Abstract
The use of myelosuppressive agents to reduce the risk of thrombosis in patients with polycythemia vera (PV) and essential thrombocythemia (ET) has been associated with an increased risk of transformation to acute myeloid leukemia (AML). Whereas chlorambucil, busulfan, and radiophosphorus (32P) have been demonstrated to increase the risk of transformation, the leukemogenic potential of hydroxyurea (HU) continues to be a matter of debate. Clinical studies have suggested that HU may cause a small increase in the risk of AML, but it has proven difficult to establish whether AML is actually caused by HU or arises during the natural progression of PV and ET. Reports that HU undergoes metabolic activation to species that induce mutation appear to support the notion that it is leukemogenic. Here, we suggest that the ability of HU to induce mutation in cell culture studies results from the generation of nitrogen dioxide via the autoxidation of nitric oxide, a product of HU metabolism. However, we argue that autoxidation would not occur in vivo, leading to the conclusion that generation of the mutagen nitrogen dioxide is peculiar to cell culture systems and has little relevance to the use of HU in the management of PV and ET.
Introduction
Polycythemia vera (PV) and essential thrombocythemia (ET) are chronic myeloproliferative disorders, shown recently to be associated with an acquired mutation in the JAK2 tyrosine kinase gene.1-3 There is a rare incidence of progression to myelofibrosis and myeloid metaplasia in both disorders, which may or may not precede transformation to acute myeloid leukemia (AML), but thrombosis is the main cause of mortality.4,5 Phlebotomy remains an important tool in the management of PV, but additional treatment with cytoreductive agents is required for most patients.6 Similarly, myelosuppressive therapy is often necessary in the management of ET, according to the risk of thrombosis.5 As the use of cytoreductive agents can increase the risk of transformation to AML in PV and ET, management of the conditions entails a fine balancing act in which the aim is to prevent thrombosis without increasing the risk of AML.4-6 Indeed, whereas early advocates of radiophosphorus (32P)–cytoreductive therapy may have considered AML to be an inevitable consequence of extended patient survival, the Polycythemia Vera Study Group (PVSG-01) trial demonstrated that treatment with either 32P or chlorambucil results in an incidence of AML far exceeding that observed with phlebotomy alone, without prolonging survival.7
Compared with 32P and the alkylating agents chlorambucil and busulfan, the use of hydroxyurea (HU) as a myelosuppressive agent is widely considered to carry a low risk of transformation to AML.4,5,8 This view is supported by the findings of the most recent studies,9,10 including one reported by the United Kingdom Medical Research Council (UK MRC) Primary Thrombocythemia 1 Study, showing that the use of HU carries no increased risk of AML in ET and PV when used in the absence of other cytoreductive agents.10 Nevertheless, concerns over the safety of HU remain, not least because the agent inhibits DNA repair,11 which may explain in part why HU increases the risk of leukemia in patients with a history of treatment with of other cytoreductive agents.12 This issue is particularly pertinent to the management of ET, where anagrelide and interferon alpha provide alternative therapeutic options. However, the recent report by the UK MRC Primary Thrombocythemia 1 study concluded that HU plus low-dose aspirin is superior to anagrelide plus low-dose aspirin in the management of ET in patients at high risk for vascular events.10 Similarly, pipobromain, a metabolic inhibitor that carries only a slightly increased risk of AML, provides an alternative to HU for the suppression of myeloproliferation in ET and PV.11,13
Against this background, it is useful to look beyond the clinical data and consider the chemical and biochemical features of HU for clues that might indicate its potential for leukemogenicity.
Reactive oxygen and nitrogen species in the metabolism of hydroxyurea
Although HU, which inhibits DNA synthesis through its action on ribonucleotide reductase (RR), is not believed to interact directly with DNA, it is possible the agent may act as a mutagen and carcinogen by preventing the repair of acquired mutations,11 which can be converted to DNA strand breaks.14 The accumulation of strand breaks, due to a shortage of deoxyribonucleoside triphosphates during DNA repair and replication, provides a mechanism for the induction of chromosomal translocation. However, an investigation into the acquired mutation rate in human subjects with in vivo HU exposure concluded that the mutagenic and carcinogenic potential of HU therapy is low.15 It is also possible that HU provides a selective advantage to clones with particular chromosomal abnormalities, such as the 17p deletion.12
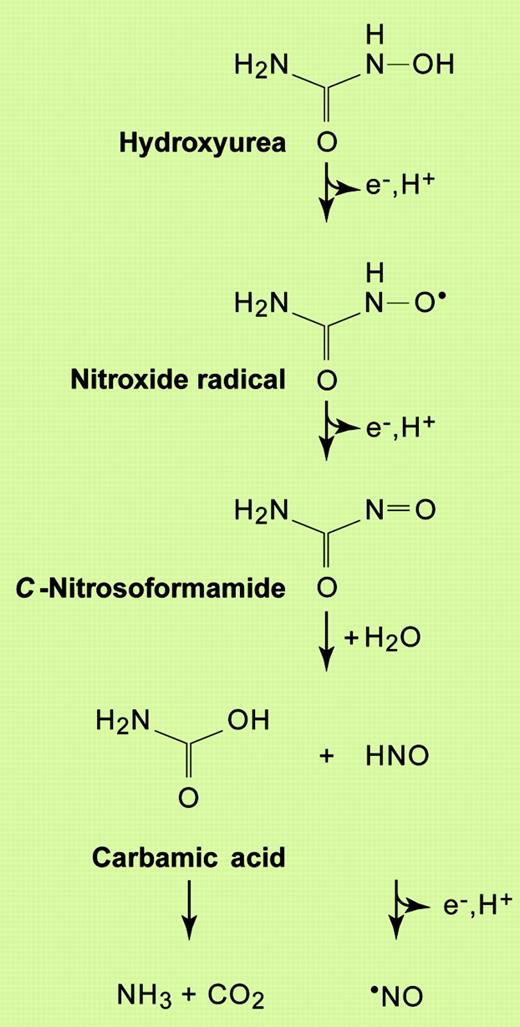
Alternatively, there is the possibility that reactive species generated during the metabolism of HU cause damage to DNA and thereby increase the risk of AML. Indeed, although HU can directly quench the catalytically active tyrosyl radical in RR,16 it is becoming increasingly apparent that the nitric oxide radical (·NO), generated upon the 3-electron oxidation of the drug, may be responsible for many of its pharmacologic effects, including the inhibition of RR (Figure 1).17-21 The radical itself is poorly reactive, with most of the molecular damage seen in biological systems exposed to ·NO attributed to the formation of powerful oxidants: the nitrogen dioxide radical (·NO2), the hydroxyl radical (·OH), and the carbonate anion radical (
Reaction scheme for the generation of nitric oxide from HU. Based on the reactions proposed by Huang et al21 for the oxidation of HU by horseradish peroxidase. Other reactions are also believed to occur (not shown), including the possible direct release of ·NO from the nitroxide radical intermediate, and the dimerization of nitroxyl (HNO) to generate nitrous oxide (N2O). Illustration by Paulette Dennis.
Reaction scheme for the generation of nitric oxide from HU. Based on the reactions proposed by Huang et al21 for the oxidation of HU by horseradish peroxidase. Other reactions are also believed to occur (not shown), including the possible direct release of ·NO from the nitroxide radical intermediate, and the dimerization of nitroxyl (HNO) to generate nitrous oxide (N2O). Illustration by Paulette Dennis.
For these reactive species to be formed, ·NO must compete effectively with the superoxide dismutases (SODs) for reaction with superoxide, which otherwise undergoes the SOD-catalyzed disproportionation:
Under physiologic conditions, where ·NO is produced as a signaling molecule, its concentration is believed to be too low to achieve this; in other words, the rate of superoxide removal by SOD is so fast that it has little opportunity to combine with ·NO to form ONOO–/ONOOH.26 In such situations, ·NO is free to bind to the iron centers of heme proteins under its regulation, including guanylate cyclase.27 Only under pathologic conditions, where local concentrations of ·NO can be much higher (eg, due to the activity of inducible nitric oxide synthase in activated macrophages), is ONOO–/ONOOH produced in significant amounts.26
It might be assumed, then, that the high local concentrations of ·NO achieved during the metabolism of nitric oxide prodrugs, such as HU, would allow the radical to combine with superoxide and produce harmful species. However, a fate of ·NO that is often overlooked in cell-culture studies is its autoxidation, which also gives rise to nitrogen dioxide:
Although ·NO autoxidation to ·NO2 occurs readily in aqueous solution at ambient oxygen tensions, within tissues the reaction is considered to be of limited significance because the prevailing oxygen and ·NO concentrations are typically too low to allow the above reactions to proceed at a significant rate.28,29 Only within biological membranes (or the hydrophobic milieu of proteins), where ·NO and O2 can achieve relatively high local concentrations, might the intracellular autoxidation of ·NO to the harmful ·NO2 occur. Even at relatively high concentrations, ·NO has very low toxicity at physiologic O2 tensions. In contrast, physiologic levels of ·NO exert toxic effects at the supraphysiologic O2 tensions typical of many experimental systems.30 This means that studies on the toxicity of ·NO carried out in vitro, including those using cultured cells, which are generally grown in a 95%-air atmosphere, can be misleading because the inevitable autoxidation of ·NO to the harmful ·NO2 (and higher oxides28 ) that occurs under such conditions would probably not occur to any significant extent in vivo.31-33 This argument also applies to nitric oxide prodrugs, such as HU. In other words, it should not be assumed that HU is a potential mutagen or carcinogen on the grounds that it provides a source of ·NO for the generation of more reactive (and harmful) higher oxides of nitrogen.
There are, in fact, very few reports suggesting that HU is mutagenic.15,34-36 In one early study, mutagenicity required metabolic activation (suggesting a role for ·NO), but involved cells grown in a 95%-air atmosphere.35 Another study reported the ability of HU to promote damage to isolated DNA by copper (II) ions, but here the drug served primarily as a reducing agent for the metal ion, which in turn reduced oxygen to reactive species.36 As the availability of free copper ions to participate in these reactions in vivo is uncertain, and because there are plenty of other reductants of Cu(II) available in the cell (eg, ascorbate and glutathione), these reactions are likely to be of limited biological relevance. Most studies reporting DNA damage by HU appear to involve DNA strand breakage, which can be attributed in the main to incomplete strand joining during repair and replication, as referred to above.14,37,38
Proposed involvement of peroxidases in hydroxyurea metabolism
There remains of course the possibility that ·NO2 and the other harmful species described above could still be formed in bone marrow through the initial combination of ·NO with superoxide (to give ONOO–/ONOOH). The key question that arises, then, is whether the concentration of superoxide in the bone marrow is high enough for this to occur. To address this issue, it is necessary to consider the enzymes in the bone marrow that might be capable of bringing about the oxidation of HU, which is necessary for ·NO to be released. The obvious candidates are myeloperoxidase (MPO) and prostaglandin H synthase (PGHS).39-41 MPO converts hydrogen peroxide (H2O2) to hypochlorous acid (HOCl), which plays an important role in the host defense against bacteria. In doing so, the enzyme reaction center forms an oxo-ferryl heme intermediate (containing a porphyrin radical) known as peroxidase compound I. This intermediate can bring about the oxidation of a wide variety of small molecules.42 Since the compound I form of horseradish peroxidase (the prototypical peroxidase often used in model studies) has been shown to oxidize HU to ·NO,21 it is possible that MPO may bring about this reaction in the bone marrow.
MPO requires hydrogen peroxide for activity.42 Activated neutrophils, including those present in the bone marrow, are capable of generating large amounts of superoxide using NAD(P)H oxidase during their respiratory burst.43,44 This radical can undergo disproportionation to H2O2, which may be catalyzed by SOD (see above). It is likely, then, that HU can undergo oxidation by MPO at sites of inflammation: H2O2 would be available to activate the enzyme and superoxide would be available for the subsequent formation of ONOO–/ONOOH and, consequently, the potential mutagens ·NO2, ·OH, and
Prostaglandin synthase exists as 2 isoforms, PGHS-1 and PGHS-2, also known as cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), respectively. PGHS-1, the constitutive form, appears to play a role in myeloid differentiation, whereas PGHS-2, the inducible form, may be specifically involved in megakaryocytopoiesis.40,41 Both are bifunctional heme enzymes: their cyclooxygenase activity catalyzes the incorporation of 2 molecules of oxygen into arachidonic acid to give prostaglandin endoperoxide G2 (PGG2); their peroxidase activity then reduces PGG2 to prostaglandin endoperoxide (PGH2). To perform these functions, the active site of PGHS is first oxidized to a peroxidase compound I intermediate (consisting of an oxo-ferryl heme and a porphyrin radical).46 A tyrosyl radical, required for the cyclooxygenase activity of PGHS, is then generated by electron transfer from Tyr385 to the porphyrin radical. Although the tyrosyl radical in PGHS reacts much slower than that in RR with HU,47 the compound I intermediate can oxidize a range of substrates and therefore is expected to be capable of generating ·NO from HU. However, in contrast with MPO, H2O2 is not required for the activation of PGHS to its compound I: this can be brought about by PGG2, the product of its cyclooxygenase action on arachidonic acid. Although a trace of lipid hydroperoxide is required to initiate PGHS activation, the enzyme becomes autocatalytic through its production of PGG2 and so, in contrast with MPO, is not dependent on a source of H2O2. Therefore, PGHS should be able to generate ·NO from HU in the absence of a source of superoxide radicals, thereby allowing the inhibition of RR without running the risk of genotoxicity.
Hydroxyurea therapy in sickle cell disease
Although sickle cell disease (SCD) is not a myeloproliferative disorder, the recent emergence of HU as a powerful tool in the management of this prevalent condition warrants discussion. HU therapy leads to the induction of hemoglobin F (HbF), which interferes with the polymerization of hemoglobin S.48 Recent findings suggest that the induction of HbF by HU involves the activation of soluble guanylate cyclase by ·NO.49 Indeed, nitrosylhemoglobin, resulting from the binding of ·NO to deoxyhemoglobin, has been detected in blood from a patient with SCD following HU administration.50 Although ·NO can be formed by the direct reaction of HU with hemoglobin, this reaction appears to be too slow to account for the rate of production of the radical in vivo. Instead, it is likely that HU is first metabolized by urease to hydroxylamine, which reacts with hemoglobin to form nitrosylhemoglobin.51
Clinical benefits from HU have been reported in some patients with SCD before levels of HbF have increased, suggesting that the ability of the agent to ease vaso-occulsive events may be due, in part, to the vasodilative actions of ·NO.48 Other potentially important mechanisms of HU action include myelosuppression, as evidenced by the strong relationship between neutrophil count and clinical outcome in SCD.48 In the context of HU and SCD, the risk of leukemia appears to be much smaller than in the myeloproliferative disorders.48
Summary and concluding remarks
It is concluded that HU is not directly mutagenic. In most studies where it has been demonstrated to damage DNA directly this can be attributed to the production of nitrogen dioxide during the autoxidation of nitric oxide31-33 ; these reactions are not expected to be of importance in vivo.28 HU could, however, induce DNA damage if the nitric oxide it yields upon oxidation has the opportunity to combine with superoxide, which leads to the production of nitrogen dioxide and other powerful oxidants. Superoxide is most likely to be available to combine with nitric oxide at sites of inflammation, where MPO might well play an important role in the oxidation of HU. However, under these conditions the same oxidants would also be generated through the concerted actions of iNOS and NAD(P)H oxidase,45 so the signifance of additional nitric oxide generation from HU needs to be investigated. Additional nitric oxide might even be protective through its binding to the heme sites of MPO and iNOS, thereby blocking their generation of reactive species.
PGHS may play a more important role than MPO in the release of nitric oxide from HU in bone marrow, where there may be less opportunity for the conversion of nitric oxide to harmful oxidants. Indeed, an investigation into the myelotoxicity of benzene identified a role for PGHS, rather than MPO, in the oxidation of its hydroquinone metabolite to p-benzoquinone, the putative genotoxic metabolite.52 If this is the case, then the use of aspirin and other PGHS inhibitors to prevent thrombosis in PV and ET may have implications for the metabolism and efficacy of HU.
Although it is reasonable to conclude that HU is unlikely to damage DNA directly when used therapeutically, the fact that the drug can inhibit DNA repair processes by decreasing the pool of deoxyribonucleoside triphosphates means that it would be unwise to use HU, as far as is practicable, when (1) the patient has a history of treatment with other cytoreductive agents that are known to damage DNA; and (2) the risk of thrombosis is small, such as in young patients.
Prepublished online as Blood First Edition Paper, November 10, 2005; DOI 10.1182/blood-2005-08-3429.
Supported by Cancer Research UK (grant no. C134/A2001).
The authors are grateful to Prof P. Wardman for critical reading of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal