required. Intriguingly, neutrophils themselves are the major blood cells to cleave CTAP-III into NAP-2 by removing a stretch of 15 amino acids from the N terminus of the precursor.9,13 So far all evidence suggests that cell surface–bound cathepsin G (CathG), a chymotryptic serine protease also found in primary neutrophil granules, is responsible for CTAP-III processing.9,13-15 Among blood cells, only monocytes share the ability of neutrophils to convert CTAP-III into NAP-2,13,16 albeit to a considerably lower extent than neutrophils, which argues against a relevant role for monocytes in NAP-2 formation during early inflammation.13 In contrast to the well-characterized processing capacity of blood leukocytes, the contribution of cells from the surrounding vascular tissue has remained largely unclear. We have previously shown that at least endothelial cells are inactive in this respect.17 A cell type located closely adjacent to blood vessels and rich in secretable proteolytic enzymes are mast cells (MCs). These act as the primary inducers of immediate hypersensitivity reactions and degranulate in response to allergen exposure. Interestingly, Schechter et al18 have demonstrated the presence of CathG in these cells immunocytochemically and by the use of inhibitors. Moreover, within their secretory granules MCs contain chymase, another chymotryptic enzyme,19,20 which is released upon cell activation. Interestingly, it has also been reported that MC degranulation in allergen-challenged animals is accompanied by the simultaneous intravascular aggregation of platelets21 and that both cell types are particularly involved in the concomitant delayed invasion of neutrophils.22,23 Considering the observation that in response to allergen exposure platelet markers CTAP-III and PF-4 are secreted into the extracellular fluid as well as into the blood,24 we hypothesized that these chemokines could interact with MC proteases and participate in orchestrating the delayed invasion of neutrophils. In our present study, we therefore examined whether MCs and their proteases would have the capacity to proteolytically modify the platelet-derived chemokines and whether the latter mediators would have an impact on the processing enzyme(s).
Here we provide first evidence that chymase released by activated MCs converts CTAP-III into biologically active NAP-2. Additionally, we found that PF-4 counteracts MC- as well as neutrophil-mediated NAP-2 generation by acting as an inhibitor of the CTAP-III–processing enzymes CathG and chymase without being cleaved itself. Because cleavage of the CTAP-III–unrelated substrate substance P (SP) also was affected by PF-4, our results suggest a more general role for PF-4 as a regulator of neutrophil- and MC-dependent processing of physiologically relevant inflammatory mediators.
Materials and methods
Cytokines, peptides, enzymes, and inhibitors
Human natural CTAP-III and PF-4 were purified to homogeneity exceeding 99% purity in our laboratory from release supernatants of thrombin-stimulated platelets as previously described.3,9,13 Human natural NAP-2 was prepared from CTAP-III by site-specific cleavage with chymotrypsin and subsequently purified as described elsewhere.12 The human recombinant proteins PF-4 and SCF were purchased from Peprotech (London, United Kingdom). SP, N-formyl-methionyl-leucyl-phenylalanine (fMLP), and the inhibitors aprotinin, soybean trypsin inhibitor (SBTI), leupeptin, and phenylmethylsulfonyl fluoride (PMSF) were from Sigma-Aldrich (Taufkirchen, Germany). Natural human neutrophil CathG and human skin chymase (both 95% or more pure) were from Calbiochem (Frankfurt, Germany). Bovine pancreas chymotrypsin was from Serva (Heidelberg, Germany).
Neutrophil preparation
Neutrophils were routinely isolated from citrated blood of healthy single donors by gradient centrifugation on Ficoll-Hypaque (Amersham, Oberursel, Germany) to a purity higher than 95% as previously described.9
Preparation and purification of human skin mast cells
Mast cells were dispersed from human skin tissue essentially as described.25-27 Fresh samples of skin tissue from abdominoplasties were obtained from the Klinik für Hand-, Brustund Plastische Chirurgie, Klinikum Neustadt (Neustadt i.H., Germany). Approval for these studies was obtained from the Institutional Review Board at the University of Lübeck (Lübeck, Germany), and informed consent was provided according to the declaration of Helsinki. Specimens were placed in ice-cold MC buffer (8 g/L NaCl, 1 g/L glucose, 2.86 g/L HEPES, 0.2 g/L KCl, 1.45 g/L Na3PO4 × 12H2O, pH 7.2, 0.1% BSA). After removal of fatty tissue, skin samples were chopped into 1- to 2-mm2 pieces. To disperse cells, 15-g skin fragments were incubated in 200 mL MC buffer containing 1.5 mg/mL type 2 collagenase and 0.7 mg/mL hyaluronidase (both from Worthington, Lakewood, NJ) with constant stirring for 4 hours at 37°C. Samples were filtered through 120 μm gauze and the resulting cell suspension washed, resuspended in 10 mL MC buffer at 20°C, and mixed with 10 mL 100% Percoll (Sigma-Aldrich). The suspension was layered on a gradient of 10 mL 60% and 10 mL 80% Percoll prepared in MC buffer. After centrifugation (400g for 20 minutes at 20°C) MC-enriched cell suspension harvested from the 60% to 80% Percoll interface was washed twice in Dulbecco PBS (D-PBS)/0.5% BSA.
Further purification of MCs was achieved by immunoaffinity magnetic enrichment using antiphycoerythrin-conjugated microbeads (α-PE microbeads; Miltenyi Biotec, Bergisch Gladbach, Germany) in combination with PE-conjugated monoclonal antibody (mAb) 97A6 (α-CD203c-PE; IOTest, Marseille, France) specific for CD203c, an antigen exclusively expressed on MCs and basophils.28,29 In a first step, cells binding unspecifically were removed by incubation (15 minutes at 4°C) with the α-PE microbeads. Then washed cells were applied to the separation column (LS-MACS column; Miltenyi Biotec), exposed to a magnetic field in the cell separation system (Miltenyi Biotec) and, finally, cells not attached were eluted. In a second step, these cells were incubated with α-CD203c-PE (60 minutes at 4°C), followed by washing and further incubation with α-PE microbeads (15 minutes at 4°C). Following transfer of the washed cells to the magnetic cell separation system, cells without attached microbeads were washed away and retained cells were eluted after withdrawal of the column from the magnetic field. MC purity in these preparations ranged from 90% to 98% as assessed by toluidine blue staining and according to CD117 expression. Viability was always more than 85% as assessed by trypan blue exclusion. Following purification, cells were cultured at a density of 105/mL for 5 days in culture medium (RPMI 1640, 2 mM l-glutamine, 200 U/mL penicillin, 200 μg/mL streptomycin, 10% FCS, 100 ng/mL SCF), allowing them to recover from rigors of the isolation procedure. Responsivity of MCs upon stimulation with cross-linking goat antiserum directed against human IgE (α-IgE; Biosource, Camarillo, CA) and calcium-ionophore A23187 (Sigma-Aldrich) was routinely verified by hexosaminidase-release assay as described elsewhere.30
Processing of CTAP-III by cells, cell supernatants, and enzymes
For processing of CTAP-III by cells and cell supernatants, indicated numbers of neutrophils or MCs were either preincubated (30 minutes at 37°C) with processing buffer alone (D-PBS supplemented with 0.1% BSA, 0.9 mM CaCl2, and 0.5 mM MgCl2) or with 100 nM fMLP (neutrophils) and 3 μg/mL α-IgE (MCs). Thereafter, 100 μL cell suspension or cell-free supernatant was mixed with 100 μL CTAP-III at the concentrations and time periods indicated. Reactions were terminated by addition of 2 μL of 10% trifluoroacetic acid (TFA) and recovered cell-free supernatants stored at –20°C. In some experiments, suspensions of α-IgE–activated MCs were incubated with the indicated enzyme inhibitors (30 minutes at 37°C) before the addition of CTAP-III to characterize the processing enzyme(s) involved. For processing assays with purified enzymes, CathG, chymase, or chymotrypsin was incubated at the concentrations indicated for different periods of time at 37°C with, finally, 3 μM CTAP-III, 4 μM PF-4, or 10 μM SPin 200 μL processing buffer.
Biologic assay for NAP-2
To determine the amount of biologically active NAP-2 formed through processing of CTAP-III, a neutrophil degranulation assay based on the stimulus-induced release of elastase enzymatic activity was used as described elsewhere.13 To avoid potential interference of MC degranulation products with the assay, CTAP-III/NAP-2 from MC supernatants was extracted by immunoaffinity chromatography prior to testing, using βTG-Ag–specific mAb C24 coupled to CNBr-activated Sepharose, as previously described.9 Recovery of CTAP-III/NAP-2 was always more than 95% as controlled by ELISA.
Electrophoresis and Western blot analysis of chemokines
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) according to Schägger and von Jagow31 and immunoblotting were performed as described in detail elsewhere.17 Blocking of the blotting membrane and subsequent incubation steps with antibodies were performed using Roti-Immunoblock buffer (Carl Roth, Karlsruhe, Germany). Blots were stained using rabbit polyclonal antiserum Rα-βTG, reacting with all βTG-Ag variants, as described previously,32 and with IRDye 800–conjugated goat α-rabbit antiserum (1:5000; Rockland, Gilbertsville, PA) as the secondary reagent. In some experiments, blots were scanned and fluorescence intensity was determined using channel 800 of an Odyssey infrared imaging system and Odyssey 1.2 software (Li-cor, Bad Homburg, Germany). The quantity of βTG-Ag variants from unknown samples was calculated from their fluorescence intensity relative to the intensities of 50 ng pure standard NAP-2 or CTAP-III run on the same blot in parallel, according to the manufacturer's instruction.
HPLC and mass analysis of CTAP-III, PF-4, and SP cleavage by cells and purified proteases
For analysis of CTAP-III, PF-4, and SP fragmentation by purified CathG or chymase, 200-μL aliquots of samples were separated by reverse-phase high-performance liquid chromatography (HPLC) using a C2/C18 column (μRPC PC 3.2/3; Pharmacia, Uppsala, Sweden) on a SMART chromatography unit (Pharmacia). The column was developed with a linear acetonitrile gradient (0% to 50%), and eluting peaks were detected at 214 nm wavelength. Peak areas were calculated using the software SMART Manager 1.51 (Pharmacia). Eluting proteins were collected and their masses determined by mass spectrometry using ESI-FT-MS (Apex II; Bruker Daltonics, Billerica, MA) as described elsewhere.33 All masses given refer to the neutral monoisotopic molecular mass. N-terminal sequence analysis of βTG-Ag isoforms was performed by Dr A. Petersen (Forschungszentrum Borstel) as described elsewhere.32
Results
Stimulated MCs convert CTAP-III into NAP-2 more efficiently than do neutrophils
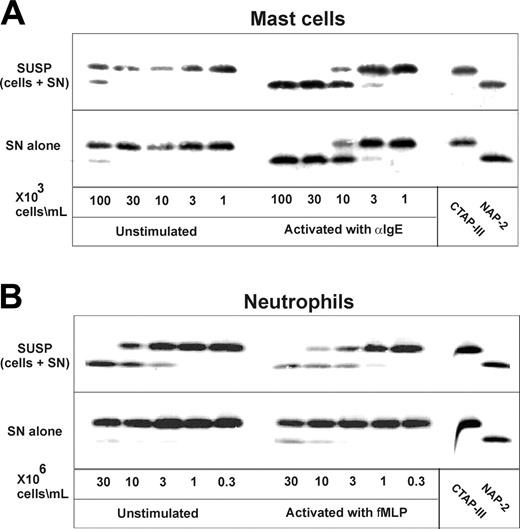
Because it has been reported that apart from neutrophils human skin MCs also contain chymotryptic proteinases within their secretory granules, we wondered whether MCs would likewise convert CTAP-III into NAP-2. As shown in Figure 1A, suspensions as well as cellfree supernatants of α-IgE–stimulated MCs induced the conversion of CTAP-III into a molecule comigrating with purified NAP-2. In both settings, the NAP-2–like molecule first became detectable with 3 × 103 cells per milliliter, while more than half-maximal CTAP-III conversion was achieved with 1 × 104 cells per milliliter and conversion was complete with 3 × 104 cells per milliliter. By contrast, corresponding samples from unstimulated cells were by far less active, effecting detectable CTAP-III conversion only at more than 30-fold higher cell concentration (1 × 105/mL). The identity of the CTAP-III conversion product was investigated after its immunopurification on mAb C24-Sepharose. Mass spectrometry revealed a molecular mass of 7623 atomic mass units (amu), which is identical to the theoretical mass of NAP-2 (7623 amu). Additional evidence for the proteolytic generation of NAP-2 was provided by sequence analysis of the truncation product showing the N-terminal sequence AELR, which corresponded to that of NAP-2. While these results demonstrate that MCs have the capacity to process CTAP-III into NAP-2, they additionally stress the requirement for cell activation for this process to become highly efficient. Moreover, the data showing that culture supernatants of stimulated MCs (Figure 1, SN) are as active as suspended MCs (Figure 1, SUSP) by themselves strongly suggest that proteolytic enzymes released into the extracellular medium rather than proteases remaining cell-associated are responsible for MC-mediated CTAP-III cleavage.
Next we compared the MC-mediated CTAP-III conversion to that by neutrophils. As evident from Figure 1B (upper panel, left), an at least 30-fold higher number of unstimulated neutrophils (3 × 106/mL) as compared with unstimulated MCs was required to generate detectable amounts of NAP-2. Remarkably, stimulation of neutrophils (Figure 1B, upper panel, right) did not significantly enhance NAP-2 generation—that is, with unstimulated and fMLP-treated neutrophils a minimum of 3 × 106 cells per milliliter rendered NAP-2 generation just detectable, 1 × 107 cells per milliliter converted about half, and 3 × 107 cells per milliliter virtually all of the substrate. Thus, about 1000-fold higher neutrophil numbers were required to meet the processing capacity of stimulated MCs. Much different than stimulated MCs, supernatants from stimulated neutrophils remained poor in processing activity. This was indicated by threshold cell numbers of 1 × 107/mL and 3 × 106/mL required to effect minimal NAP-2 formation by neutrophil supernatants and cell suspensions, respectively (Figure 1B). Altogether these data not only demonstrate a high processing capacity for MCs as compared with neutrophils but moreover suggest that processing by either cell type is regulated by different mechanisms.
Processing of CTAP-III by human skin mast cells and neutrophils. Increasing concentrations of human skin mast cells were incubated with cross-linking goat α-IgE antiserum (3 μg/mL) or left untreated for 30 minutes at 37°C (A). For comparison, increasing concentrations of human neutrophils were either incubated with fMLP or left untreated under the same conditions (B). Immediately following the stimulation period, cell samples were split and the original cell suspensions (SUSP) as well as the cell-free supernatants (SN) prepared thereof were incubated with 3 μM CTAP-III for 30 minutes (A-B). Thereafter, 2 μL cell-free supernatant of each sample and standard preparations of CTAP-III and NAP-2 (50 ng per lane) were separated by SDS-PAGE, subjected to Western blotting, and immunochemically stained to visualize CTAP-III and potential degradation products with rabbit antiserum Rα-βTG and an IRDye 800-conjugated goat α-rabbit antiserum. Data from 1 representative experiment of 3 are shown.
Processing of CTAP-III by human skin mast cells and neutrophils. Increasing concentrations of human skin mast cells were incubated with cross-linking goat α-IgE antiserum (3 μg/mL) or left untreated for 30 minutes at 37°C (A). For comparison, increasing concentrations of human neutrophils were either incubated with fMLP or left untreated under the same conditions (B). Immediately following the stimulation period, cell samples were split and the original cell suspensions (SUSP) as well as the cell-free supernatants (SN) prepared thereof were incubated with 3 μM CTAP-III for 30 minutes (A-B). Thereafter, 2 μL cell-free supernatant of each sample and standard preparations of CTAP-III and NAP-2 (50 ng per lane) were separated by SDS-PAGE, subjected to Western blotting, and immunochemically stained to visualize CTAP-III and potential degradation products with rabbit antiserum Rα-βTG and an IRDye 800-conjugated goat α-rabbit antiserum. Data from 1 representative experiment of 3 are shown.
CTAP-III conversion by MCs is a rapid process and generates biologically active NAP-2
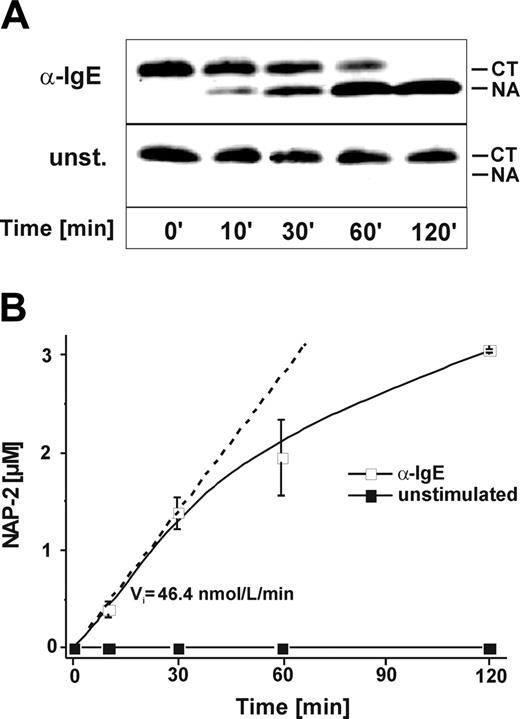
Next we investigated the time kinetics of CTAP-III processing by MCs and the functional characteristics of the truncation product formed. For this we incubated a fixed concentration of α-IgE–stimulated or unstimulated MCs with CTAP-III for increasing periods. Analysis of the cellfree supernatants by SDS-PAGE and immunoblotting (Figure 2A) demonstrated rapid formation of NAP-2 by stimulated cells, while none became visible with unstimulated cells. CTAP-III conversion by stimulated cells was already detectable after 10 minutes of incubation, appeared near half maximal after 30 minutes, and was complete after 2 hours. To see whether the NAP-2 generated was biologically active, βTG-Ag from the same samples was immunopurified and tested for its capacity to induce the release of elastase from neutrophils. As depicted in Figure 2B, no activity was detectable in samples from unstimulated cells, whereas in samples from α-IgE–stimulated MCs NAP-2–like activity was first detectable after 10 minutes of incubation, was equivalent to slightly less than 50% of converted CTAP-III after 30 minutes, and was practically equivalent to 100% of converted CTAP-III after 2 hours. Activity formed by stimulated cells increased in a practically linear relationship for up to 30 minutes (initial velocity [Vi], about 46.7 nmol NAP-2 equivalents/L/min), thereafter leveling off. These data are in good agreement with those obtained by immunoblotting, strongly suggesting that stimulated MCs rapidly process CTAP-III into biologically active NAP-2. The biologic activity of the generated NAP-2 was additionally verified by its ability to induce cell migration in neutrophils (maximum chemotactic index, about 10) in a concentration-dependent manner, whereas no chemotaxis was induced by CTAP-III (data not shown). Interestingly, induction of NAP-2 processing was not restricted to α-IgE stimulation but also occurred upon exposure to 100 ng/mL calcium-ionophore A23187 (Vi, 114.3 nmol/L/min) and upon lysis with 0.05% Triton X-100 (Vi, 210.1 nmol/L/min) (data not shown). These data correlated with those obtained for the induction of hexosaminidase release from the same cells by α-IgE (19.8% ± 0.4%), calcium-ionophore (45.8% ± 0.6%), and Triton X-100 (set to 100%) (not shown), strongly suggesting that CTAP-III processing requires the release of protease(s) from secretory granules.
Time course of mast cell–mediated NAP-2 formation as detected by immunoreactivity and biologic activity. A fixed concentration (1 × 104/mL) of either α-IgE–stimulated (□) or unstimulated (▪) MCs was incubated with 3 μM CTAP-III for increasing periods of time. Thereafter, 2 μL cell-free supernatant of each sample and standard preparations of CTAP-III and NAP-2 (50 ng per lane) were separated by SDS-PAGE, subjected to Western blotting, and immunochemically stained with rabbit antiserum Rα-βTG to visualize CTAP-III and potential degradation products (A). Migration of standard CTAP-III (CT) and NAP-2 (NA) is indicated. Data from 1 representative experiment of 3 are shown. For quantification of NAP-2 biologic activity, CTAP-III and derivates were isolated from recovered cell-free supernatants by immunoaffinity chromatography, and the amount of NAP-2–like activity formed was assessed by its neutrophil-stimulating capacity as determined by the elastase release assay against a standard of purified NAP-2 (B). Shown are means ± SD of data obtained in 3 independent experiments. In the approach using α-IgE–stimulated MCs, the initial velocity (Vi) of NAP-2 formation was calculated as the increase in NAP-2 concentration per minute on the basis of the first time point at which significantly elevated NAP-2-levels could be detected.
Time course of mast cell–mediated NAP-2 formation as detected by immunoreactivity and biologic activity. A fixed concentration (1 × 104/mL) of either α-IgE–stimulated (□) or unstimulated (▪) MCs was incubated with 3 μM CTAP-III for increasing periods of time. Thereafter, 2 μL cell-free supernatant of each sample and standard preparations of CTAP-III and NAP-2 (50 ng per lane) were separated by SDS-PAGE, subjected to Western blotting, and immunochemically stained with rabbit antiserum Rα-βTG to visualize CTAP-III and potential degradation products (A). Migration of standard CTAP-III (CT) and NAP-2 (NA) is indicated. Data from 1 representative experiment of 3 are shown. For quantification of NAP-2 biologic activity, CTAP-III and derivates were isolated from recovered cell-free supernatants by immunoaffinity chromatography, and the amount of NAP-2–like activity formed was assessed by its neutrophil-stimulating capacity as determined by the elastase release assay against a standard of purified NAP-2 (B). Shown are means ± SD of data obtained in 3 independent experiments. In the approach using α-IgE–stimulated MCs, the initial velocity (Vi) of NAP-2 formation was calculated as the increase in NAP-2 concentration per minute on the basis of the first time point at which significantly elevated NAP-2-levels could be detected.
Chymase is responsible for CTAP-III processing by MCs
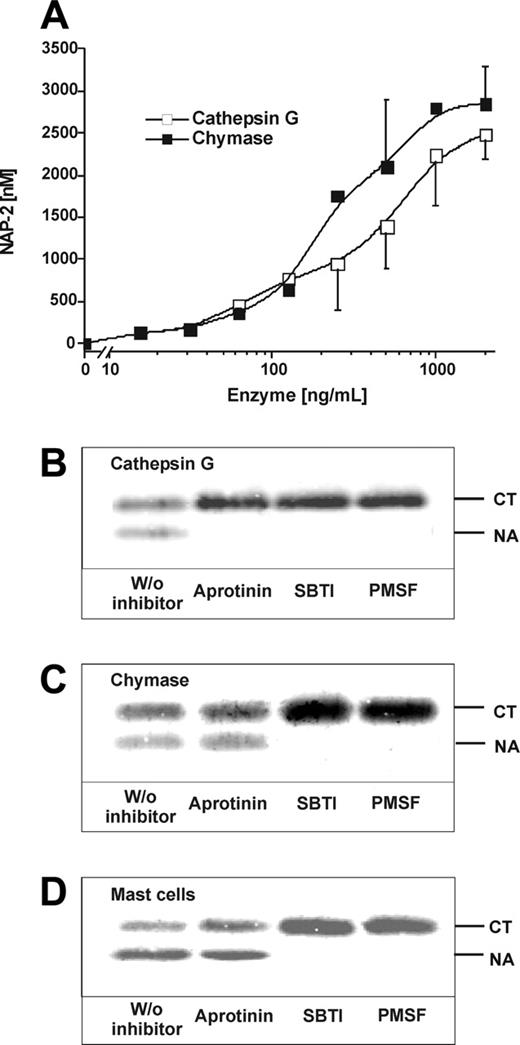
Apart from CathG, the protease responsible for CTAP-III cleavage by neutrophils, human skin MCs express and release chymase, another chymotryptic enzyme that could potentially cleave CTAP-III. Comparing increasing concentrations of both enzymes with respect to their CTAP-III–converting capacity (Figure 3A), about 500 ng/mL CathG and 250 ng/mL chymase (ie, 16 nM and 8 nM, respectively) were equivalent in catalyzing half-maximal conversion of the substrate (generation of 1.5 μM NAP-2). Beyond 1000 ng/mL of either enzyme, conversion was practically complete. These results supported our hypothesis that chymase participates in the processing of CTAP-III by MCs. To estimate the relative contributions of CathG and chymase to CTAP-III processing by MCs and to examine a potential role for mast cell tryptase, we used a set of serine protease inhibitors. As shown in control experiments using the purified enzymes (Figure 3B-C), CathG- as well as chymase-catalyzed CTAP-III conversion expectedly became completely inhibited by PMSF, a general serine hydrolase inhibitor, and by SBTI, an inhibitor affecting chymase but also CathG (as do all other chymase inhibitors). For specific inhibition of CathG, aprotinin was chosen,18,34 which in fact selectively prevented CTAP-III cleavage by the latter enzyme without affecting processing by chymase. Corresponding experiments with α-IgE–stimulated MCs resulted in an inhibitory pattern most similar to that observed with purified chymase (Figure 3D). Thus, MC-mediated NAP-2 formation was completely prevented in the presence of PMSF and SBTI, while hardly any reduction (5.3% ± 8.3%) became visible in the presence of aprotinin. Leupeptin, an inhibitor of tryptase,34 did not affect NAP-2 formation by MCs up to dosages of 400 μg/mL (data not shown). These results clearly show that chymase is the major protease responsible for CTAP-III processing by human MCs. CathG appears to confer only a marginal contribution, if any, and tryptase appears to be without importance.
Inhibition of mast cell–and mast cell protease–mediated CTAP-III conversion. Increasing concentrations of purified natural neutrophil CathG and mast cell chymase were incubated with a fixed concentration of 3 μM CTAP-III for 30 minutes at 37°C in processing buffer. Subsequently, 2 μL of each sample was subjected to SDS-PAGE and Western blotting and thereafter stained with Rα-βTG and IRDye 800-conjugated goat α-rabbit antiserum. The amount of generated NAP-2 is shown as assessed by Li-cor quantification against a standard of NAP-2 run in parallel. Data represent mean ± SD from 3 independent experiments (A). Likewise, fixed concentrations of CathG (500 ng/mL) (B) and chymase (250 ng/mL) (C) were incubated with 3 μM CTAP-III under the same conditions in the absence or presence of inhibitors SBTI (1 μg/mL), aprotinin (1 μg/mL), or PMSF (2 mM) for 30 minutes. Correspondingly, human skin MCs (1 × 104/mL) prestimulated with 3 μg/mL α-IgE for 30 minutes were incubated with 3 μM CTAP-III in the absence or presence of inhibitors as indicated above (D). The Western blots shown in panels B-D were performed and developed using the primary and secondary antisera given in Figure 1. The migration of untreated CTAP-III (CT) and NAP-2 (NA) is indicated. For quantification of formed NAP-2, fluorescence of NAP-2-bands was determined by Li-cor analysis and compared with that of 50 ng standardized NAP-2 on the same blot. One representative experiment of 3 is shown.
Inhibition of mast cell–and mast cell protease–mediated CTAP-III conversion. Increasing concentrations of purified natural neutrophil CathG and mast cell chymase were incubated with a fixed concentration of 3 μM CTAP-III for 30 minutes at 37°C in processing buffer. Subsequently, 2 μL of each sample was subjected to SDS-PAGE and Western blotting and thereafter stained with Rα-βTG and IRDye 800-conjugated goat α-rabbit antiserum. The amount of generated NAP-2 is shown as assessed by Li-cor quantification against a standard of NAP-2 run in parallel. Data represent mean ± SD from 3 independent experiments (A). Likewise, fixed concentrations of CathG (500 ng/mL) (B) and chymase (250 ng/mL) (C) were incubated with 3 μM CTAP-III under the same conditions in the absence or presence of inhibitors SBTI (1 μg/mL), aprotinin (1 μg/mL), or PMSF (2 mM) for 30 minutes. Correspondingly, human skin MCs (1 × 104/mL) prestimulated with 3 μg/mL α-IgE for 30 minutes were incubated with 3 μM CTAP-III in the absence or presence of inhibitors as indicated above (D). The Western blots shown in panels B-D were performed and developed using the primary and secondary antisera given in Figure 1. The migration of untreated CTAP-III (CT) and NAP-2 (NA) is indicated. For quantification of formed NAP-2, fluorescence of NAP-2-bands was determined by Li-cor analysis and compared with that of 50 ng standardized NAP-2 on the same blot. One representative experiment of 3 is shown.
Inhibitory impact of PF-4 on CTAP-III processing by mast cells and neutrophils. Human skin MCs (1 × 104/mL) prestimulated with α-IgE (A) and human neutrophils (5 × 106/mL) (C) were incubated with 3 μM CTAP-III in the absence and in the presence of increasing dosages of PF-4 for 30 minutes at 37°C. For time-course studies, prestimulated MCs (B) and neutrophils (D) were incubated for increasing times with either 3 μM CTAP-III alone (▪) or in the presence of 4 μM PF-4 (□). To determine the amount of generated NAP-2, 2-μL samples of cell-free supernatants were subjected to SDS-PAGE, Western blotting, and immunochemical staining with rabbit antiserum Rα-βTG. NAP-2 was then quantified by Li-cor analysis against a standard of NAP-2 run in parallel (A-B). In panel A, results are given as the percentage of NAP-2 formed in the absence of PF-4. Data represent mean ± SD from 3 independent experiments with cells from different donors.
Inhibitory impact of PF-4 on CTAP-III processing by mast cells and neutrophils. Human skin MCs (1 × 104/mL) prestimulated with α-IgE (A) and human neutrophils (5 × 106/mL) (C) were incubated with 3 μM CTAP-III in the absence and in the presence of increasing dosages of PF-4 for 30 minutes at 37°C. For time-course studies, prestimulated MCs (B) and neutrophils (D) were incubated for increasing times with either 3 μM CTAP-III alone (▪) or in the presence of 4 μM PF-4 (□). To determine the amount of generated NAP-2, 2-μL samples of cell-free supernatants were subjected to SDS-PAGE, Western blotting, and immunochemical staining with rabbit antiserum Rα-βTG. NAP-2 was then quantified by Li-cor analysis against a standard of NAP-2 run in parallel (A-B). In panel A, results are given as the percentage of NAP-2 formed in the absence of PF-4. Data represent mean ± SD from 3 independent experiments with cells from different donors.
PF-4 inhibits MC- as well as neutrophil-mediated CTAP-III conversion into NAP-2
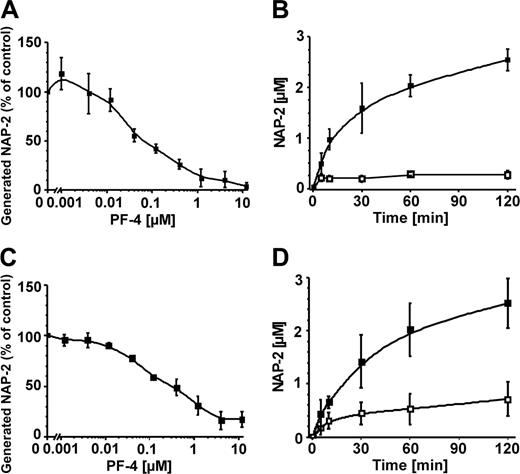
Because CTAP-III is released from activated platelets together with the structurally related chemokine PF-4, we wondered whether PF-4 would interfere with CTAP-III processing. To investigate this, we incubated α-IgE–stimulated MCs with a constant dosage of CTAP-III in the absence and presence of increasing concentrations of PF-4 for a fixed time. During incubation with CTAP-III alone, MCs converted approximately half of the precursor into NAP-2 (set to 100%), while in the presence of PF-4 the NAP-2 formation was inhibited in a concentration-dependent manner (Figure 4A). Inhibition by PF-4 was measurable at about 12 nM, was half maximal at about 75 nM, and approached maximum levels (90% or more) from 1.2 μM PF-4 on. To investigate the time course, cells were allowed to process CTAP-III for up to 120 minutes in the absence and in the presence of 4 μM PF-4. As shown in Figure 4B, with MCs receiving only CTAP-III, NAP-2 levels increased over time, being half maximal after about 30 minutes. By contrast, in the presence of PF-4, NAP-2 generation increased only within 5 minutes and became inhibited thereafter.
Corresponding experiments performed with unstimulated neutrophils yielded results similar to those obtained with MCs—that is, measurable reduction of NAP-2 formation occurred from a PF-4 dosage as low as about 12 nM on, whereas inhibition was half maximal at 220 nM and approached maximum levels (90% or more) at 4 μM (Figure 4C). As shown in Figure 4D, PF-4 also had a drastic effect on CTAP-III processing by neutrophils over time. Thus, the initial velocity of NAP-2 formation (about 47.3 nM NAP-2 per minute) was reduced to about 15.1 nM/min in the presence of PF-4. Even after 120 minutes of incubation, the inhibitory effect of PF-4 still was about 74%. These data demonstrate that PF-4 at physiological concentrations mediates efficient and long-lasting inhibition of CTAP-III processing by MCs as well as by neutrophils. To exclude that the inhibitory effect of the purified PF-4 was due to contamination by plasma inhibitors, the experiments depicted in Figure 4A,C were repeated using recombinant PF-4. Inhibition of CTAP-III processing by MCs as well as by neutrophils in the presence of the recombinant chemokine became measurable from about 12 nM on and reached maximum values (more than 90%) at 1.2 μM (data not shown). Thus, recombinant PF-4 was as efficient as the natural chemokine in inhibition of CTAP-III processing, thereby emphasizing that the effect of PF-4 was clearly not caused by contamination with plasma inhibitors.
PF-4 inhibits CTAP-III conversion by acting on MC chymase and CathG in a noncompetitive manner
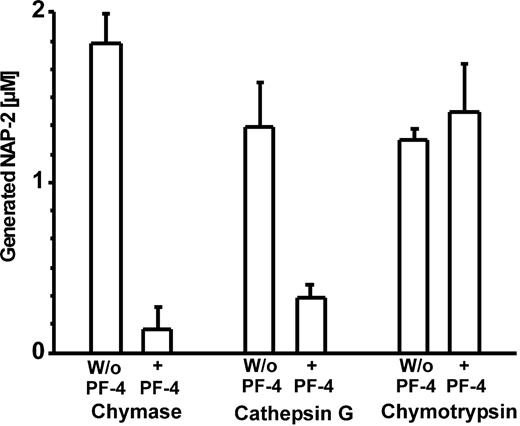
To obtain more insight into the mechanism(s) by which PF-4 affects CTAP-III processing, we first examined whether PF-4 would also inhibit CTAP-III cleavage by purified MC chymase and neutrophil CathG. As depicted in Figure 5, NAP-2 generation by either enzyme became drastically reduced in the presence of PF-4. More specifically, under conditions where chymase and CathG effected approximately half-maximal NAP-2 generation from 3 μM CTAP-III (1.82 ± 0.17 μM and 1.33 ± 0.26 μM, respectively) PF-4 had inhibitory effects of 92.3% and 75.3%, respectively. These results demonstrate that inhibition by PF-4 does not depend on the presence of cellular entities but rather relies on direct interference with the process of enzyme-catalyzed CTAP-III cleavage. Interestingly, bovine chymotrypsin was unaffected by PF-4—that is, no significant differences in NAP-2 generation were noticed in the absence (1.25 ± 0.07 μM NAP-2) and in the presence (1.41 ± 0.29 μM NAP-2) of PF-4 (Figure 5). The latter result demonstrates that PF-4 does not protect CTAP-III from cleavage by every chymotryptic enzyme. This argues against the idea that PF-4's inhibitory effect is mediated through an interaction with the substrate, but rather suggests that PF-4 directly modulates the catalytic activity of the respective protease(s).
Effect of PF-4 on NAP-2 formation by purified chymotryptic proteases. Purified human neutrophil cathepsin G (500 ng/mL), human mast cell chymase (250 ng/mL), or bovine pancreas chymotrypsin (200 ng/mL) was incubated with 3 μM CTAP-III alone or in the presence of 4 μM PF-4 for 30 minutes at 37°C. Thereafter, 2μL of each sample was subjected to SDS-PAGE and Western blotting and subsequently stained in sequence with Rα-βTG and IRDye 800–conjugated goat α-rabbit antiserum. NAP-2 was then quantified by Li-cor analysis against a standard of NAP-2 run in parallel. Data represent mean ± SD from 3 independent experiments.
Effect of PF-4 on NAP-2 formation by purified chymotryptic proteases. Purified human neutrophil cathepsin G (500 ng/mL), human mast cell chymase (250 ng/mL), or bovine pancreas chymotrypsin (200 ng/mL) was incubated with 3 μM CTAP-III alone or in the presence of 4 μM PF-4 for 30 minutes at 37°C. Thereafter, 2μL of each sample was subjected to SDS-PAGE and Western blotting and subsequently stained in sequence with Rα-βTG and IRDye 800–conjugated goat α-rabbit antiserum. NAP-2 was then quantified by Li-cor analysis against a standard of NAP-2 run in parallel. Data represent mean ± SD from 3 independent experiments.
To investigate the latter possibility, we examined PF-4's potential to act as a competitive substrate. Especially the tyrosine residue at position 60 within the sequence of PF-4 could form a potential cleavage site for chymotryptic enzymes. We thus incubated PF-4 and, for a control, also CTAP-III with chymase and CathG for 0 and 120 minutes, separated these samples by reverse-phase HPLC, and looked for potential degradation products by mass spectrometry (Figure 6). Expectedly, no cleavage products were detected in these samples at time point 0 minutes. After 120 minutes of incubation CTAP-III–containing samples displayed remarkably reduced CTAP-III peaks as well as 2 additional peaks representing the N-terminal 15 amino acid residue fragment of CTAP-III and NAP-2. Exposure of PF-4 to chymase or CathG for 120 minutes did not lead to a change in the elution profile of the sample. Neither were additional peaks observed; nor was there a change in peak area or retention time of the single component, which was identified as intact PF-4 (mass, 7764 amu). These data demonstrate that PF-4 is degraded neither by chymase nor by CathG under conditions where CTAP-III undergoes almost quantitative conversion into NAP-2. Thus, the mechanism by which PF-4 inhibits CTAP-III conversion cannot be based on substrate competition.
To more directly assess whether inhibition by PF-4 followed competitive or noncompetitive characteristics, we examined the processing kinetics of CTAP-III at the cellular level. For this MCs and neutrophils were incubated with increasing concentrations of CTAP-III in the absence and in the presence of a constant dosage of PF-4. As shown in Table 1, the inhibitory capacity of PF-4 did not remarkably change in the presence of different concentrations of CTAP-III but retained constant levels of about 62% to 70% with neutrophils and about 82% to 88% with MCs. On the basis of these results, PF-4's effect appears not to be that of a classic competitive inhibitor.
Impact of increasing CTAP-III concentration on PF-4 inhibition of NAP-2 generation by MCs and neutrophils
Cells and CTAP-III added, nM . | NAP-2 formed, nM . | . | . | |
|---|---|---|---|---|
| . | Without PF-4 . | With PF-4 . | % inhibition . | |
| MCs | ||||
| 1000 | 792 ± 124 | 96 ± 136 | 88.3 ± 17.1 | |
| 3000 | 2168 ± 615 | 326 ± 149 | 85.5 ± 4.0 | |
| 9000 | 3883 ± 1147 | 525 ± 89 | 85.3 ± 6.2 | |
| 27 000 | 8230 ± 3740 | 1570 ± 1071 | 82.0 ± 4.8 | |
| Neutrophils | ||||
| 1000 | 795 ± 83 | 253 ± 219 | 66.1 ± 29.3 | |
| 3000 | 2088 ± 194 | 648 ± 446 | 69.3 ± 19.4 | |
| 9000 | 5098 ± 697 | 1572 ± 731 | 70.1 ± 10.7 | |
| 27 000 | 10 607 ± 3199 | 3928 ± 1160 | 62.6 ± 4.5 | |
Cells and CTAP-III added, nM . | NAP-2 formed, nM . | . | . | |
|---|---|---|---|---|
| . | Without PF-4 . | With PF-4 . | % inhibition . | |
| MCs | ||||
| 1000 | 792 ± 124 | 96 ± 136 | 88.3 ± 17.1 | |
| 3000 | 2168 ± 615 | 326 ± 149 | 85.5 ± 4.0 | |
| 9000 | 3883 ± 1147 | 525 ± 89 | 85.3 ± 6.2 | |
| 27 000 | 8230 ± 3740 | 1570 ± 1071 | 82.0 ± 4.8 | |
| Neutrophils | ||||
| 1000 | 795 ± 83 | 253 ± 219 | 66.1 ± 29.3 | |
| 3000 | 2088 ± 194 | 648 ± 446 | 69.3 ± 19.4 | |
| 9000 | 5098 ± 697 | 1572 ± 731 | 70.1 ± 10.7 | |
| 27 000 | 10 607 ± 3199 | 3928 ± 1160 | 62.6 ± 4.5 | |
Different amounts of CTAP-III were incubated with α-IgE-stimulated MCs or unstimulated neutrophils in the absence or presence of a constant dosage of PF-4 (4 μM) for 30 minutes at 37°C. Generated NAP-2 was quantified by Li-cor analysis as described. Data represent mean ± SD from 3 different experiments.
Purified chymase and CathG cleave CTAP-III but not PF-4. Human chymase (Chy; 250 ng/mL) purified from mast cells (left panels) or human CathG (500 ng/mL) purified from neutrophil granulocytes (right panels) were incubated with either 3 μM CTAP-III (top row) or 4 μM PF-4 (bottom row) in processing buffer for 0 minutes (solid line) or 120 minutes (broken line) at 37°C. The products were separated, detected by their absorbance at 214 nm, and fractionated by reverse-phase HPLC using a linear gradient of acetonitrile in 0.1% TFA (dashed line) as described in “Materials and methods.” Fractions containing protein peaks eluting at retention times of 23.0 minutes, 22.2 minutes, 16.6 minutes, and 25.4 minutes were analyzed by ESI-FT-MS and identified by their respective mass of 9286 amu, 7623 amu, 1682 amu, and 7764 amu as CTAP-III, NAP-2, CTAP-III[1-15], and PF-4, respectively. One representative experiment of 3 is shown.
Purified chymase and CathG cleave CTAP-III but not PF-4. Human chymase (Chy; 250 ng/mL) purified from mast cells (left panels) or human CathG (500 ng/mL) purified from neutrophil granulocytes (right panels) were incubated with either 3 μM CTAP-III (top row) or 4 μM PF-4 (bottom row) in processing buffer for 0 minutes (solid line) or 120 minutes (broken line) at 37°C. The products were separated, detected by their absorbance at 214 nm, and fractionated by reverse-phase HPLC using a linear gradient of acetonitrile in 0.1% TFA (dashed line) as described in “Materials and methods.” Fractions containing protein peaks eluting at retention times of 23.0 minutes, 22.2 minutes, 16.6 minutes, and 25.4 minutes were analyzed by ESI-FT-MS and identified by their respective mass of 9286 amu, 7623 amu, 1682 amu, and 7764 amu as CTAP-III, NAP-2, CTAP-III[1-15], and PF-4, respectively. One representative experiment of 3 is shown.
PF-4 also inhibits the cleavage of substance P, a chymase/CathG substrate unrelated to CTAP-III
Finally, we addressed the question of whether PF-4 would also function as an inhibitor with substrates unrelated to CTAP-III. For this purpose we examined the cleavage of substance P (SP[1-11]), a well-characterized neuropeptide substrate of chymase and of CathG35,36 consisting of 11 amino acid residues (sequence, RPKPQQFFGLM). As analyzed by HPLC and mass spectrometry (Figure 7), SP became degraded by both proteases in a time-dependent manner to cleavage products SP[1-7], SP[8-11], and SP[1-8]. In the presence of PF-4, degradation was much less effective, as seen by much smaller decreases in SP[1-11] peak areas after 120 minutes with chymase (by 22.4%) and after 30 minutes with CathG (by 40.7%). Concomitantly, formation of the 3 SP degradation products was also retarded. These data clearly show that PF-4 as an inhibitor of chymase and CathG also interferes with the cleavage of small peptides unrelated to CTAP-III.
Discussion
In our present study, we demonstrate for the first time that human skin MCs are capable of converting exogenous CTAP-III into functionally active NAP-2 by limited proteolysis. Thus, our findings show that this important step in NAP-2 generation is not exclusively associated with blood leukocytes such as neutrophils9,13 but may also be executed by tissue cells located outside the bloodstream. Most interestingly, a direct comparison on a cell number basis revealed that MCs were by far more active than neutrophils, with unstimulated MCs exceeding the processing activity of both unstimulated and fMLP-stimulated neutrophils by about 30-fold, while α-IgE–stimulated MCs were even about 1000-fold more active. Because densities of neutrophils in the blood (0.5 × 104 to 1 × 104/mm3) and those of MCs in normal skin (0.4 × 104 to 1.3 × 104/mm3)37 are rather similar, these results indicate a high potential of MCs for CTAP-III activation under physiological conditions. This is underlined by our findings that 1 × 104 activated MCs per milliliter are already sufficient to generate 400 ± 85 nM NAP-2 from a physiological dosage of CTAP-III within only 10 minutes (Figure 2), a quantity about 40-fold and 300-fold higher than required to induce measurable neutrophil elastase release (threshold, 10 nM)38 and chemotaxis (threshold, 1.3 nM),12 respectively. This rapid accumulation of the chemokine is also reflected by the initial velocity of NAP-2 generation (46.4 nM NAP-2 per minute [357 ng/mL/min]), pointing out that functionally relevant amounts of the active chemokine may be formed within seconds. Interestingly, MCs secrete neutrophil-attracting chemokines by themselves, the most prominent one being IL-8. However, as compared with the data reported for IL-8 production (5 to 30 ng IL-8 per 106 skin MCs),39,40 there appears to exist a high potential for the CTAP-III/NAP-2 pathway in contributing to neutrophil recruitment by MCs.
Impact of PF-4 on the cleavage of SP by purified chymase and CathG. SP (10 μM) was incubated with the same enzyme concentrations as used previously for CTAP-III processing of either 250 ng/mL chymase (left panels) or 500 ng/mL CathG (right panels) for 0, 30, and 120 minutes in the absence (solid line) and in the presence (broken line) of PF-4 (4 μM) in processing buffer at 37°C. SP and its cleavage products were separated by reverse-phase HPLC using a linear acetonitrile gradient in 0.1% TFA (dashed line). Peptides as detected by their absorbance at 214 nm at retention times of 20.4 minutes, 13.7 minutes, 15.4 minutes, and 17.1 minutes were analyzed by ESI-FT-MS and identified by their respective mass of 1363 amu, 901 amu, 466 amu, and 1048 amu as full-size SP (SP[1-11], calculated mass of oxidized form, 1363 amu) and fragments SP[1-7] (901 amu), SP[8-11] (467 amu), and SP[1-8] (1046 amu). One representative experiment of 3 is shown.
Impact of PF-4 on the cleavage of SP by purified chymase and CathG. SP (10 μM) was incubated with the same enzyme concentrations as used previously for CTAP-III processing of either 250 ng/mL chymase (left panels) or 500 ng/mL CathG (right panels) for 0, 30, and 120 minutes in the absence (solid line) and in the presence (broken line) of PF-4 (4 μM) in processing buffer at 37°C. SP and its cleavage products were separated by reverse-phase HPLC using a linear acetonitrile gradient in 0.1% TFA (dashed line). Peptides as detected by their absorbance at 214 nm at retention times of 20.4 minutes, 13.7 minutes, 15.4 minutes, and 17.1 minutes were analyzed by ESI-FT-MS and identified by their respective mass of 1363 amu, 901 amu, 466 amu, and 1048 amu as full-size SP (SP[1-11], calculated mass of oxidized form, 1363 amu) and fragments SP[1-7] (901 amu), SP[8-11] (467 amu), and SP[1-8] (1046 amu). One representative experiment of 3 is shown.
As a most striking difference between both cell types, MCs utilize chymase instead of CathG as a CTAP-III–processing enzyme. To our knowledge this is the first report demonstrating a role for chymase in the processing of chemokines. In fact, in experiments using the purified proteases we could show that chymase cleaves CTAP-III at the same site as CathG (ie, C-terminal to the tyrosine in position 15). Notably, both enzymes generated the same cleavage products, the CTAP-III[1-15] N-terminal peptide and NAP-2, without the appearance of alternative degradation products (Figure 6). Moreover, we observed no major differences in the processing activities of both enzymes, because only about 2-fold more purified CathG than chymase was required to convert the same amount of substrate. Thus, the dominant role of chymase in MC-mediated CTAP-III processing appears surprising, inasmuch as MCs contain CathG along with chymase within their secretory granules.18 However, the reported amount of CathG in MCs is dramatically lower (0.1 to 0.7 pg per cell)41 than that of chymase (about 4.5 pg per cell),42 which may explain its negligible role in CTAP-III processing.
The utilization of different enzymes by neutrophils and MCs may be related to their different tissue localization, which may require distinct mechanisms for the functional regulation of CTAP-III processing. We have previously shown that CTAP-III processing by neutrophils is completely blocked by only 0.1% plasma, corresponding to the reported high susceptibility of CathG to plasma inhibitors.43 Nevertheless, after platelet activation and concomitant inclusion of neutrophils in thrombi substantial NAP-2 formation may occur (B.I. Schenk, unpublished observation, March 2001), obviously due to the exclusion of plasma inhibitors from this site. These observations clearly demonstrate the tight control of NAP-2 formation within the bloodstream. Remarkably, purified chymase in contrast to CathG reportedly retains considerable proteolytic activity against synthetic substrates in the presence of plasma.44 Moreover, the cleavage of ApoA1 by chymase in plasma corroborates the physiological importance of the enzyme's resistance to inhibitors.45 It may thus be envisaged that CTAP-III, being extravasated as a plasma constituent after MC activation and concomitant platelet aggregation, will also become processed by chymase. Because plasma inhibitors appear to play no major role in controlling chymase activity, one alternative mechanism to control its hazardous potential for inadequate and excessive NAP-2 generation could consist in the requirement for MC activation to release chymase.46 This is supported by our findings that CTAP-III–processing activity becomes mobilized from MCs only upon cell activation and is completely released into the environment. Interestingly, this appears to be substantially different with neutrophils, where CTAP-III conversion into NAP-2 was only marginally increased by stimulation and only some of the processing activity appeared in the supernatant. This goes together with findings from others suggesting that most extracellular CathG remains bound to the neutrophil cell surface.47 These differences in regulation of protease activity in MCs and neutrophils may indicate individual adaptations to their respective environment. In MCs, being practically immobilized within the tissue, cell activation regulates the local bioavailability of chymase, whereas in neutrophils, representing highly mobile blood cells, the absence or presence of plasma inhibitors in the respective compartment regulates CathG activity.
Regarding the concept outlined above, our finding that the chemokine PF-4 acted as a potent inhibitor of CTAP-III processing was highly surprising, inasmuch as PF-4 similarly affected neutrophils and MCs. Because PF-4 inhibited purified CathG and chymase to a degree comparable to that observed with the intact cells, a potential contribution by PF-4–mediated cellular effects is unlikely. To our knowledge so far no chemokine has been described to act as a direct enzyme inhibitor. Addressing the underlying mechanism(s), it is tempting to speculate that inhibition of CTAP-III processing by PF-4 may originate from these chemokines' close structural relationship (sequence identity, 53%).1 Interestingly, former reports demonstrated that both chemokines form homo-oligomers48,49 and that PF-4 even integrates other chemokines such as RANTES50 and IL-8 51 into hetero-oligomeric complexes. Along these lines, formation of hetero-oligomers between PF-4 and CTAP-III could protect the latter against proteolytic attack by rendering its cleavage sites inaccessible to proteases. Although we found in cross-linking studies that PF-4/CTAP-III hetero-oligomers do exist, their interaction appeared rather weak—that is, even with a 9000-fold molar excess of PF-4 still about 60% of CTAP-III remained in the monomeric state (T.A.G., unpublished data). Moreover, because according to our present results an about equimolar PF-4/CTAP-III ratio was sufficient for practically complete inhibition of CTAP-III conversion, direct interaction between the chemokines cannot explain the inhibitory action of PF-4. Additionally, our finding that chymotrypsin-mediated CTAP-III cleavage remained unaffected by PF-4 likewise argues against this hypothesis, inasmuch as PF-4/CTAP-III complexation should be expected to provide protection against any enzyme sharing the same cleavage site in CTAP-III. Because PF-4's inhibitory impact is obviously not based on its interaction with CTAP-III, we examined several potential modes of direct interaction of PF-4 with the proteases. Such interaction could consist in the competition of PF-4 with CTAP-III for binding to the enzyme's active center, where it might act as a competitive inhibitor or substrate. Examining the proteolytic susceptibility of PF-4 to CathG and chymase, we found neither cleavage of intact PF-4 (Figure 6) nor accumulation of PF-4 degradation products even upon long-term incubation with the proteases. Further experiments regarding the ability of PF-4 to compete with CTAP-III for binding to the protease(s) revealed that the inhibitory efficiency of a constant PF-4 dosage remained practically unchanged with increasing CTAP-III concentrations. Finally, PF-4 also inhibited the proteolysis of SP, a peptide substrate structurally unrelated to chemokines. Altogether, PF-4–mediated inhibition is obviously not due to competition between the structurally similar chemokines but must be based on other mechanisms of interaction with the proteases.
From a physiological viewpoint an especially important finding was the strikingly high potency of PF-4 as an inhibitor of CTAP-III processing. With neutrophils as well as MCs the dosages required for half-maximal inhibition (75 and 220 nM) were substantially below PF-4 serum levels (0.4 to 1.9 μM)1 and are thus likely to be relevant in vivo. Moreover, PF-4 affected the generation of NAP-2 for a time (120 minutes) sufficiently long to potentially dampen the establishment of a NAP-2 chemotactic gradient at the onset of inflammation. Intriguingly, instead of residing preformed in the plasma, PF-4 becomes released by activated platelets simultaneously with CTAP-III, strongly suggesting that NAP-2 formation by processing is tightly counterregulated on a temporal and spatial basis. The present results support our current concept of PF-4 as a regulator of CTAP-III/NAP-2 functions. As we reported previously, there does exist a cross-talk between PF-4 and CTAP-III regarding the regulation of neutrophil adhesion.17 In that study, we observed that CTAP-III–induced neutrophil adhesion to endothelial cells was dependent on the formation of NAP-2 through processing by the neutrophils themselves, while PF-4 abrogated CTAP-III–induced adherence. This phenomenon can now be easily explained by the action of PF-4 as an inhibitor of processing. Because PF-4 is also an efficient inhibitor of CTAP-III processing by MCs, our results altogether indicate a more general regulatory role for PF-4 in CTAP-III/NAP-2–dependent cell functions. However, as the present results suggest, PF-4's impact is not restricted to CTAP-III but also extends to other substrates of chymase and CathG such as SP, a neuropeptide with activating functions in neutrophils, MCs, and endothelial cells. Irrespective of this novel and unexpected role for PF-4, our observation that MCs represent highly efficient converters of platelet-secreted CTAP-III into a potent neutrophil attractant may point to a so far unrecognized mechanism for the interaction of MCs with blood cells. Thus, it has long been recognized that in acute allergic as well as infectious diseases simultaneous activation of MCs and thrombocytes is accompanied by the delayed recruitment of neutrophils to the inflamed tissue. With regard to the potential role of MC-mediated NAP-2 generation during these inflammatory processes, it may be worthwhile to more closely investigate the modulatory impact of MC proteases on the biologic activity of cytokines and other inflammatory mediators.
Prepublished online as Blood First Edition Paper, November 29, 2005; DOI 10.1182/blood-2005-06-2424.
Supported in part by Deutsche Forschungsgemeinschaft SFB/TR 22, Projekt A11 (E.B., F.P., and F.S.) and Projekt Z1 (B.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Christine Engellenner, Gabriele Huß, Alette Hettfleisch, and Lida Winter for excellent technical assistance and administrative help. We also thank Kathleen Marienfeld, Bernhard F. Gibbs, Zane Orinska, and Daniell Mitchell for help with the isolation and characterization of mast cells, Arnd Petersen for performing N-terminal sequence analysis, Natalia Nashkevich and Brigitte Kasper for fruitful discussion, and Annette Wallisch for critical reading of the manuscript. We moreover wish to thank Brigitte Flesch and Gesa Washington (Institute of Transfusion Medicine, University of Kiel, Germany) for the generous supply of platelet concentrates.

![Figure 6. Purified chymase and CathG cleave CTAP-III but not PF-4. Human chymase (Chy; 250 ng/mL) purified from mast cells (left panels) or human CathG (500 ng/mL) purified from neutrophil granulocytes (right panels) were incubated with either 3 μM CTAP-III (top row) or 4 μM PF-4 (bottom row) in processing buffer for 0 minutes (solid line) or 120 minutes (broken line) at 37°C. The products were separated, detected by their absorbance at 214 nm, and fractionated by reverse-phase HPLC using a linear gradient of acetonitrile in 0.1% TFA (dashed line) as described in “Materials and methods.” Fractions containing protein peaks eluting at retention times of 23.0 minutes, 22.2 minutes, 16.6 minutes, and 25.4 minutes were analyzed by ESI-FT-MS and identified by their respective mass of 9286 amu, 7623 amu, 1682 amu, and 7764 amu as CTAP-III, NAP-2, CTAP-III[1-15], and PF-4, respectively. One representative experiment of 3 is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/6/10.1182_blood-2005-06-2424/4/m_zh80060692720006.jpeg?Expires=1769087302&Signature=Na83ltQOjgcQVv84TrNGrQGOr~zK4PwWBYlzv-p9Fh-eHEvn0xSxccstxAGl8IvH56sY5Vq7KobVSD5NS0hq2HbTn4QVfr4MI30hSIWvs81ci2BjtbWxvOfR-qfcz2zvNdZxcLQZlLJaeDFWd-d~KzMpNTo8SDE8TDkDjn927ABT71VL69CBLu8Ie0oD2k6n0ywG3Kkl9eH7Tdo1~qmAgvmyyO7V0knO7UOVUse7ph9Y1uLEFr74qx6T64nQ0zcqhkvdC~XMPYlR-CYNuDTj23myyiBv~ao1osngfW3fxKb7wI68p2LeJjPQVqSa7gMl3cO~FXdqRu1VqYphy2y3rA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Impact of PF-4 on the cleavage of SP by purified chymase and CathG. SP (10 μM) was incubated with the same enzyme concentrations as used previously for CTAP-III processing of either 250 ng/mL chymase (left panels) or 500 ng/mL CathG (right panels) for 0, 30, and 120 minutes in the absence (solid line) and in the presence (broken line) of PF-4 (4 μM) in processing buffer at 37°C. SP and its cleavage products were separated by reverse-phase HPLC using a linear acetonitrile gradient in 0.1% TFA (dashed line). Peptides as detected by their absorbance at 214 nm at retention times of 20.4 minutes, 13.7 minutes, 15.4 minutes, and 17.1 minutes were analyzed by ESI-FT-MS and identified by their respective mass of 1363 amu, 901 amu, 466 amu, and 1048 amu as full-size SP (SP[1-11], calculated mass of oxidized form, 1363 amu) and fragments SP[1-7] (901 amu), SP[8-11] (467 amu), and SP[1-8] (1046 amu). One representative experiment of 3 is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/6/10.1182_blood-2005-06-2424/4/m_zh80060692720007.jpeg?Expires=1769087302&Signature=TiHdZsT3zZuvdA1PDjvBtBVLsdKPQotpGvIJIoc1Ccr8wdrE16P7sAjBIb6Tv6eB4aaLhM8ZvMVDQoVmUweI1MjDwrN5Uphtxvj53wEjhjTdG71Ba~Ks4tbeWMWV719Qu0X9Teh91aSJo21Ls7jfXdOzUyvYDm6pfXiwf85IPWKa-WiBZXpBTvXfjMHghAna-GytgL104XUxzHIaQjM6J0nFvTqZ5muE8BOREJ4v9vHVv-BKbkyL~3CraCJDXKpl~80BvwaYfnonDHY4ABxiZWhoHNeBfd26tQ34sA2zPPZj-llapaS330uw8lV~pgYI1xF35i28VPjVRMcXc6sC~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal